Articles
- Page Path
- HOME > Restor Dent Endod > Volume 46(2); 2021 > Article
- Review Article Wear of contemporary dental composite resin restorations: a literature review
-
Dimitrios Dionysopoulos
 , Olga Gerasimidou
, Olga Gerasimidou
-
Restor Dent Endod 2021;46(2):e18.
DOI: https://doi.org/10.5395/rde.2021.46.e18
Published online: February 25, 2021
Department of Operative Dentistry, Faculty of Dentistry, School of Health Sciences, Aristotle University of Thessaloniki, Thessaloniki, Greece.
- Correspondence to Dimitrios Dionysopoulos, PhD, MSc, DDS. Assistant Professor, Department of Operative Dentistry, Faculty of Dentistry, School of Health Sciences, Aristotle University of Thessaloniki, Thessaloniki 54124, Greece. ddionys@dent.auth.gr
Copyright © 2021. The Korean Academy of Conservative Dentistry
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
- Composite resins are the most commonly used dental restorative materials after minimally invasive dental procedures, and they offer an aesthetically pleasing appearance. An ideal composite restorative material should have wear properties similar to those of tooth tissues. Wear refers to the damaging, gradual loss or deformation of a material at solid surfaces. Depending on the mechanism of action, wear can be categorized as abrasive, adhesive, fatigue, or corrosive. Currently used composite resins cover a wide range of materials with diverse properties, offering dental clinicians multiple choices for anterior and posterior teeth. In order to improve the mechanical properties and the resistance to wear of composite materials, many types of monomers, silane coupling agents, and reinforcing fillers have been developed. Since resistance to wear is an important factor in determining the clinical success of composite resins, the purpose of this literature review was to define what constitutes wear. The discussion focuses on factors that contribute to the extent of wear as well as to the prevention of wear. Finally, the behavior of various types of existing composite materials such as nanohybrid, flowable, and computer-assisted design/computer-assisted manufacturing materials, was investigated, along with the factors that may cause or contribute to their wear.
