Articles
- Page Path
- HOME > Restor Dent Endod > Volume 46(2); 2021 > Article
-
Research Article
Influence of reciprocating and rotary instrumentation on microbial reduction: a systematic review and meta-analysis of
in vitro studies -
Selen Küçükkaya Eren1
 , Emel Uzunoğlu-Özyürek1
, Emel Uzunoğlu-Özyürek1 , Sevilay Karahan2
, Sevilay Karahan2
-
Restor Dent Endod 2021;46(2):e19.
DOI: https://doi.org/10.5395/rde.2021.46.e19
Published online: March 10, 2021
1Department of Endodontics, Faculty of Dentistry, Hacettepe University, Ankara, Turkey.
2Department of Biostatistics, Faculty of Medicine, Hacettepe University, Ankara, Turkey.
- Correspondence to Selen Küçükkaya Eren, DDS, PhD. Associate Professor, Department of Endodontics, Faculty of Dentistry, Hacettepe University, Sıhhiye, Ankara 06100, Turkey. selenkkkaya@yahoo.com
Copyright © 2021. The Korean Academy of Conservative Dentistry
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Objectives The purpose of this study was to conduct a systematic review and meta-analysis of in vitro studies regarding the effectiveness of reciprocating and rotary instrumentation on microbial reduction in root canals.
-
Materials and Methods PubMed, Scopus, Web of Science, the Cochrane Library, and the gray literature were searched through December 2019. Studies comparing the influence of reciprocating and rotary instrumentation on the removal of microorganisms from root canals that quantified the antimicrobial effect were included. Data extraction was completed using a systematic form for data collection. The risk of bias of the studies was evaluated. Standardized mean differences (SMDs) and confidence intervals (CIs) were calculated using a random effects meta-analysis.
-
Results Seventeen in vitro studies were included in this systematic review, of which 7 provided adequate data for inclusion in the meta-analysis. Both reciprocating and rotary systems were similarly effective in reducing the microbial load in infected root canals (SMD [95% CI], 0.0481 [−0.271, 0.367]). Three studies showed a low risk of bias, whereas most of the studies (82%) presented a medium risk.
-
Conclusions Although both techniques decrease the microbial content (with reductions of 23.32%–88.47% and 23.33%–89.86% for reciprocating and rotary instrumentation, respectively), they are not able to provide complete disinfection of root canals.
INTRODUCTION
MATERIALS AND METHODS
Example of the search strategy (PubMed)
1. In vitro studies performed on fully formed human permanent teeth
2. Teeth that had not received any endodontic treatment previously
3. Teeth contaminated with microorganisms
4. Studies comparing the efficacy of reciprocating and rotary instrumentation for the removal of microorganisms from root canals
5. Studies that quantified the antimicrobial effect and reported the outcome as reduction in microbial load
Summary of the main characteristics of the included in vitro studies
| Studies | Tooth type | No. | Sterilization procedure | Preparation before contamination | Smear layer removal before contamination | Microorganism type | Incubation period following contamination | Confirmation of contamination |
|---|---|---|---|---|---|---|---|---|
| Alves et al. [3] | Mandibular incisors and maxillary second premolars with single root canals | 34 | Autoclave | 25 K-file | 17% EDTA and 2.5% NaOCl | E. faecalis strain (ATCC 29212) | 30 days | SEM |
| Alves et al. [13] | Distobuccal canals of maxillary molars | 43 | Autoclave | Rotary instrument, size 10/0.04 | 17% EDTA | E. faecalis strain (ATCC 29212) | 30 days | Culture technique and SEM |
| Basmaci et al. [14] | Mandibular premolars with single root canals | 81 | Autoclave | 20 K-file | NM | E. faecalis strain (ATCC 29212) | 24 hours | Culture technique |
| Dagna et al. [15] | Single-rooted teeth | 60 | Autoclave | 20 K-file | 10% EDTA | E. faecalis strain (ATCC 19433) | 120 hours | Culture technique |
| de Brito et al. [16] | Mandibular premolars | 100 | Autoclave | 20 K-file | NM | E. faecalis strain (ATCC 29212) | 28 days | Culture technique |
| de Oliveira et al. [17] | Mandibular premolars | 60 | Autoclave | NM | NM | E. faecalis (ATCC 6057), P. aeruginosa (ATCC 27853), S. aureus (ATCC 29213) and C. albicans (ATCC 10231) | 48 hours | Culture technique |
| Ferrer-Luque et al. [18] | Single-rooted mandibular premolars | 76 | Autoclave | 25 K-file | 17% EDTA and then irrigated with 1% NaOCl followed by DW | E. faecalis strain (ATCC 29212) | 4 weeks | Culture technique |
| Guillen et al. [19] | Distobuccal canals of maxillary molars | 56 | Ethylene oxide | 15 K-file | 17% EDTA | E. faecalis strain (ATCC 29212) | 21 days | Culture technique |
| Karatas et al. [20] | Mandibular incisor teeth | 70 | Autoclave | 20 K-file | NM | E. faecalis strain (ATCC 29212) | 48 hours | Culture technique |
| Krokidis et al. [21] | Canines, lower incisors and premolars with single root canals | 50 | Autoclave | 25 K-file | 17% EDTA and 2.5% NaOCl | E. faecalis strain (ATCC 29212) | 30 days | Culture technique |
| Machado et al. [4] | Distobuccal canals of maxillary molars | 65 | Ethylene oxide | 15 K-file | 17% EDTA and DW | E. faecalis strain (ATCC 29212) | 21 days | Culture technique |
