Articles
- Page Path
- HOME > Restor Dent Endod > Volume 46(2); 2021 > Article
- Research Article A novel antimicrobial-containing nanocellulose scaffold for regenerative endodontics
-
Victoria Kichler1
 , Lucas Soares Teixeira1
, Lucas Soares Teixeira1 , Maick Meneguzzo Prado2
, Maick Meneguzzo Prado2 , Guilherme Colla2
, Guilherme Colla2 , Daniela Peressoni Vieira Schuldt1
, Daniela Peressoni Vieira Schuldt1 , Beatriz Serrato Coelho1
, Beatriz Serrato Coelho1 , Luismar Marques Porto2
, Luismar Marques Porto2 , Josiane de Almeida1,2
, Josiane de Almeida1,2 -
Restor Dent Endod 2021;46(2):e20.
DOI: https://doi.org/10.5395/rde.2021.46.e20
Published online: March 16, 2021
1Department of Endodontics, Faculty of Dentistry, University of Southern Santa Catarina, Palhoça, SC, Brazil.
2Department of Chemical Engineering, Federal University of Santa Catarina, Florianópolis, SC, Brazil.
- Correspondence to Josiane de Almeida, PhD. Associate Professor, Department of Endodontics, Faculty of Dentistry, University of Southern Santa Catarina, Av. Pedra Branca, 25 Cidade Universitária, Palhoça, SC 88137-270, Brazil. dealmeidajosiane@hotmail.com
Copyright © 2021. The Korean Academy of Conservative Dentistry
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Objectives The aim of this study was to evaluate bacterial nanocellulose (BNC) membranes incorporated with antimicrobial agents regarding cytotoxicity in fibroblasts of the periodontal ligament (PDLF), antimicrobial activity, and inhibition of multispecies biofilm formation.
-
Materials and Methods The tested BNC membranes were BNC + 1% clindamycin (BNC/CLI); BNC + 0.12% chlorhexidine (BNC/CHX); BNC + nitric oxide (BNC/NO); and conventional BNC (BNC; control). After PDLF culture, the BNC membranes were positioned in the wells and maintained for 24 hours. Cell viability was then evaluated using the MTS calorimetric test. Antimicrobial activity against Enterococcus