INTRODUCTION
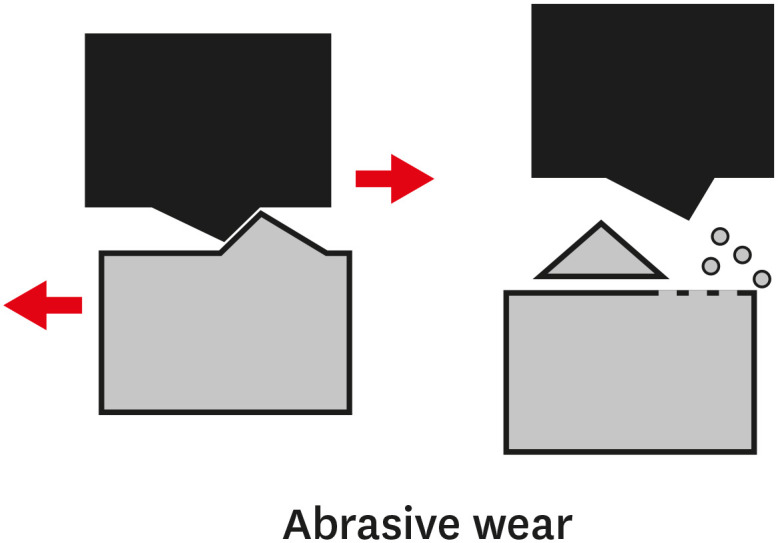
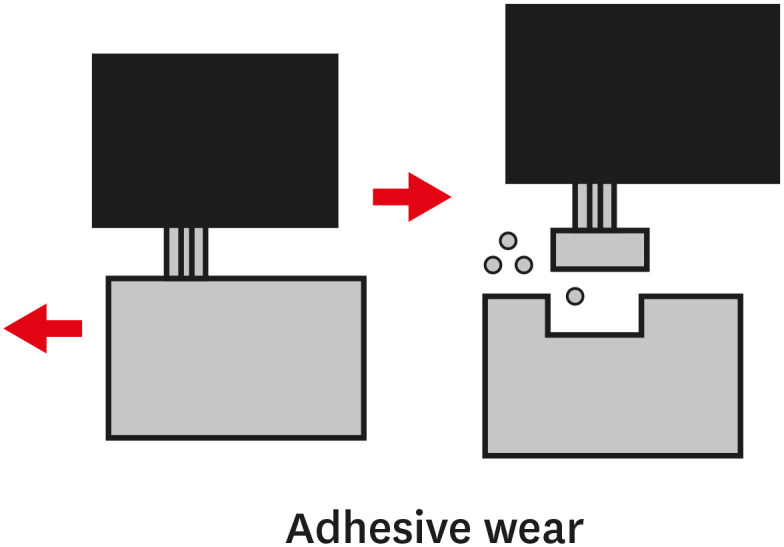
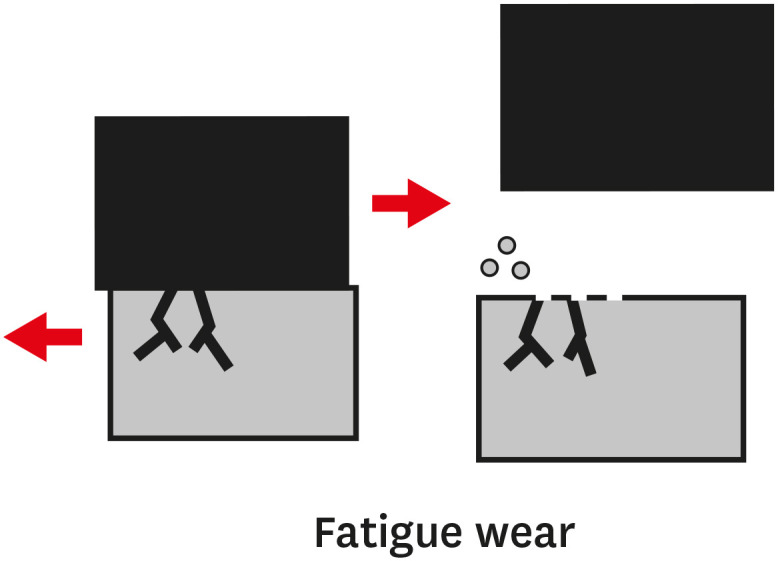
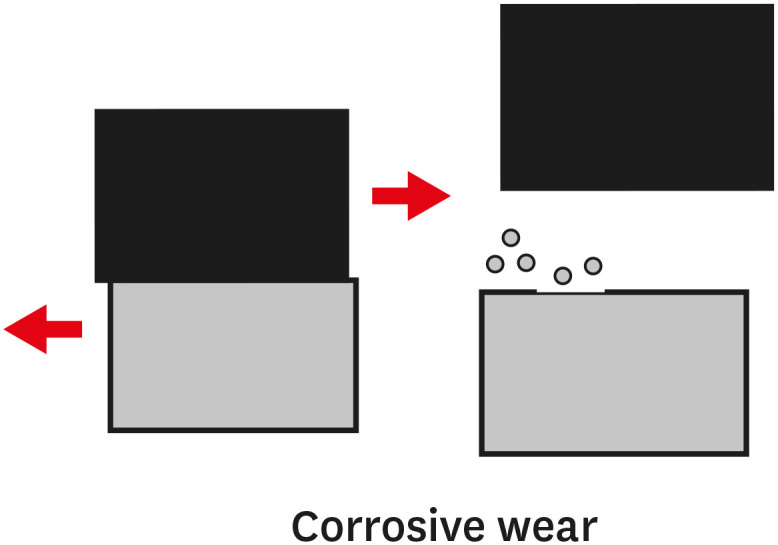
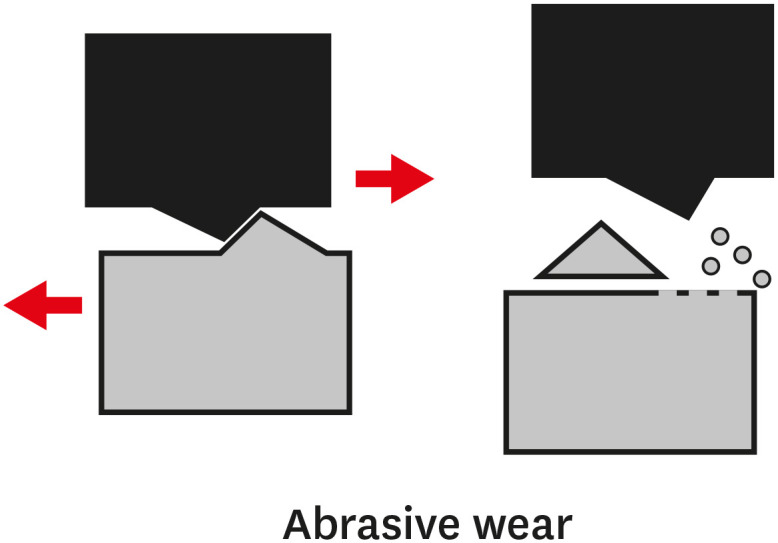
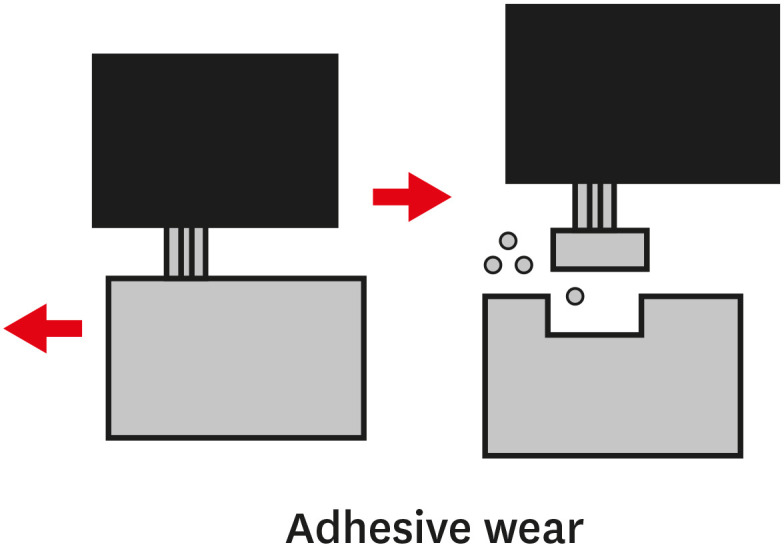
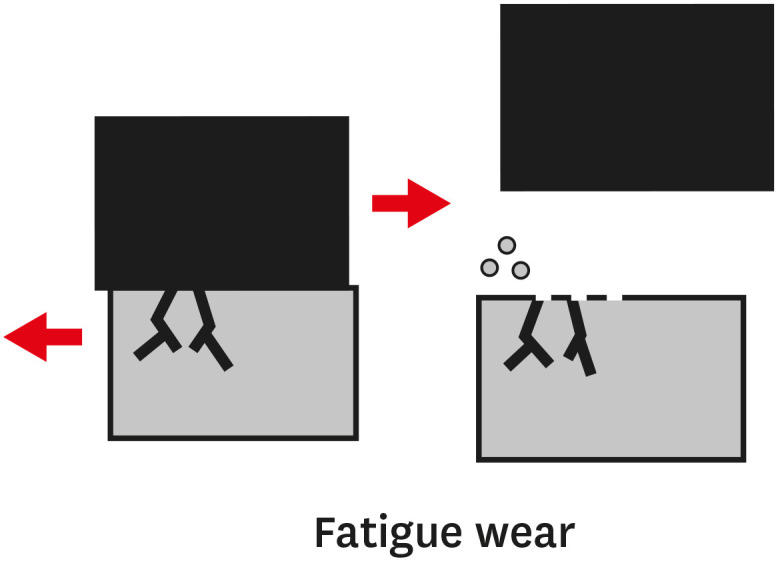
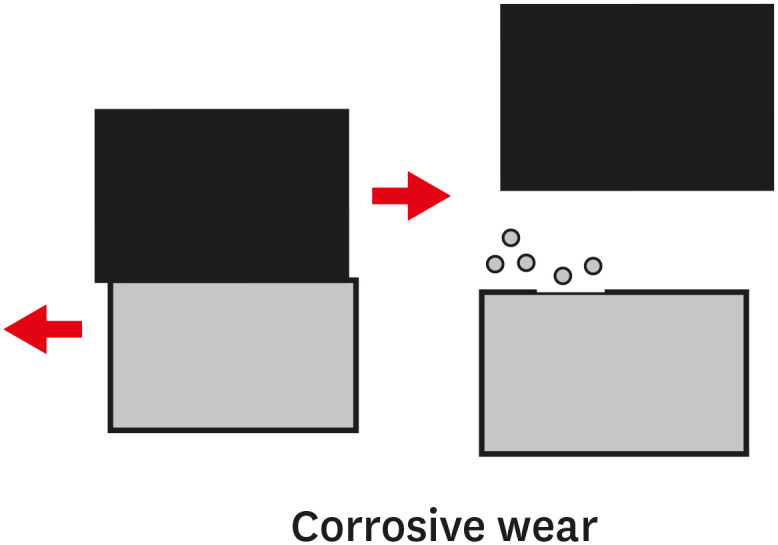
TYPES OF WEAR OF COMPOSITE MATERIALS
1. The primary or early stage, where surfaces adapt to each other and the wear rate might vary between high and low.
2. The secondary or mid-age stage, where steady wear can be observed. Most of the component's operational life is spent in this stage.
3. The tertiary or old-age stage, where surfaces are subjected to rapid failure due to a high rate of wear.
WEAR OF DIFFERENT DENTAL COMPOSITE RESIN MATERIALS
WEAR OF DIRECT COMPOSITE RESIN RESTORATIONS
WEAR OF INDIRECT COMPOSITE RESIN RESTORATIONS
WEAR OF COMPOSITE CAD/CAM MATERIALS
CONCLUSIONS
-
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
-
Author Contributions:
Conceptualization: Dionysopoulos D.
Data curation: Dionysopoulos D, Gerasimidou O.
Formal analysis: Dionysopoulos D.
Investigation: Dionysopoulos D, Gerasimidou O.
Methodology: Dionysopoulos D, Gerasimidou O.
Project administration: Dionysopoulos D.
Resources: Dionysopoulos D.
Software: Gerasimidou O.
Supervision: Dionysopoulos D.
Validation: Dionysopoulos D.
Visualization: Dionysopoulos D.
Writing - original draft: Dionysopoulos D, Gerasimidou O.
Writing - review & editing: Dionysopoulos D, Gerasimidou O.
- 1. Gwon B, Bae EB, Lee JJ, Cho WT, Bae HY, Choi JW, Huh JB. Wear characteristics of dental ceramic CAD/CAM materials opposing various dental composite resins. Materials (Basel) 2019;12:1839.ArticlePubMedPMC
- 2. Lambrechts P, Braem M, Vuylsteke-Wauters M, Vanherle G. Quantitative in vivo wear of human enamel. J Dent Res 1989;68:1752-1754.ArticlePubMedPDF
- 3. Ferracane JL. Resin composite--State of the art. Dent Mater 2011;27:29-38.ArticlePubMed