| Marinho et al. [9] | Mandibular premolars | 40 | Gamut radiation and autoclave | 15 K-file | 17% EDTA, 5.25% NaOCl and DW | E. coli strain (ATCC 25922) | 21 days | Culture technique and SEM |
| Nabeshima et al. [22] | Distobuccal canals of maxillary molars | 51 | Ethylene oxide | 15 K-file | 17% EDTA and DW | E. faecalis strain (ATCC 29212) | 21 days | Culture technique |
| Nakamura et al. [23] | Mandibular premolars | 50 | Autoclave | 30 K-file | 17% EDTA-T, 5.25% NaOCl and DW | E. faecalis strain (ATCC 29212) | 28 days | Culture technique and SEM |
| Siqueira et al. [8] | Mesial canals of mandibular molars | 36 | Autoclave | 20 K-file | 17% EDTA and 2.5% NaOCl | E. faecalis strain ATCC 29212 | 30 days | Culture technique and SEM |
| Üreyen Kaya et al. [24] | Mandibular premolars | 74 | Autoclave | NM | NM | E. faecalis | 4 weeks | Culture technique and SEM |
| Vasconcelos et al. [25] | Mandibular incisors | 84 | Autoclave | 20 K-file | 1% NaOCl, 17% EDTA and saline | E. faecalis strain ATCC 29212 | 5 days | Culture technique and SEM |
Details extracted from the included studies regarding methodology and main outcomes
| Studies | Instrumentation systems tested (final apical diameter/taper) | Irrigation techniques and irrigants | Sampling time | Evaluation method | Main findings |
|---|---|---|---|---|---|
| Alves et al. [3] | Reciproc (40/0.06), BioRace (40/0.04) | 2.5% NaOCl and 17% EDTA | S1 and S2 | CFU, qPCR | No difference was found between the instrumentation systems. |
| Alves et al. [13] | Reciproc (25/0.08), XP-endo Shaper (30/0.04) | Saline | S1 and S2 | qPCR | XP-endo Shaper resulted in higher bacteria reduction. |
| Basmacı et al. [14] | SAF (1.5 mm), Reciproc (25/0.08), ProTaper Universal (30/0.09) | a) PBS | S1 and S2 | CFU | No difference was found among the instrumentation systems. |
| b) 5% NaOCl and 15% EDTA | |||||
| c) 5% NaOCl and 7% maleic acid | |||||
| Dagna et al. [15] | Mtwo (30/0.05), Revo-S (25/0.06), Reciproc (25/0.08), OneShape (25/0.06) | 5.25% NaOCl and 17% EDTA | S1 and S2 | CFU | No difference was found among the instrumentation systems. |
| de Brito et al. [16] | ProTaper Next (40/0.06), ProTaper Universal (40/0.06), WaveOne Large (40/0.08) | a) 2.5% NaOCl and 17% EDTA | S1 and S2 | CFU | WaveOne resulted in a lower level of bacterial reduction when saline solution was used. No difference was found among the instrumentation systems when NaOCl and EDTA were used. |
| b) Saline | |||||
| de Oliveira et al. [17] | ProTaper Universal (30/0.09), Reciproc (40/0.06) | a) 1% NaOCl | S1 and S2 | Presence/Absence | ProTaper Universal showed the best results when NaOCl was used. |
| b) Saline | |||||
| Ferrer-Luque et al. [18] | Mtwo (40/0.04), Twisted File (40/0.04), WaveOne (40/0.08) | a) DW | S1 and S2 and S3 (after 60 days) | CFU | No difference was found among the instrumentation systems after S2. Mtwo showed the best results when NaOCl was used at 60 days (S3). |
| b) 5.25% NaOCl | |||||
| Guillen et al. [19] | WaveOne Gold (25/0.07), WaveOne (25/0.08), One Shape New Generation (25/0.06), One Shape (25/0.06) | DW | S1 and S2 and S3 (after 7 days) | CFU | WaveOne Gold and One Shape New Generation promoted higher bacterial reduction than WaveOne and One Shape systems. |
| Karatas et al. [20] | ProTaper Next (25/0.06), Twisted File Adaptive (25/0.06), SAF (1.5 mm), WaveOne (25/0.08), Reciproc (25/0.08), OneShape (25/0.06) | DW | S1 and S2 | CFU | No difference was found between the rotary and reciprocating instrumentation. |
| Krokidis et al. [21] | BT-Race (40/0.04), WaveOne (40/0.08) | 2.5% NaOCl and 17% EDTA | S1 and S2 | CFU | BT-RaCe resulted in higher bacteria reduction. |
| Machado et al. [4] | WaveOne (25/0.08), Reciproc (25/0.08), ProTaper Universal (25/.08), Mtwo (25/0.06), K-file (35/0.02) | DW | S1 and S2 and S3 (after 7 days) | CFU | No difference was found among the instrumentation systems. |
| Marinho et al. [9] | Reciproc (25/0.08), Mtwo (25/0.06), ProTaper Universal (25/0.08), Race (25/0.04) | Endotoxin-free water (LAL water) | S1 and S2 | CFU, LAL assay (for endotoxin reduction) | No difference was found among the instrumentation systems. |
| Nabeshima et al. [22] | WaveOne (25/0.08), One Shape (25/0.06), K-file (35/0.02) | DW | S1 and S2 | CFU | No difference was found among the instrumentation systems. |
| Nakamura et al. [23] | K-file (50/0.02), Mtwo (50/0.04), Reciproc (50/0.05) | 2.5% NaOCl and 17% EDTA | S1 and S2 | CFU | No difference was found among the instrumentation systems. |
| Siqueira et al. [8] | Reciproc (25/0.08), SAF (1.5 mm), Twisted File (25/0.06) | 2.5% NaOCl and 17% EDTA | S1 and S2 | CFU, PCR | No difference was found among the instrumentation systems. |
| Üreyen Kaya et al. [24] | WaveOne Gold (25/0.07), Hyflex EDM One File (25/variable), XP-endo Shaper (30/0.04) | Saline | S1 and S2 | CFU | Hyflex EDM and XP-endo Shaper resulted in significantly greater bacteria reduction than WaveOne Gold. |
| Vasconcelos et al. [25] | ProTaper Universal (25/0.08), BioRaCe (25/0.06), Reciproc (25/0.08) | Saline | S1 and S2 | CFU | ProTaper Universal was the most effective system in bacteria reduction. |