faecalis, Actinomyces naeslundii, and Streptococcus sanguinis (S. sanguinis) was evaluated using the agar diffusion test. To assess the antibiofilm activity, BNC membranes were exposed for 24 hours to the mixed culture. After sonicating the BNC membranes to remove the remaining biofilm and plating the suspension on agar, the number of colony-forming units (CFU)/mL was determined. Data were analyzed by 1-way analysis of variance and the Tukey, Kruskal-Wallis, and Dunn tests (α = 5%).
-
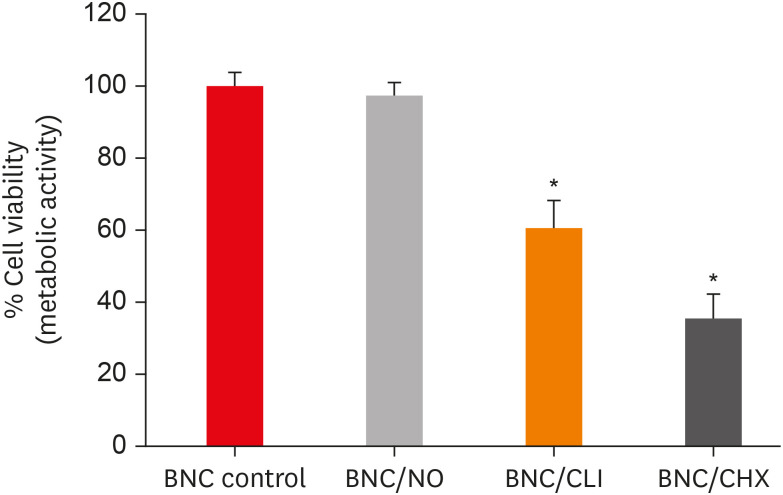
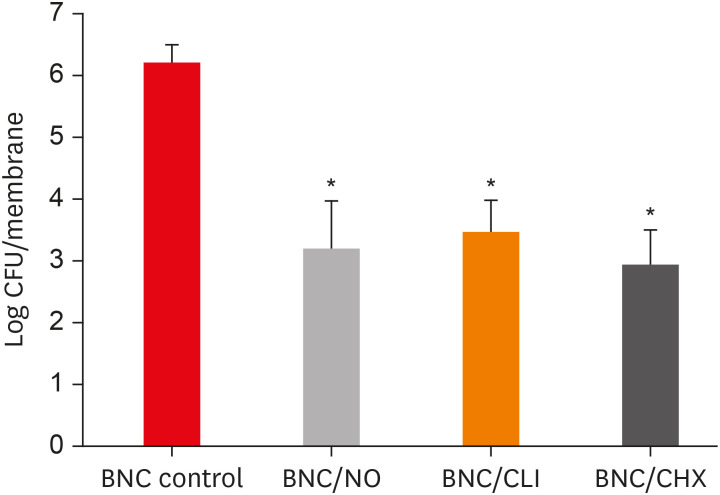
Results PDLF metabolic activity after contact with BNC/CHX, BNC/CLI, and BNC/NO was 35%, 61% and 97%, respectively, compared to BNC. BNC/NO showed biocompatibility similar to that of BNC (p = 0.78). BNC/CLI showed the largest inhibition halos, and was superior to the other BNC membranes against S. sanguinis (p < 0.05). The experimental BNC membranes inhibited biofilm formation, with about a 3-fold log CFU reduction compared to BNC (p < 0.05).
-
Conclusions BNC/NO showed excellent biocompatibility and inhibited multispecies biofilm formation, similarly to BNC/CLI and BNC/CHX.
INTRODUCTION
MATERIALS AND METHODS
RESULTS
Percentage of periodontal ligament fibroblast (PDLF) metabolic activity after contact with the different membranes.
The inhibition halos value (mm) formed after the contact of the different bacterial nanocelluloses (BNCs) with Enterococcus faecalis (E. faecalis), Streptococcus sanguinis (S. sanguinis), and Actinomyces naeslundii (A. naeslundii)
Mean colony-forming unit (CFU) values (log CFU/membrane) present in the multispecies biofilm that adhered to the different experimental bacterial nanocelluloses (BNCs).
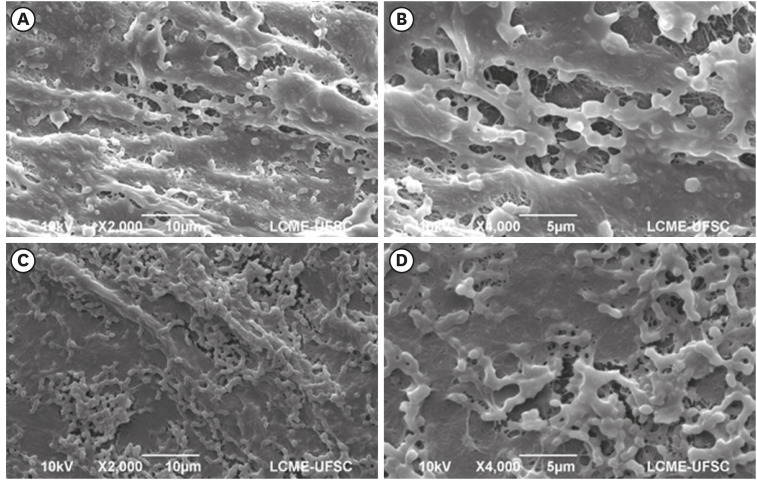
Dense and homogeneous bacterial biofilm covering the membrane surface in samples of the control group (conventional bacterial nanocellulose [BNC]) (magnification ×2,000 and ×4,000).