- 4. Burke FJ. Amalgam to tooth-coloured materials--implications for clinical practice and dental education: governmental restrictions and amalgam-usage survey results. J Dent 2004;32:343-350.ArticlePubMed
- 5. Kakaboura A, Vougiouklakis G. Basic principles of operative dentistry. Athens: Paschalidis; 2012.
- 6. de Gee AJ, Wendt SL, Werner A, Davidson CL. Influence of enzymes and plaque acids on in vitro wear of dental composites. Biomaterials 1996;17:1327-1332.ArticlePubMed
- 7. Turssi CP, De Moraes Purquerio B, Serra MC. Wear of dental resin composites: insights into underlying processes and assessment methods--a review. J Biomed Mater Res B Appl Biomater 2003;65:280-285.ArticlePubMed
- 8. Tsujimoto A, Barkmeier WW, Fischer NG, Nojiri K, Nagura Y, Takamizawa T, Latta MA, Miazaki M. Wear of resin composites: current insights into underlying mechanisms, evaluation methods and influential factors. Jpn Dent Sci Rev 2018;54:76-87.ArticlePubMed
- 9. Ferracane JL. Is the wear of dental composites still a clinical concern? Is there still a need for in vitro wear simulating devices? Dent Mater 2006;22:689-692.ArticlePubMed
- 10. Heintze SD, Ilie N, Hickel R, Reis A, Loguercio A, Rousson V. Laboratory mechanical parameters of composite resins and their relation to fractures and wear in clinical trials-A systematic review. Dent Mater 2017;33:e101-e114.ArticlePubMed
- 11. Heintze SD, Rousson V. Clinical effectiveness of direct class II restorations - a meta-analysis. J Adhes Dent 2012;14:407-431.PubMed
- 12. McCabe JF, Molyvda S, Rolland SL, Rusby S, Carrick TE. Two-and three-body wear of dental restorative materials. Int Dent J 2002;52:406-416.Article
- 13. Popov VL. Is tribology approaching its golden age? Grand challenges in engineering education and tribological research. Front Mech Eng 2018;4:16.Article
- 14. Blau PJ, Bayer RG. Wear of materials. Amsterdam: Elsevier; 2003.