1. Was the calculation of the required minimum sample size performed before experiments?
2. Were the samples randomly distributed to groups?
3. Was specimen sterilization confirmed after the sterilization procedures?
4. Was specimen contamination confirmed after the procedure of root canal contamination with microorganisms?
5. Were the root canal preparation procedures performed by a single operator?
6. Was the total irrigant volume standard in all groups?
7. Were the analyses performed by evaluators blinded to the groups?
8. Were one or more outcomes of interest reported incompletely?
RESULTS
Risk of bias of individual studies
| Studies | Sample size calculation | Teeth randomization | Confirmation of sterilization | Confirmation of contamination | Single operator | Standardization of total irrigant volume | Blinding of the evaluator | Complete outcome reporting | Risk of bias |
|---|---|---|---|---|---|---|---|---|---|
| Alves et al. [3] | N | N | N | Y | N | Y | N | Y | Moderate |
| Alves et al. [13] | Y | Y | Y | Y | N | Y | N | Y | Low |
| Basmaci et al. [14] | N | Y | N | Y | N | N | N | Y | Moderate |
| Dagna et al. [15] | N | Y | Y | Y | Y | Y | N | N | Moderate |
| de Brito et al. [16] | N | Y | Y | Y | Y | Y | N | Y | Low |
| de Oliveira et al. [17] | N | Y | Y | Y | Y | N | N | Y | Moderate |
| Ferrer-Luque et al. [18] | N | N | Y | Y | N | Y | N | Y | Moderate |
| Guillen et al. [19] | Y | Y | Y | Y | N | Y | N | Y | Low |
| Karatas et al. [20] | N | Y | N | Y | N | Y | N | N | Moderate |
| Krokidis et al. [21] | N | Y | N | Y | Y | Y | N | Y | Moderate |
| Machado et al. [4] | N | N | Y | Y | N | Y | N | Y | Moderate |
| Marinho et al. [9] | N | Y | Y | Y | N | Y | N | Y | Moderate |
| Nabeshima et al. [22] | N | Y | Y | Y | N | Y | N | Y | Moderate |
| Nakamura et al. [23] | N | Y | Y | Y | N | Y | N | Y | Moderate |
| Siqueira et al. [8] | N | Y | N | Y | N | Y | N | Y | Moderate |
| Üreyen Kaya et al. [24] | N | Y | Y | Y | N | Y | N | Y | Moderate |
| Vasconcelos et al. [25] | N | Y | Y | Y | Y | N | N | N | Moderate |
Forest plot of standardized mean differences with 95% confidence intervals (CIs) in microbial reduction as an outcome measure for in vitro studies.
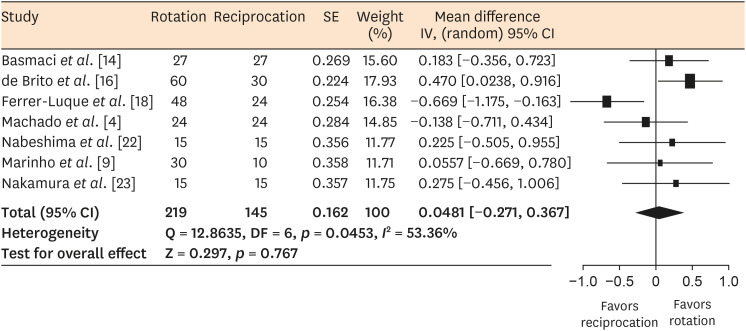
DISCUSSION
CONCLUSIONS
ACKNOWLEDGEMENTS
-
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
-
Author Contributions:
Conceptualization: Küçükkaya Eren S, Uzunoğlu-Özyürek E.
Data curation: Küçükkaya Eren S, Uzunoğlu-Özyürek E, Karahan S.
Formal analysis: Küçükkaya Eren S, Uzunoğlu-Özyürek E, Karahan S.
Funding acquisition: Küçükkaya Eren S, Uzunoğlu-Özyürek E, Karahan S.
Investigation: Küçükkaya Eren S, Uzunoğlu-Özyürek E, Karahan S.
Methodology: Küçükkaya Eren S, Uzunoğlu-Özyürek E, Karahan S.
Project administration: Küçükkaya Eren S.
Resources: Küçükkaya Eren S, Uzunoğlu-Özyürek E, Karahan S.
Software: Karahan S.
Supervision: Küçükkaya Eren S.
Validation: Karahan S.
Visualization: Küçükkaya Eren S, Uzunoğlu-Özyürek E.
Writing - original draft: Küçükkaya Eren S.
Writing - review & editing: Küçükkaya Eren S, Uzunoğlu-Özyürek E, Karahan S.