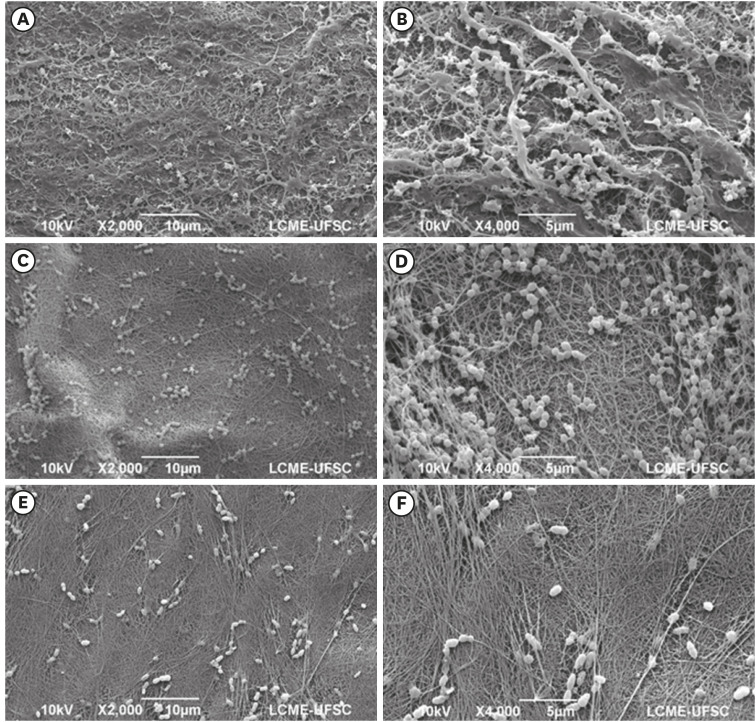
Isolated bacterial cells and small bacterial clusters, of varying sizes, irregularly distributed on the surface of the bacterial nanocellulose (BNC)/nitric oxide (NO) (A, B), BNC/clindamycin (CLI) (C, D), and BNC/chlorhexidine (CHX) (E, F) membranes (magnification ×2,000 and ×4,000).
DISCUSSION
CONCLUSIONS
ACKNOWLEDGEMENTS
-
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
-
Author Contributions:
Conceptualization: de Almeida J, Kichler V, Teixeira LS.
Data curation: Colla G, de Almeida J.
Formal analysis: de Almeida J, Porto LM.
Funding acquisition: Porto LM, Colla G.
Investigation: Kichler V, Teixeira LS, Prado MM.
Methodology: Schuldt DPV, Coelho BS, Kichler V, Teixeira LS, Prado MM.
Project administration: de Almeida J.
Resources: Colla G, de Almeida J, Porto LM.
Software: Prado MM.
Supervision: de Almeida J, Porto LM, Colla G.
Validation: Schuldt DPV, Coelho BS.
Visualization: Schuldt DPV, Coelho BS.
Writing - original draft: Kichler V, Teixeira LS.
Writing - review & editing: de Almeida J, Porto LM, Colla G.
- 1. Jeeruphan T, Jantarat J, Yanpiset K, Suwannapan L, Khewsawai P, Hargreaves KM. Mahidol study 1: comparison of radiographic and survival outcomes of immature teeth treated with either regenerative endodontic or apexification methods: a retrospective study. J Endod 2012;38:1330-1336.ArticlePubMed
- 2. Diogenes A, Ruparel NB, Shiloah Y, Hargreaves KM. Regenerative endodontics: a way forward. J Am Dent Assoc 2016;147:372-380.PubMed
- 3. Verma P, Nosrat A, Kim JR, Price JB, Wang P, Bair E, Xu HH, Fouad AF. Effect of residual bacteria on the outcome of pulp regeneration in vivo . J Dent Res 2017;96:100-106.ArticlePubMedPDF
- 4. Vishwanat L, Duong R, Takimoto K, Phillips L, Espitia CO, Diogenes A, Ruparel SB, Kolodrubetz D, Ruparel NB. Effect of bacterial biofilm on the osteogenic differentiation of stem cells of apical papilla. J Endod 2017;43:916-922.ArticlePubMed
- 5. Curvello R, Raghuwanshi VS, Garnier G. Engineering nanocellulose hydrogels for biomedical applications. Adv Colloid Interface Sci 2019;267:47-61.ArticlePubMed
- 6. Vismara E, Bernardi A, Bongio C, Farè S, Pappalardo S, Serafini A, Pollegioni L, Rosini E, Torri G. Bacterial nanocellulose and its surface modification by glycidyl methacrylate and ethylene glycol dimethacrylate. Incorporation of vancomycin and ciprofloxacin. Nanomaterials (Basel) 2019;9:1668.ArticlePubMedPMC
- 7. Osorio M, Ortiz I, Gañán P, Naranjo T, Zuluaga R, van Kooten TG, Castro C. Novel surface modification of three-dimensional bacterial nanocellulose with cell-derived adhesion proteins for soft tissue engineering. Mater Sci Eng C 2019;100:697-705.Article
- 8. Bottino MC, Pankajakshan D, Nör JE. Advanced scaffolds for dental pulp and periodontal regeneration. Dent Clin North Am 2017;61:689-711.ArticlePubMedPMC
- 9. Osorio M, Fernández-Morales P, Gañán P, Zuluaga R, Kerguelen H, Ortiz I, Castro C. Development of novel three-dimensional scaffolds based on bacterial nanocellulose for tissue engineering and regenerative medicine: effect of processing methods, pore size, and surface area. J Biomed Mater Res A 2019;107:348-359.ArticlePubMedPDF