- 15. Yang LJ. Wear coefficient equation for aluminium-based matrix composites against steel disc. Wear 2003;255:579-592.Article
- 16. Nihei T, Dabanoglu A, Teranaka T, Kurata S, Ohashi K, Kondo Y, Yoshino N, Hickel R, Kunzelmann KH. Three-body-wear resistance of the experimental composites containing filler treated with hydrophobic silane coupling agents. Dent Mater 2008;24:760-764.ArticlePubMed
- 17. Ghazal M, Kern M. Wear of human enamel and nano-filled composite resin denture teeth under different loading forces. J Oral Rehabil 2009;36:58-64.ArticlePubMed
- 18. Turssi CP, Ferracane JL, Serra MC. Abrasive wear of resin composites as related to finishing and polishing procedures. Dent Mater 2005;21:641-648.ArticlePubMed
- 19. de Paula AB, Fucio SB, Ambrosano GM, Alonso RC, Sardi JC, Puppin-Rontani RM. Biodegradation and abrasive wear of nano restorative materials. Oper Dent 2011;36:670-677.ArticlePubMedPDF
- 20. Mair LH, Stolarski TA, Vowles RW, Lloyd CH. Wear: mechanisms, manifestations and measurement. Report of a workshop. J Dent 1996;24:141-148.ArticlePubMed
- 21. American Society for Testing and Materials. Standard terminology relating to wear and erosion. Annual book of ASTM standards. Vol 03.02. West Conshohocken, PA: American Society for Testing and Materials; 1987. p. 243-250.
- 22. Ferracane JL. Materials in dentistry: principle and application. Philadelphia, PA: Lippincott Williams & Wilkins; 2001.
- 23. ASM Handbook Committee. ASM handbook. Volume 18: Friction, lubrication and wear technology. Almere: ASM International; 2002.
- 24. ISO 9352: Plastics—Determination of resistance to wear by abrasive wheels. Geneve: International Organization for Standardization; 2012.
- 25. ASTM D4060: Standard test method for abrasion resistance of organic coatings by the taber abraser. West Conshohocken, PA: American Society for Testing and Materials; 2019.
- 26. Cavalcante LM, Masouras K, Watts DC, Pimenta LA, Silikas N. Effect of nanofillers' size on surface properties after toothbrush abrasion. Am J Dent 2009;22:60-64.PubMed
- 27. Han JM, Zhang H, Choe HS, Lin H, Zheng G, Hong G. Abrasive wear and surface roughness of contemporary dental composite resin. Dent Mater J 2014;33:725-732.ArticlePubMed
- 28. Turssi CP, Ferracane JL, Vogel K. Filler features and their effects on wear and degree of conversion of particulate dental resin composites. Biomaterials 2005;26:4932-4937.ArticlePubMed
- 29. Manhart J, Kunzelmann KH, Chen HY, Hickel R. Mechanical properties and wear behavior of light-cured packable composite resins. Dent Mater 2000;16:33-40.ArticlePubMed
- 30. Krejci I, Albert P, Lutz F. The influence of antagonist standardization on wear. J Dent Res 1999;78:713-719.PubMed
- 31. Stachowiak GW, Batchelor AW. Engineering tribology. Burlington: Elsevier Butterworth-Heinemann; 2005.
- 32. Yap AU, Teoh SH, Hastings GW, Lu CS. Comparative wear ranking of dental restorative materials utilizing different wear simulation modes. J Oral Rehabil 1997;24:574-580.ArticlePubMed
- 33. Mandel ID. The functions of saliva. J Dent Res 1987;66(Spec No):623-627.ArticlePubMedPDF
- 34. Mair LH. Subsurface compression fatigue in seven dental composites. Dent Mater 1994;10:111-115.ArticlePubMed
- 35. McCabe JF, Wang Y, Braem M. Surface contact fatigue and flexural fatigue of dental restorative materials. J Biomed Mater Res 2000;50:375-380.ArticlePubMed
- 36. Söderholm KJ, Richards ND. Wear resistance of composites: a solved problem? Gen Dent 1998;46:256-263.PubMed
- 37. Ilie N, Hickel R. Resin composite restorative materials. Aust Dent J 2011;56(Supplement 1):59-66.ArticlePubMed
- 38. Finlay N, Hahnel S, Dowling AH, Fleming GJ. The in vitro wear behavior of experimental resin-based composites derived from a commercial formulation. Dent Mater 2013;29:365-374.ArticlePubMed
- 39. Barkmeier WW, Erickson RI, Latta MA, Wilwerding TM. Wear rates of resin composites. Oper Dent 2013;38:226-233.ArticlePubMedPDF
- 40. Barkmeier WW, Takamizawa T, Erickson RL, Tsujimoto A, Latta M, Miyazaki M. Localized and generalized simulated wear of resin composites. Oper Dent 2015;40:322-335.ArticlePubMedPDF
- 41. Mitra SB, Wu D, Holmes BN. An application of nanotechnology in advanced dental materials. J Am Dent Assoc 2003;134:1382-1390.ArticlePubMed
- 42. Egilmez F, Ergun G, Cekic-Nagas I, Vallittu PK, Lassila LV. Estimation of the surface gloss of dental nano composites as a function of color measuring geometry. Am J Dent 2012;25:220-226.PubMed
- 43. Sideridou ID, Karabela MM, Vouvoudi EC. Physical properties of current dental nanohybrid and nanofill light-cured resin composites. Dent Mater 2011;27:598-607.ArticlePubMed
- 44. Ilie N, Rencz A, Hickel R. Investigations towards nano-hybrid resin-based composites. Clin Oral Investig 2013;17:185-193.ArticlePubMedPDF
- 45. Hahnel S, Schultz S, Trempler C, Ach B, Handel G, Rosentritt M. Two-body wear of dental restorative materials. J Mech Behav Biomed Mater 2011;4:237-244.ArticlePubMed