- 1. Siqueira JF Jr, Rôças IN. Clinical implications and microbiology of bacterial persistence after treatment procedures. J Endod 2008;34:1291-1301.e3.ArticlePubMed
- 2. Siqueira JF Jr, Rôças IN, Santos SR, Lima KC, Magalhães FA, de Uzeda M. Efficacy of instrumentation techniques and irrigation regimens in reducing the bacterial population within root canals. J Endod 2002;28:181-184.ArticlePubMed
- 3. Alves FR, Rôças IN, Almeida BM, Neves MA, Zoffoli J, Siqueira JF Jr. Quantitative molecular and culture analyses of bacterial elimination in oval-shaped root canals by a single-file instrumentation technique. Int Endod J 2012;45:871-877.ArticlePubMed
- 4. Machado ME, Nabeshima CK, Leonardo MF, Reis FA, Britto ML, Cai S. Influence of reciprocating single-file and rotary instrumentation on bacterial reduction on infected root canals. Int Endod J 2013;46:1083-1087.ArticlePubMedPDF
- 5. Short JA, Morgan LA, Baumgartner JC. A comparison of canal centering ability of four instrumentation techniques. J Endod 1997;23:503-507.ArticlePubMed
- 6. De-Deus G, Brandão MC, Barino B, Di Giorgi K, Fidel RA, Luna AS. Assessment of apically extruded debris produced by the single-file ProTaper F2 technique under reciprocating movement. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010;110:390-394.ArticlePubMed
- 7. Martinho FC, Chiesa WM, Marinho AC, Zaia AA, Ferraz CC, Almeida JF, Souza-Filho FJ, Gomes BP. Clinical investigation of the efficacy of chemomechanical preparation with rotary nickel-titanium files for removal of endotoxin from primarily infected root canals. J Endod 2010;36:1766-1769.ArticlePubMed
- 8. Siqueira JF Jr, Alves FR, Versiani MA, Rôças IN, Almeida BM, Neves MA, Sousa-Neto MD. Correlative bacteriologic and micro-computed tomographic analysis of mandibular molar mesial canals prepared by self-adjusting file, reciproc, and twisted file systems. J Endod 2013;39:1044-1050.ArticlePubMed
- 9. Marinho AC, Martinho FC, Gonçalves LM, Rabang HR, Gomes BP. Does the Reciproc file remove root canal bacteria and endotoxins as effectively as multifile rotary systems? Int Endod J 2015;48:542-548.ArticlePubMed
- 10. De-Deus G, Moreira EJ, Lopes HP, Elias CN. Extended cyclic fatigue life of F2 ProTaper instruments used in reciprocating movement. Int Endod J 2010;43:1063-1068.ArticlePubMed
- 11. Siddique R, Nivedhitha MS. Effectiveness of rotary and reciprocating systems on microbial reduction: a systematic review. J Conserv Dent 2019;22:114-122.ArticlePubMedPMC
- 12. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006-1012.ArticlePubMed
- 13. Alves FR, Paiva PL, Marceliano-Alves MF, Cabreira LJ, Lima KC, Siqueira JF Jr, Rôças IN, Provenzano JC. Bacteria and hard tissue debris extrusion and intracanal bacterial reduction promoted by XP-endo shaper and reciproc instruments. J Endod 2018;44:1173-1178.ArticlePubMed
- 14. Basmaci F, Oztan MD, Kiyan M. Ex vivo evaluation of various instrumentation techniques and irrigants in reducing E. faecalis within root canals. Int Endod J 2013;46:823-830.ArticlePubMed
- 15. Dagna A, Arciola CR, Visai L, Selan L, Colombo M, Bianchi S, Poggio C. Antibacterial efficacy of conventional and single-use Ni-Ti endodontic instruments: an in vitro microbiological evaluation. Int J Artif Organs 2012;35:826-831.ArticlePubMedPDF
- 16. de Brito PRR, Lima PM, Leal Silva EJN, Rivera Fidel S, Fidel RAS, Sassone LM. Effectiveness of ProTaper Next, ProTaper Universal and WaveOne systems in reducing intracanal bacterial load. Endod Pract Today 2016;10:167-173.