- 10. Reis EMD, Berti FV, Colla G, Porto LM. Bacterial nanocellulose-IKVAV hydrogel matrix modulates melanoma tumor cell adhesion and proliferation and induces vasculogenic mimicry in vitro . J Biomed Mater Res B Appl Biomater 2018;106:2741-2749.ArticlePubMedPDF
- 11. Shoda M, Sugano Y. Recent advances in bacterial cellulose production. Biotechnol Bioprocess Eng 2005;10:1-8.ArticlePDF
- 12. Dobmeier KP, Schoenfisch MH. Antibacterial properties of nitric oxide-releasing sol-gel microarrays. Biomacromolecules 2004;5:2493-2495.ArticlePubMed
- 13. Seabra AB, Martins D, Simões MM, da Silva R, Brocchi M, de Oliveira MG. Antibacterial nitric oxide-releasing polyester for the coating of blood-contacting artificial materials. Artif Organs 2010;34:E204-E214.ArticlePubMed
- 14. Moon CY, Nam OH, Kim M, Lee HS, Kaushik SN, Cruz Walma DA, Jun HW, Cheon K, Choi SC. Effects of the nitric oxide releasing biomimetic nanomatrix gel on pulp-dentin regeneration: pilot study. PLoS One 2018;13:e0205534.ArticlePubMedPMC
- 15. Kim JO, Noh JK, Thapa RK, Hasan N, Choi M, Kim JH, Lee JH, Ku SK, Yoo JW. Nitric oxide-releasing chitosan film for enhanced antibacterial and in vivo wound-healing efficacy. Int J Biol Macromol 2015;79:217-225.ArticlePubMed
- 16. Pankajakshan D, Albuquerque MT, Evans JD, Kamocka MM, Gregory RL, Bottino MC. Triple antibiotic polymer nanofibers for intracanal drug delivery: effects on dual species biofilm and cell function. J Endod 2016;42:1490-1495.ArticlePubMedPMC
- 17. Karczewski A, Feitosa SA, Hamer EI, Pankajakshan D, Gregory RL, Spolnik KJ, Bottino MC. Clindamycin-modified triple antibiotic nanofibers: a stain-free antimicrobial intracanal drug delivery system. J Endod 2018;44:155-162.ArticlePubMed
- 18. Gomes BP, Vianna ME, Zaia AA, Almeida JFA, Souza-Filho FJ, Ferraz CCR. Chlorhexidine in endodontics. Braz Dent J 2013;24:89-102.ArticlePubMed
- 19. Galler KM, Buchalla W, Hiller KA, Federlin M, Eidt A, Schiefersteiner M, Schmalz G. Influence of root canal disinfectants on growth factor release from dentin. J Endod 2015;41:363-368.ArticlePubMed
- 20. Widbiller M, Althumairy RI, Diogenes A. Direct and indirect effect of chlorhexidine on survival of stem cells from the apical papilla and its neutralization. J Endod 2019;45:156-160.ArticlePubMed
- 21. De Souza SS, Berti FV, Oliveira KPV, Pittella C, Vasconcellos J, Pelissari C, Rambo CR, Porto LM. Nanocellulose biosynthesis by Komagataeibacter hansenii in a defined minimal culture medium. Cellulose 2018;26:1641-1655.ArticlePDF
- 22. Lourenço SDM, de Oliveira MG. Topical photochemical nitric oxide release from porous poly(vinyl alcohol) membrane for visible light modulation of dermal vasodilation. J Photochem Photobiol Chem 2017;346:548-558.Article
- 23. Kumar V, Yang T. HNO3/H3PO4-NANO2 mediated oxidation of cellulose - preparation and characterization of bioabsorbable oxidized celluloses in high yields and with different levels of oxidation. Carbohydr Polym 2002;48:403-412.Article
- 24. Pittela CQP, Porto LM. Application of bacterial nanocellulose membranes for epithelial tissue repair. Revista de Enfermagem da UFJF 2015;1:223-232.