- 46. Oliveira GU, Mondelli RF, Charantola Rodrigues M, Franco EB, Ishikiriama SK, Wang L. Impact of filler size and distribution on roughness and wear of composite resin after simulated toothbrushing. J Appl Oral Sci 2012;20:510-516.ArticlePubMedPMC
- 47. Takahashi H, Finger WJ, Endo T, Kanehira M, Koottathape N, Komatsu M, Balkenhol M. Comparative evaluation of mechanical characteristics of nanofiller containing resin composites. Am J Dent 2011;24:264-270.PubMed
- 48. Seemann R, Pfefferkorn F, Hickel R. Behaviour of general dental practitioners in Germany regarding posterior restorations with flowable composites. Int Dent J 2011;61:252-256.ArticlePubMedPMC
- 49. Lawson NC, Radhakrishnan R, Givan DA, Ramp LC, Burgess JO. Two-year randomized, controlled clinical trial of a flowable and conventional composite in class I restorations. Oper Dent 2015;40:594-602.ArticlePubMedPDF
- 50. Sumino N, Tsubota K, Takamizawa T, Shiratsuchi K, Miyazaki M, Latta MA. Comparison of the wear and flexural characteristics of flowable resin composites for posterior lesions. Acta Odontol Scand 2013;71:820-827.ArticlePubMed
- 51. Shinkai K, Taira Y, Suzuki S, Suzuki M. In vitro wear of flowable resin composite for posterior restorations. Dent Mater J 2016;35:37-44.PubMed
- 52. Tsujimoto A, Barkmeier WW, Takamizawa T, Latta MA, Miyazaki M. Mechanical properties, volumetric shrinkage and depth of cure of short fiber-reinforced resin composite. Dent Mater J 2016;35:418-424.ArticlePubMed
- 53. Dionysopoulos D, Tolidis K, Gerasimou P. Polymerization efficiency of bulk-fill dental resin composites with different curing modes. J Appl Polym Sci 2016;133:43392.Article
- 54. Magno MB, Nascimento GC, Rocha YS, Ribeiro BD, Loretto SC, Maia LC. Silorane-based composite resin restorations are not better than conventional composites – a meta-analysis of clinical studies. J Adhes Dent 2016;18:375-386.PubMed
- 55. Torres C, Augusto MG, Mathias-Santamaria IF, Di Nicoló R, Borges AB. Pure ormocer vs methacrylate composites on posterior teeth: a double-blinded randomized clinical trial. Oper Dent 2020;45:359-367.ArticlePubMedPDF
- 56. Dejak B, Młotkowski A. A comparison of stresses in molar teeth restored with inlays and direct restorations, including polymerization shrinkage of composite resin and tooth loading during mastication. Dent Mater 2015;31:e77-e87.ArticlePubMed
- 57. Tanoue N, Murakami M, Koizumi H, Atsuta M, Matsumura H. Depth of cure and hardness of an indirect composite polymerized with three laboratory curing units. J Oral Sci 2007;49:25-29.ArticlePubMed
- 58. Yamamoto T, Nakamura Y, Nishide A, Kubota Y, Momoi Y. Contraction stresses in direct and indirect composite restorations compared by crack analysis. J Adhes Dent 2013;15:47-54.PubMed
- 59. Hirata M, Koizumi H, Tanoue N, Ogino T, Murakami M, Matsumura H. Influence of laboratory light sources on the wear characteristics of indirect composites. Dent Mater J 2011;30:127-135.ArticlePubMed
- 60. Ferracane JL, Condon JR. Post-cure heat treatments for composites: properties and fractography. Dent Mater 1992;8:290-295.ArticlePubMed
- 61. Angeletaki F, Gkogkos A, Papazoglou E, Kloukos D. Direct versus indirect inlay/onlay composite restorations in posterior teeth. A systematic review and meta-analysis. J Dent 2016;53:12-21.ArticlePubMed
- 62. da Veiga AM, Cunha AC, Ferreira DM, da Silva Fidalgo TK, Chianca TK, Reis KR, Maia LC. Longevity of direct and indirect resin composite restorations in permanent posterior teeth: a systematic review and meta-analysis. J Dent 2016;54:1-12.ArticlePubMed
- 63. Mandikos MN, McGivney GP, Davis E, Bush PJ, Carter JM. A comparison of the wear resistance and hardness of indirect composite resins. J Prosthet Dent 2001;85:386-395.ArticlePubMed
- 64. Furuichi T, Takamizawa T, Tsujimoto A, Miyazaki M, Barkmeier WW, Latta MA. Mechanical properties and sliding-impact wear resistance of self-adhesive resin cements. Oper Dent 2016;41:E83-E92.ArticlePubMedPDF
- 65. Tsujimoto A, Barkmeier WW, Takamizawa T, Watanabe H, Johnson WW, Latta MA, Miyazaki M. Simulated localized wear of resin luting cements for universal adhesive systems with different curing mode. J Oral Sci 2018;60:29-36.ArticlePubMed
- 66. Takamizawa T, Barkmeier WW, Latta MA, Berry TP, Tsujimoto A, Miyazaki M. Simulated wear of self-adhesive resin cements. Oper Dent 2016;41:327-338.ArticlePubMedPDF
- 67. Tsujimoto A, Barkmeier WW, Takamizawa T, Latta MA, Miayazaki M. Relationship between simulated gap wear and generalized wear of resin luting cements. Oper Dent 2017;42:E148-E158.ArticlePubMedPDF
- 68. Miyazaki T, Hotta Y. CAD/CAM systems available for the fabrication of crown and bridge restorations. Aust Dent J 2011;56(Supplement 1):97-106.Article
- 69. Yoshida F, Tsujimoto A, Ishii R, Nojiri K, Takamizawa T, Miyazaki M, Latta MA. Influence of surface treatment of contaminated lithium disilicate and leucite glass ceramics on surface free energy and bond strength of universal adhesives. Dent Mater J 2015;34:855-862.ArticlePubMed
- 70. Kömürcüoğlu MB, Sağırkaya E, Tulga A. Influence of different surface treatments on bond strength of novel CAD/CAM restorative materials to resin cement. J Adv Prosthodont 2017;9:439-446.ArticlePubMedPMCPDF