- 17. DE Oliveira BP, Aguiar CM, Câmara AC, DE Albuquerque MM, Correia AC, Soares MF. Evaluation of microbial reduction in root canals instrumented with reciprocating and rotary systems. Acta Stomatol Croat 2015;49:294-303.ArticlePubMedPMC
- 18. Ferrer-Luque CM, Bejarano I, Ruiz-Linares M, Baca P. Reduction in Enteroccocus faecalis counts - a comparison between rotary and reciprocating systems. Int Endod J 2014;47:380-386.PubMed
- 19. Guillén RE, Nabeshima CK, Caballero-Flores H, Cayón MR, Mercadé M, Cai S, Machado MEL. Evaluation of the WaveOne Gold and One Shape New Generation in reducing Enterococcus faecalis from root canal. Braz Dent J 2018;29:249-253.ArticlePubMed
- 20. Karataş E, Gültekin E, Arslan H, Kirici DÖ, Alsancak M, Topçu MÇ. Evaluation of instrumentation systems in reducing E. faecalis from root canals: TF adaptive and ProTaper next versus single file systems. Int J Artif Organs 2015;38:161-164.ArticlePubMedPDF
- 21. Krokidis A, Bonfanti C, Cerutti AC, Barabanti N, Panopoulos P. Rotary versus reciprocating shaping files in bacterial elimination from long oval canals. Aust Endod J 2018;44:240-244.PubMed
- 22. Nabeshima CK, Caballero-Flores H, Cai S, Aranguren J, Borges Britto ML, Machado ME. Bacterial removal promoted by 2 single-file systems: Wave One and One Shape. J Endod 2014;40:1995-1998.ArticlePubMed
- 23. Nakamura VC, Candeiro GT, Cai S, Gavini G. Ex vivo evaluation of three instrumentation techniques on E. faecalis biofilm within oval shaped root canals. Braz Oral Res 2015;29:29.Article
- 24. Üreyen Kaya B, Erik CE, Sesli Çetin E, Köle M, Maden M. Mechanical reduction in intracanal Enterococcus faecalis when using three different single-file systems: an ex vivo comparative study. Int Endod J 2019;52:77-85.ArticlePubMedPDF
- 25. Vasconcelos LR, Midena RZ, Minotti PG, Pereira TC, Duarte MA, Andrade FB. Effect of ultrasound streaming on the disinfection of flattened root canals prepared by rotary and reciprocating systems. J Appl Oral Sci 2017;25:477-482.ArticlePubMedPMC
- 26. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-560.ArticlePubMedPMC
- 27. Sjögren U, Figdor D, Persson S, Sundqvist G. Influence of infection at the time of root filling on the outcome of endodontic treatment of teeth with apical periodontitis. Int Endod J 1997;30:297-306.ArticlePubMed
- 28. Molander A, Warfvinge J, Reit C, Kvist T. Clinical and radiographic evaluation of one- and two-visit endodontic treatment of asymptomatic necrotic teeth with apical periodontitis: a randomized clinical trial. J Endod 2007;33:1145-1148.ArticlePubMed
- 29. Bürklein S, Hinschitza K, Dammaschke T, Schäfer E. Shaping ability and cleaning effectiveness of two single-file systems in severely curved root canals of extracted teeth: Reciproc and WaveOne versus Mtwo and ProTaper. Int Endod J 2012;45:449-461.ArticlePubMed
- 30. Alves FR, Siqueira JF Jr, Carmo FL, Santos AL, Peixoto RS, Rôças IN, Rosado AS. Bacterial community profiling of cryogenically ground samples from the apical and coronal root segments of teeth with apical periodontitis. J Endod 2009;35:486-492.ArticlePubMed
- 31. Portenier I, Waltimo T, Ørstavik D, Haapasalo M. The susceptibility of starved, stationary phase, and growing cells of Enterococcus faecalis to endodontic medicaments. J Endod 2005;31:380-386.ArticlePubMed
- 32. Siqueira JF Jr, Rôças IN. Diversity of endodontic microbiota revisited. J Dent Res 2009;88:969-981.ArticlePubMedPDF
- 33. Rôças IN, Siqueira JF Jr. Identification of bacteria enduring endodontic treatment procedures by a combined reverse transcriptase-polymerase chain reaction and reverse-capture checkerboard approach. J Endod 2010;36:45-52.ArticlePubMed
- 34. Nair PN. Pathogenesis of apical periodontitis and the causes of endodontic failures. Crit Rev Oral Biol Med 2004;15:348-381.PubMed
- 35. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. London: The Cochrane Collaboration; 2011.
- 36. Uzunoglu-Özyürek E, Küçükkaya Eren S, Karahan S. Effect of root canal sealers on the fracture resistance of endodontically treated teeth: a systematic review of in vitro studies. Clin Oral Investig 2018;22:2475-2485.ArticlePubMedPDF
REFERENCES
Tables & Figures
REFERENCES
Citations

- Mapping risk of bias criteria in systematic reviews of in vitro endodontic studies: an umbrella review
Rafaella Rodrigues da Gama, Lucas Peixoto de Araújo, Evandro Piva, Leandro Perello Duro, Adriana Fernandes da Silva, Wellington Luiz de Oliveira da Rosa
Evidence-Based Dentistry.2025; 26(4): 179. CrossRef - Fifteen years of engine‐driven nickel–titanium reciprocating instruments, what do we know so far? An umbrella review
Felipe Immich, Lucas Peixoto de Araújo, Rafaella Rodrigues da Gama, Wellington Luiz de Oliveira da Rosa, Evandro Piva, Giampiero Rossi‐Fedele
Australian Endodontic Journal.2024; 50(2): 409. CrossRef - Does minimally invasive canal preparation provide higher fracture resistance of endodontically treated teeth? A systematic review ofin vitrostudies
Sıla Nur Usta, Emmanuel João Nogueira Leal Silva, Seda Falakaloğlu, Mustafa Gündoğar
Restorative Dentistry & Endodontics.2023;[Epub] CrossRef - The Effect of Combined Ultrasonic Tip and Mechanized Instrumentation on the Reduction of the Percentage of Non-Instrumented Surfaces in Oval/Flat Root Canals: A Systematic Review and Meta-Analysis
Marcella Dewes Cassal, Pedro Cardoso Soares, Marcelo dos Santos
Cureus.2023;[Epub] CrossRef - Impact of Different Access Cavity Designs and Ni–Ti Files on the Elimination of Enterococcus faecalis from the Root Canal System: An In Vitro Study
Gizem Andac, Atakan Kalender, Buket Baddal, Fatma Basmaci
Applied Sciences.2022; 12(4): 2049. CrossRef - Shaping Properties and Outcomes of Nickel-Titanium Reciprocation Systems in Primary Teeth: A Systematic Review and Meta-Analysis of In Vitro Studies
SelvaKumar Haridoss, Bhavyaa R, Kavitha Swaminathan, Aruna P
Cureus.2022;[Epub] CrossRef - Influence of Root Canal Sealers and Obturation Techniques on Vertical Root Fracture Resistance. An In Vitro Experiment
Mazen F. Alkahtany, Khalid H. Almadi, Fahad A. Alahmad, Abdullah M. Alshehri, Abdulrahman A. AlSwayyed, Omar M. AlZahran, Ali AlHadan, Abdulaziz S. Almustafa, Fahim Vohra, Tariq Abduljabbar
Applied Sciences.2021; 11(17): 8022. CrossRef
- Figure
- Related articles
-
- Effect of surface treatment on glass ionomers in sandwich restorations: a systematic review and meta-analysis of laboratory studies
- Fracture resistance and failure modes of endodontically-treated permanent teeth restored with Ribbond posts vs other post systems: a systematic review and meta-analysis of in vitro studies
-
Influence of disinfecting solutions on the surface topography of gutta-percha cones: a systematic review of
in vitro studies - Success rate of direct pulp capping on permanent teeth using bioactive materials: a systematic review and meta-analysis of randomized clinical trials
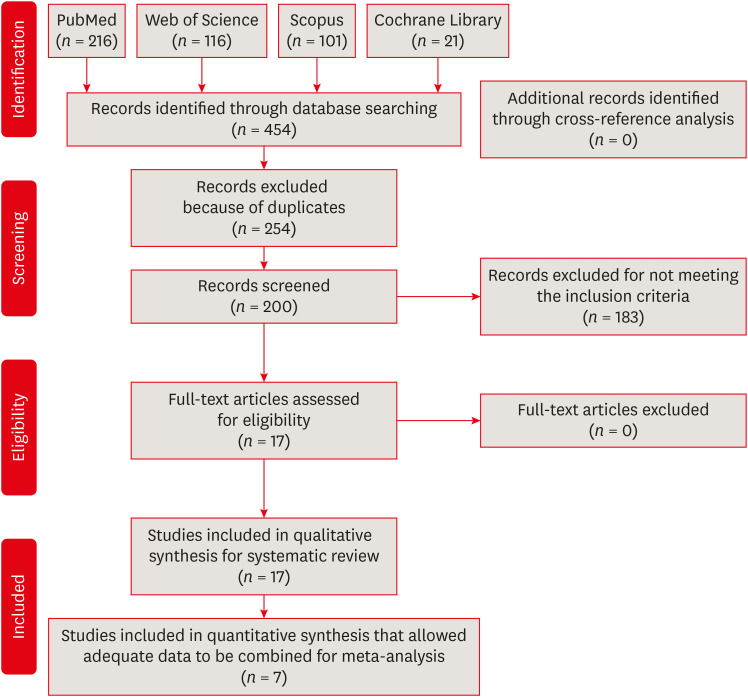
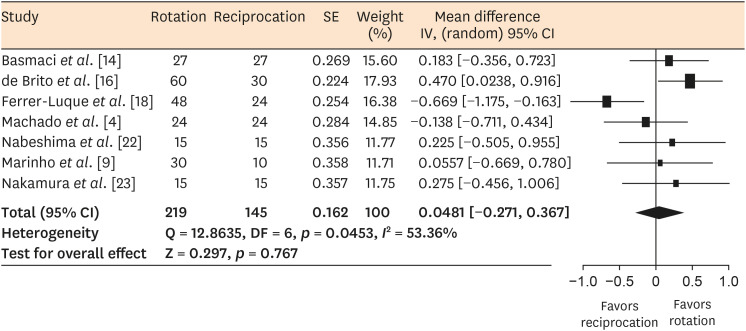
Figure 1
Figure 2
Example of the search strategy (PubMed)
| No. | Search strategy | Results |
|---|---|---|
| 1 | bacteria OR microbial OR microorganism OR microorganisms OR microbiota OR antibacterial OR antifungal OR antimicrobial OR CFU OR colony forming unit OR colony forming units OR PCR OR faecalis OR polymerase chain reaction OR toxin OR toxins OR infection | 6,358,577 |
| 2 | reciproc OR reciprocating OR reciprocal OR waveone | 59,954 |
| 3 | root canal OR root canals OR endodontic OR endodontics OR canal OR canals OR tooth OR teeth OR endodontology | 350,001 |
| 4 | #1 AND #2 AND #3 | 216 |
Summary of the main characteristics of the included in vitro studies
| Studies | Tooth type | No. | Sterilization procedure | Preparation before contamination | Smear layer removal before contamination | Microorganism type | Incubation period following contamination | Confirmation of contamination |
|---|---|---|---|---|---|---|---|---|
| Alves et al. [ | Mandibular incisors and maxillary second premolars with single root canals | 34 | Autoclave | 25 K-file | 17% EDTA and 2.5% NaOCl | E. faecalis strain (ATCC 29212) | 30 days | SEM |
| Alves et al. [ | Distobuccal canals of maxillary molars | 43 | Autoclave | Rotary instrument, size 10/0.04 | 17% EDTA | E. faecalis strain (ATCC 29212) | 30 days | Culture technique and SEM |
| Basmaci et al. [ | Mandibular premolars with single root canals | 81 | Autoclave | 20 K-file | NM | E. faecalis strain (ATCC 29212) | 24 hours | Culture technique |
| Dagna et al. [ | Single-rooted teeth | 60 | Autoclave | 20 K-file | 10% EDTA | E. faecalis strain (ATCC 19433) | 120 hours | Culture technique |
| de Brito et al. [ | Mandibular premolars | 100 | Autoclave | 20 K-file | NM | E. faecalis strain (ATCC 29212) | 28 days | Culture technique |