- 71. Alamoush RA, Silikas N, Salim N, Al-Nasrawi S, Satterthwaite JD. Effect of the composition of CAD/CAM composite blocks on mechanical properties. Biomed Res Int 2018;2018:4893143.ArticlePubMedPMCPDF
- 72. Papadopoulos C, Dionysopoulos D, Tolidis K, Kouros P, Koliniotou-Koumpia E, Tsitrou EA. Structural integrity evaluation of large MOD restorations fabricated with a bulk-fill and a CAD-CAM resin composite material. Oper Dent 2019;44:312-321.ArticlePubMedPDF
- 73. Magne P, Dietschi D, Holz J. Esthetic restorations for posterior teeth: practical and clinical considerations. Int J Periodontics Restorative Dent 1996;16:104-119.PubMed
- 74. Ilie N, Bucuta S, Draenert M. Bulk-fill resin-based composites: an in vitro assessment of their mechanical performance. Oper Dent 2013;38:618-625.ArticlePubMedPDF
- 75. Wassell RW, Walls AW, McCabe JF. Direct composite inlays versus conventional composite restorations: 5-year follow-up. J Dent 2000;28:375-382.ArticlePubMed
- 76. Ruse ND, Sadoun MJ. Resin-composite blocks for dental CAD/CAM applications. J Dent Res 2014;93:1232-1234.ArticlePubMedPMCPDF
- 77. Lawson NC, Bansal R, Burgess JO. Wear, strength, modulus and hardness of CAD/CAM restorative materials. Dent Mater 2016;32:e275-e283.ArticlePubMed
- 78. Lauvahutanon S, Takahashi H, Shiozawa M, Iwasaki N, Asakawa Y, Oki M, Finger WJ, Arksornnukit M. Mechanical properties of composite resin blocks for CAD/CAM. Dent Mater J 2014;33:705-710.ArticlePubMed
- 79. Tsujimoto A, Barkmeier WW, Takamizawa T, Latta MA, Miyazaki M. Influence of thermal cycling on flexural properties and simulated wear of computer-aided design/computer-aided manufacturing resin composites. Oper Dent 2017;42:101-110.ArticlePubMedPDF
- 80. Papadopoulos K, Pahinis K, Saltidou K, Dionysopoulos D, Tsitrou E. Evaluation of the surface characteristics of dental CAD/CAM materials after different surface treatments. Materials (Basel) 2020;13:981.ArticlePubMedPMC
- 81. Dionysopoulos D. Smart materials in dentistry. Stoma (Thessaloniki) 2016;44:83-92.
- 82. Althaqafi KA, Satterthwaite J, Silikas N. A review and current state of autonomic self-healing microcapsules-based dental resin composites. Dent Mater 2020;36:329-342.ArticlePubMed
REFERENCES
Tables & Figures
REFERENCES
Citations

- Synergistic Effects of Simulated Energy Drink Exposure and Fatigue Loading on Bioactive and Conventional Resin Composites
Fatin A. Hasanain, Alaa Turkistani
Journal of Functional Biomaterials.2026; 17(1): 29. CrossRef - Direct and Semi-Direct Composite Techniques in Posterior Teeth: A Two-Year Follow-Up Comparative Study
Adriana Saceleanu, Anca Maria Fratila, Vasile Calin Arcas, Cristina Ana-Maria Arcas, Dragos Anton Dadarlat, Laura Stef
Journal of Clinical Medicine.2026; 15(2): 687. CrossRef - Micro-scratch and wear resistance of restorative dental materials: an in vitro study
Valeriya Aleksandrova, Neshka Manchorova, Veselina Todorova, Lyubomir Vangelov, Svetlin Alexandrov
Folia Medica.2026;[Epub] CrossRef - Nanotechnology in contemporary dentistry: a comprehensive review of current clinical applications, bioactive materials, and future perspectives
Murtada A. Ahmed, Samar M. Albuhiri, Manal M. Alanazi, Ameerah A. Albogami, Jawaher N. Alharbi, Eman A. Alruwaili, Maryam A. Shabeen
International Journal Of Community Medicine And Public Health.2026; 13(2): 1044. CrossRef - Advances in Ecofriendly and High-Strength Dental Composites: Structural and Functional Perspectives
Sayem A. Mulla, Amit Patil, Himmat Jaiswal, Bhavani Sangala Nagendra, Ashima Jakhar, Waseem Z. Khan
European Journal of General Dentistry.2026;[Epub] CrossRef - Letter to the Editor regarding, “A digital workflow for tooth-supported complete overdentures with a composite resin injection technique to manage the treatment of a child with ectodermal dysplasia” by Liu et al
Meng Liu, Guoyan Quan
The Journal of Prosthetic Dentistry.2026;[Epub] CrossRef - How surface electronegativity and calcium release in enamel mediate the adsorption and lubrication of salivary proteins: The role of interfacial water
Yue Tang, Lei Lei, Hujun Wang, Haonan Qiu, Jing Zheng, Zhongrong Zhou
Friction.2025; 13(3): 9440912. CrossRef - Effect of SiC particle size and content on the mechanical and tribological properties of porous Si3N4-SiC composites fabricated following a facile low-temperature processing route
Siddharth, Sakshi Tiwari, Pritam Biswas, Nilrudra Mandal, Siddhartha Roy
Ceramics International.2025; 51(14): 19508. CrossRef - Evaluation of Color Stability and Surface Abrasion of Nano-modified Glass Ionomer Cement with Dentifrices: An In Vitro Study
Jessy Paulraj, Subhabrata Maiti, Harini Palani
International Journal of Prosthodontics and Restorative Dentistry.2025; 15(1): 10. CrossRef - Wear resistance of orthodontic attachments: a comparative analysis of different composite resins in clear aligner therapy
Irmak Ocak, Hande Gorucu-Coskuner, Muge Aksu
Clinical Oral Investigations.2025;[Epub] CrossRef - A Review: Resin‐Based Dental Materials and Their Characterization