| de Oliveira et al. [ | Mandibular premolars | 60 | Autoclave | NM | NM | E. faecalis (ATCC 6057), P. aeruginosa (ATCC 27853), S. aureus (ATCC 29213) and C. albicans (ATCC 10231) | 48 hours | Culture technique |
| Ferrer-Luque et al. [ | Single-rooted mandibular premolars | 76 | Autoclave | 25 K-file | 17% EDTA and then irrigated with 1% NaOCl followed by DW | E. faecalis strain (ATCC 29212) | 4 weeks | Culture technique |
| Guillen et al. [ | Distobuccal canals of maxillary molars | 56 | Ethylene oxide | 15 K-file | 17% EDTA | E. faecalis strain (ATCC 29212) | 21 days | Culture technique |
| Karatas et al. [ | Mandibular incisor teeth | 70 | Autoclave | 20 K-file | NM | E. faecalis strain (ATCC 29212) | 48 hours | Culture technique |
| Krokidis et al. [ | Canines, lower incisors and premolars with single root canals | 50 | Autoclave | 25 K-file | 17% EDTA and 2.5% NaOCl | E. faecalis strain (ATCC 29212) | 30 days | Culture technique |
| Machado et al. [ | Distobuccal canals of maxillary molars | 65 | Ethylene oxide | 15 K-file | 17% EDTA and DW | E. faecalis strain (ATCC 29212) | 21 days | Culture technique |
| Marinho et al. [ | Mandibular premolars | 40 | Gamut radiation and autoclave | 15 K-file | 17% EDTA, 5.25% NaOCl and DW | E. coli strain (ATCC 25922) | 21 days | Culture technique and SEM |
| Nabeshima et al. [ | Distobuccal canals of maxillary molars | 51 | Ethylene oxide | 15 K-file | 17% EDTA and DW | E. faecalis strain (ATCC 29212) | 21 days | Culture technique |
| Nakamura et al. [ | Mandibular premolars | 50 | Autoclave | 30 K-file | 17% EDTA-T, 5.25% NaOCl and DW | E. faecalis strain (ATCC 29212) | 28 days | Culture technique and SEM |
| Siqueira et al. [ | Mesial canals of mandibular molars | 36 | Autoclave | 20 K-file | 17% EDTA and 2.5% NaOCl | E. faecalis strain ATCC 29212 | 30 days | Culture technique and SEM |
| Üreyen Kaya et al. [ | Mandibular premolars | 74 | Autoclave | NM | NM | E. faecalis | 4 weeks | Culture technique and SEM |
| Vasconcelos et al. [ | Mandibular incisors | 84 | Autoclave | 20 K-file | 1% NaOCl, 17% EDTA and saline | E. faecalis strain ATCC 29212 | 5 days | Culture technique and SEM |
EDTA, ethylenediaminetetraacetic acid; SEM, scanning electron microscopy; DW, distilled water; NM: not mentioned.
Details extracted from the included studies regarding methodology and main outcomes
| Studies | Instrumentation systems tested (final apical diameter/taper) | Irrigation techniques and irrigants | Sampling time | Evaluation method | Main findings |
|---|---|---|---|---|---|
| Alves et al. [ | Reciproc (40/0.06), BioRace (40/0.04) | 2.5% NaOCl and 17% EDTA | S1 and S2 | CFU, qPCR | No difference was found between the instrumentation systems. |
| Alves et al. [ | Reciproc (25/0.08), XP-endo Shaper (30/0.04) | Saline | S1 and S2 | qPCR | XP-endo Shaper resulted in higher bacteria reduction. |
| Basmacı et al. [ | SAF (1.5 mm), Reciproc (25/0.08), ProTaper Universal (30/0.09) | a) PBS | S1 and S2 | CFU | No difference was found among the instrumentation systems. |
| b) 5% NaOCl and 15% EDTA | |||||
| c) 5% NaOCl and 7% maleic acid | |||||
| Dagna et al. [ | Mtwo (30/0.05), Revo-S (25/0.06), Reciproc (25/0.08), OneShape (25/0.06) | 5.25% NaOCl and 17% EDTA | S1 and S2 | CFU | No difference was found among the instrumentation systems. |
| de Brito et al. [ | ProTaper Next (40/0.06), ProTaper Universal (40/0.06), WaveOne Large (40/0.08) | a) 2.5% NaOCl and 17% EDTA | S1 and S2 | CFU | WaveOne resulted in a lower level of bacterial reduction when saline solution was used. No difference was found among the instrumentation systems when NaOCl and EDTA were used. |
| b) Saline | |||||
| de Oliveira et al. [ | ProTaper Universal (30/0.09), Reciproc (40/0.06) | a) 1% NaOCl | S1 and S2 | Presence/Absence | ProTaper Universal showed the best results when NaOCl was used. |
| b) Saline | |||||
| Ferrer-Luque et al. [ | Mtwo (40/0.04), Twisted File (40/0.04), WaveOne (40/0.08) | a) DW | S1 and S2 and S3 (after 60 days) | CFU | No difference was found among the instrumentation systems after S2. Mtwo showed the best results when NaOCl was used at 60 days (S3). |
| b) 5.25% NaOCl | |||||
| Guillen et al. [ | WaveOne Gold (25/0.07), WaveOne (25/0.08), One Shape New Generation (25/0.06), One Shape (25/0.06) | DW | S1 and S2 and S3 (after 7 days) | CFU | WaveOne Gold and One Shape New Generation promoted higher bacterial reduction than WaveOne and One Shape systems. |