Arda Bingül, Merve Nezir, Aykan Onur Atilla, Suat Özcan, Zafer Evis
Polymers for Advanced Technologies.2025;[Epub] CrossRef - Biomechanical and Occlusal Factors Influencing the Longevity of Single-Unit Restorations: A Comprehensive Review
Wedad S Alaida, Safa A Gadi, Rokia E Al-Ghannam, Moayad F Alamri, Feras I Mirdad, Ruba M Argaibeh, Bushra A Alqahtani, Abdulrahman M Alqahtani, Abdulelah A Al Jaban, Turki M Alkuraydimi, Abdulrahman S Alamari
Cureus.2025;[Epub] CrossRef - Effect of Bleaching on Surface Roughness of Universal Composite Resins After Chlorhexidine-Induced Staining
Gözde Aksoy Vaizoğlu
Dentistry Journal.2025; 13(7): 277. CrossRef - Wear properties of hybrid antibacterial dental composite with micro-particles of S. persica and hydroxyapatite as fillers
Rihem Chaaben, Ayman Ayedi, Khaled Elleuch
Euro-Mediterranean Journal for Environmental Integration.2025; 10(4): 3055. CrossRef - Comparative Evaluation of Direct and Indirect Composite Restorations in Class II Tooth Preparations - An In vivo Study
Akshun Gupta, Garima Arora, Aprajita Mehta, Satish Sane, Siddhi Nevrekar, Apurva Nagrale
Advances in Human Biology.2025; 15(4): 550. CrossRef - Effect of Thermal Ageing on Flexural Strength and Microhardness of Novel High-Performance Polymer (Nanoksa G-Plus) in Comparison to a Widely Used Bio-HPP/PEEK
Ramy Abdallah Abdelrahim, Ahmed Ali Ezzeldine, Mahmoud Abdellah, SaadEldein Sadeq Elghazawi
Dentistry Journal.2025; 13(8): 370. CrossRef - A comparative 48 month randomized trial of clinical performance and wear of BISGMA based and BISGMA free nanoceramic resin composites
Samah Mohamed Bahig, Heba Helal El Sherbiney, Mohamed Moustafa Zayed, Shereen Hafez Ibrahim
Scientific Reports.2025;[Epub] CrossRef - Bruxism Simulation in Aligner Therapy: Effects on Restored Posterior Teeth
Amelia Anita Boitor (Andreica), Adriana Objelean, Cristina Gasparik, Alexandru Victor Burde, Horațiu Alexandru Colosi, Diana Dudea
Journal of Clinical Medicine.2025; 14(21): 7877. CrossRef - Comparison of wear behavior of occlusal device materials manufactured by different processes
Catherine Arreaza, Robert R. Seghi, Scott R. Schricker, William M. Johnston, Paola C. Saponaro
The Journal of Prosthetic Dentistry.2025;[Epub] CrossRef - Thermal Effects of Rapid High‐Intensity Light Curing on Bulk‐Fill Resin‐Based Composites: A Systematic Review and Meta‐Analysis
Samille Biasi Miranda, Marina Rodrigues Santi, Giovana Lordsleem de Mendonça, Luiz Antonio Soares Falson, Matheus José Gusmão Simões Barza, Veronica Maria de Sá Rodrigues, Ana Karina Maciel de Andrade, Rodrigo Barros Esteves Lins, Marcos Antonio Japiassú
The Scientific World Journal.2025;[Epub] CrossRef - Effect of Various Toothpaste Tablets on Gloss and Surface Roughness of Resin-based Composite Materials
J Ko, A Tsao, R Kim, C Perry, U Oyoyo, SR Kwon
Operative Dentistry.2024; 49(3): 282. CrossRef - Surface wear of attachments in patients during clear aligner therapy: a prospective clinical study
Qiuying Li, Kai Yang
Progress in Orthodontics.2024;[Epub] CrossRef - Awareness of possible complications associated with direct composite restorations: A multinational survey among dentists from 13 countries with meta-analysis
Anna Lehmann, Kacper Nijakowski, Jakub Jankowski, David Donnermeyer, Paulo J. Palma, Milan Drobac, João Filipe Brochado Martins, Fatma Pertek Hatipoğlu, Indira Tulegenova, Muhammad Qasim Javed, Hamad Mohammad Alharkan, Olga Bekjanova, Sylvia Wyzga, Moataz
Journal of Dentistry.2024; 145: 105009. CrossRef - Evaluation of pre-heated composite resins with soft-start polymerization and conventional composite restorations in class-I carious lesions – A randomized clinical trial
Niral Kotecha, Nimisha C. Shah, Namita N. Gandhi, Priya Porwal, Ajinkya M. Pawar, Novaldy Wahjudianto, Dian Agustin Wahjuningrum, Suraj Arora, Mohmed Isaqali Karobari
Heliyon.2024; 10(10): e30794. CrossRef - Reabilitação estética de dente conóide: relato de caso
Anna Danielle Oliveira dos Santos, Diana Fernandes de Melo , Jorge Alberto Carrazana Moya , Kathleen Rebelo de Sousa , Lizete Karla Filgueiras de Souza, Marcela Lopes Linhares, Márcio Langbeck Castelo Branco , Márcio Lopes Linhares
Revista Clínica de Odontologia.2024; 5(1): 80. CrossRef - Non-collagenous protein analog-induced biomimetic mineralization strategy to restore the dentin interface
Ruhua Chen, Yimeng Xie, Liang Ma, Bing Li, Wei Yao
Biomedical Physics & Engineering Express.2024; 10(6): 062004. CrossRef - Influence of Low pH on the Microhardness and Roughness Surface of Dental Composite—A Preliminary Study
Leszek Szalewski, Dorota Wójcik, Monika Sowa, Vladyslav Vivcharenko, Krzysztof Pałka
Materials.2024; 17(14): 3443. CrossRef - Fabrication of a novel aesthetic orthodontic bracket and evaluation of friction properties between PEEK and stainless steel wires
Jiaqi Wu, Xiujing Wang, Jiuhui Jiang, Yunyang Bai
Technology and Health Care.2024; 32(1): 269. CrossRef - Can wheel polishers improve surface properties and color stability of monochromatic resin composites?