| Karatas et al. [ | ProTaper Next (25/0.06), Twisted File Adaptive (25/0.06), SAF (1.5 mm), WaveOne (25/0.08), Reciproc (25/0.08), OneShape (25/0.06) | DW | S1 and S2 | CFU | No difference was found between the rotary and reciprocating instrumentation. |
| Krokidis et al. [ | BT-Race (40/0.04), WaveOne (40/0.08) | 2.5% NaOCl and 17% EDTA | S1 and S2 | CFU | BT-RaCe resulted in higher bacteria reduction. |
| Machado et al. [ | WaveOne (25/0.08), Reciproc (25/0.08), ProTaper Universal (25/.08), Mtwo (25/0.06), K-file (35/0.02) | DW | S1 and S2 and S3 (after 7 days) | CFU | No difference was found among the instrumentation systems. |
| Marinho et al. [ | Reciproc (25/0.08), Mtwo (25/0.06), ProTaper Universal (25/0.08), Race (25/0.04) | Endotoxin-free water (LAL water) | S1 and S2 | CFU, LAL assay (for endotoxin reduction) | No difference was found among the instrumentation systems. |
| Nabeshima et al. [ | WaveOne (25/0.08), One Shape (25/0.06), K-file (35/0.02) | DW | S1 and S2 | CFU | No difference was found among the instrumentation systems. |
| Nakamura et al. [ | K-file (50/0.02), Mtwo (50/0.04), Reciproc (50/0.05) | 2.5% NaOCl and 17% EDTA | S1 and S2 | CFU | No difference was found among the instrumentation systems. |
| Siqueira et al. [ | Reciproc (25/0.08), SAF (1.5 mm), Twisted File (25/0.06) | 2.5% NaOCl and 17% EDTA | S1 and S2 | CFU, PCR | No difference was found among the instrumentation systems. |
| Üreyen Kaya et al. [ | WaveOne Gold (25/0.07), Hyflex EDM One File (25/variable), XP-endo Shaper (30/0.04) | Saline | S1 and S2 | CFU | Hyflex EDM and XP-endo Shaper resulted in significantly greater bacteria reduction than WaveOne Gold. |
| Vasconcelos et al. [ | ProTaper Universal (25/0.08), BioRaCe (25/0.06), Reciproc (25/0.08) | Saline | S1 and S2 | CFU | ProTaper Universal was the most effective system in bacteria reduction. |
CFU, colony forming unit; DW, distilled water; EDTA, ethylenediaminetetraacetic acid; NM, not mentioned; NaOCl, sodium hypochlorite; PBS, phosphate-buffered saline; SEM, scanning electron microscopy; qPCR, quantitative polymerase chain reaction; S1: Sampling after cavity preparation immediately before root canal preparation; S2, Sampling immediately after root canal preparation; S3, Sampling after a period of time following root canal preparation (for regrowth evaluation).
Risk of bias of individual studies
| Studies | Sample size calculation | Teeth randomization | Confirmation of sterilization | Confirmation of contamination | Single operator | Standardization of total irrigant volume | Blinding of the evaluator | Complete outcome reporting | Risk of bias |
|---|---|---|---|---|---|---|---|---|---|
| Alves et al. [ | N | N | N | Y | N | Y | N | Y | Moderate |
| Alves et al. [ | Y | Y | Y | Y | N | Y | N | Y | Low |
| Basmaci et al. [ | N | Y | N | Y | N | N | N | Y | Moderate |
| Dagna et al. [ | N | Y | Y | Y | Y | Y | N | N | Moderate |
| de Brito et al. [ | N | Y | Y | Y | Y | Y | N | Y | Low |
| de Oliveira et al. [ | N | Y | Y | Y | Y | N | N | Y | Moderate |
| Ferrer-Luque et al. [ | N | N | Y | Y | N | Y | N | Y | Moderate |
| Guillen et al. [ | Y | Y | Y | Y | N | Y | N | Y | Low |
| Karatas et al. [ | N | Y | N | Y | N | Y | N | N | Moderate |
| Krokidis et al. [ | N | Y | N | Y | Y | Y | N | Y | Moderate |
| Machado et al. [ | N | N | Y | Y | N | Y | N | Y | Moderate |
| Marinho et al. [ | N | Y | Y | Y | N | Y | N | Y | Moderate |
| Nabeshima et al. [ | N | Y | Y | Y | N | Y | N | Y | Moderate |
| Nakamura et al. [ | N | Y | Y | Y | N | Y | N | Y | Moderate |
| Siqueira et al. [ | N | Y | N | Y | N | Y | N | Y | Moderate |
| Üreyen Kaya et al. [ | N | Y | Y | Y | N | Y | N | Y | Moderate |
| Vasconcelos et al. [ | N | Y | Y | Y | Y | N | N | N | Moderate |
EDTA, ethylenediaminetetraacetic acid; SEM, scanning electron microscopy; DW, distilled water; NM: not mentioned.
CFU, colony forming unit; DW, distilled water; EDTA, ethylenediaminetetraacetic acid; NM, not mentioned; NaOCl, sodium hypochlorite; PBS, phosphate-buffered saline; SEM, scanning electron microscopy; qPCR, quantitative polymerase chain reaction; S1: Sampling after cavity preparation immediately before root canal preparation; S2, Sampling immediately after root canal preparation; S3, Sampling after a period of time following root canal preparation (for regrowth evaluation).

 KACD
KACD
 ePub Link
ePub Link Cite
Cite