Lezize Sebnem Turkun, Cankut Canevi, Alperen Degirmenci, Hayal Boyacioglu
BMC Oral Health.2024;[Epub] CrossRef - Comparison of volumetric loss and surface roughness of composite dental restorations obtained by additive and subtractive manufacturing methods
Neslihan Güntekin, Ali Rıza Tunçdemir
Heliyon.2024; 10(4): e26269. CrossRef - Comparative evaluation of microhardness of three restorative materials after immersion in chlorhexidine mouthwash: An in vitro study
Shilpa S. Shah, Nishtha K. Patel, Kruti P. Yagnik, Aarshati Vyas, Prerak Doshi, Pooja R. Keshrani
Journal of Conservative Dentistry and Endodontics.2024; 27(5): 520. CrossRef - NON-INTERVENTION VERSUS REPAIR/REPLACEMENT DECISIONS IN POSTERIOR COMPOSITE RESTORATIONS AGED 3-5 YEARS: A RETROSPECTIVE STUDY
Galina Pancu, Andrei Georgescu , Antonia Moldovanu , Angela Ghiorghe , Simona Stoleriu , Irina Nica , Ionut Tărăboanţă , Alexandru Iovan , Sorin Andrian
Romanian Journal of Oral Rehabilitation.2024; 16(2): 186. CrossRef - Effect of tooth brushing simulation on the surface properties of various resin‐matrix computer‐aided design/computer‐aided manufacturing ceramics
Evangelos Ximinis, Dimitrios Dionysopoulos, Constantinos Papadopoulos, Alexandros Tournavitis, Avraam Konstantinidis, Olga Naka
Journal of Esthetic and Restorative Dentistry.2023; 35(6): 937. CrossRef - Effect of toothpaste with different components on toothbrushing wear resistance of micro-hybrid/nano-filled resin composites
Seon-Mi Byeon, Jung-Eun Park, Kyeong-Seon Kim, Tae-Hwan Kim, Chung-Cha Oh, Seung-O Ko3, Min-Ho Lee
Korean Journal of Dental Materials.2023; 50(4): 247. CrossRef - Release Kinetics of Monomers from Dental Composites Containing Fluoride-Doped Calcium Phosphates
Adrián M. Alambiaga-Caravaca, Alicia López-Castellano, Yu Fu Chou, Arlinda Luzi, Juan Manuel Núñez, Avijit Banerjee, María del Mar Jovani Sancho, Salvatore Sauro
Pharmaceutics.2023; 15(7): 1948. CrossRef - Comparative study on the impact-sliding wear behaviour of CAD/CAM resin-ceramic materials and tooth enamel
Chunxiao Jin, Jiuhong Deng, Peiyue Pan, Yuhuan Xiong, Liqing Zhu, Shanshan Gao
Dental Materials.2023; 39(1): 25. CrossRef - The impact of dental varnishes on the immediate surface microhardness and roughness of restorative dental materials: An in vitro study
Jovana Lovric, Milisav Markovic, Marko Bulajic, Sasa Zeljkovic, Jana Ilic, Olivera Dolic
Vojnosanitetski pregled.2023; 80(12): 1022. CrossRef - An In Vitro Study regarding the Wear of Composite Materials Following the Use of Dental Bleaching Protocols
Alexandru Dan Popescu, Mihaela Jana Ţuculină, Lelia Mihaela Gheorghiță, Andrei Osman, Claudiu Nicolicescu, Smaranda Adelina Bugălă, Mihaela Ionescu, Jaqueline Abdul-Razzak, Oana Andreea Diaconu, Bogdan Dimitriu
Journal of Functional Biomaterials.2023; 14(10): 532. CrossRef - Investigation of aging resistance for dental resin composites with and without glass flakes
Dan Feng, Shujun Dong, Zuosen Shi, Zhanchen Cui, Song Zhu
Clinical Oral Investigations.2023; 27(11): 6903. CrossRef - Tribological behavior and wear mechanisms of dental resin composites with different polymeric matrices
Vladja Torno, Paulo Soares
Journal of the Mechanical Behavior of Biomedical Materials.2023; 144: 105962. CrossRef - Performance of two-flux and four-flux models for predicting the spectral reflectance and transmittance factors of flowable dental resin composites
Vincent Duveiller, Raphaël Clerc, Julien Eymard, Jean-Pierre Salomon, Mathieu Hébert
Dental Materials.2023; 39(8): 743. CrossRef - Biocompatibility of bulk-fill resins in vitro
Carla Junqueira, Paulo Mascarenhas, Mariana Avelar, Ana Clara Ribeiro, Isabel Barahona
Clinical Oral Investigations.2023; 27(12): 7851. CrossRef - Optimizing Dental Bond Strength: Insights from Comprehensive Literature Review and Future Implications for Clinical Practice
Yung-Shin Fan-Chiang, Peng-Chen Chou, Yu-Wen Hsiao, Yu-Hsuan Cheng, Yi Huang, Yu-Chieh Chiu, Yu-Ju Lin, Yuichi Mine, Sheng-Wei Feng, I-Ta Lee, Tzu-Yu Peng
Biomedicines.2023; 11(11): 2995. CrossRef - Polymères et résines composites en technique directe
T. Giraud, E. Casazza, B. Ballester, A. Raskin
EMC - Médecine buccale.2023; 16(6): 1. CrossRef - In Vitro Evaluation of the Strength of Dentin Replacement in Complex Posterior Tooth Restoration
Nurhayaty Natsir, Farida Rahim, Juni Jekti Nugroho, Christine Anastasia Rovani, Syamsiah Syam, Muhammad Ruslin, Takashi Saito, Keng-Liang Ou
Applied Sciences.2022; 12(14): 6877. CrossRef - Calcium release-mediated adsorption and lubrication of salivary proteins on resin-based dental composites
Yue Tang, Lei Lei, Dan Yang, Jing Zheng, Qihang Zeng, Heng Xiao, Zhongrong Zhou
Journal of the Mechanical Behavior of Biomedical Materials.2022; 135: 105437. CrossRef - Modifications of Glass Ionomer Cements Using Nanotechnology: Recent Advances
Dimitrios Dionysopoulos, Olga Gerasimidou, Constantinos Papadopoulos
Recent Progress in Materials.2022; 04(02): 1. CrossRef - Microleakage Evaluation in Class V Cavities Restored with Five Different Resin Composites: In vitro Dye Leakage Study
Sahar Bajabaa, Shaza Balbaid, Muruj Taleb, Lujain Islam, Salem Elharazeen, Ebaa Alagha
Clinical, Cosmetic and Investigational Dentistry.2021; Volume 13: 405. CrossRef

 KACD
KACD
 ePub Link
ePub Link Cite
Cite