- 25. Osorio M, Cañas A, Puerta J, Díaz L, Naranjo T, Ortiz I, Castro C. Ex vivo and in vivo biocompatibility assessment (blood and tissue) of three-dimensional bacterial nanocellulose biomaterials for soft tissue implants. Sci Rep 2019;9:10553.ArticlePubMedPMCPDF
- 26. Albuquerque MTP, Nagata J, Bottino MC. Antimicrobial efficacy of triple antibiotic-eluting polymer nanofibers against multispecies biofilm. J Endod 2017;43:S51-S56.ArticlePubMedPMC
- 27. Sun B, Slomberg DL, Chudasama SL, Lu Y, Schoenfisch MH. Nitric oxide-releasing dendrimers as antibacterial agents. Biomacromolecules 2012;13:3343-3354.ArticlePubMedPMC
- 28. Frost MC, Batchelor MM, Lee YM, Zhang H, Kang Y, Oh B, Wilson GS, Gifford R, Rudich SM, Meyerhoff M. Preparation and characterization of implantable sensors with nitric oxide release coatings. Microchem J 2003;74:277-288.Article
- 29. Sadrearhami Z, Nguyen TK, Namivandi-Zangeneh R, Jung K, Wong EHH, Boyer C. Recent advances in nitric oxide delivery for antimicrobial applications using polymer-based systems. J Mater Chem B 2018;6:2945-2959.ArticlePubMed
- 30. Chuensombat S, Khemaleelakul S, Chattipakorn S, Srisuwan T. Cytotoxic effects and antibacterial efficacy of a 3-antibiotic combination: an in vitro study. J Endod 2013;39:813-819.ArticlePubMed
- 31. Tanase S, Tsuchiya H, Yao J, Ohmoto S, Takagi N, Yoshida S. Reversed-phase ion-pair chromatographic analysis of tetracycline antibiotics. Application to discolored teeth. J Chromatogr B Biomed Sci Appl 1998;706:279-285.PubMed
- 32. Rahhal JG, Rovai ED, Holzhausen M, Caldeira CL, Santos CF, Sipert CR. Root canal dressings for revascularization influence in vitro mineralization of apical papilla cells. J Appl Oral Sci 2019;27:e20180396.ArticlePubMedPMC
- 33. Zargar N, Rayat Hosein Abadi M, Sabeti M, Yadegari Z, Akbarzadeh Baghban A, Dianat O. Antimicrobial efficacy of clindamycin and triple antibiotic paste as root canal medicaments on tubular infection: an in vitro study. Aust Endod J 2019;45:86-91.ArticlePubMedPDF
- 34. Skucaite N, Peciuliene V, Vitkauskiene A, Machiulskiene V. Susceptibility of endodontic pathogens to antibiotics in patients with symptomatic apical periodontitis. J Endod 2010;36:1611-1616.ArticlePubMed
- 35. Brook I, Lewis MA, Sándor GK, Jeffcoat M, Samaranayake LP, Vera Rojas J. Clindamycin in dentistry: more than just effective prophylaxis for endocarditis? Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2005;100:550-558.ArticlePubMed
- 36. Lessa FC, Aranha AM, Nogueira I, Giro EM, Hebling J, Costa CAS. Toxicity of chlorhexidine on odontoblast-like cells. J Appl Oral Sci 2010;18:50-58.ArticlePubMedPMC
REFERENCES
Tables & Figures
REFERENCES
Citations

- Topic: Perspectives on Success and Failure of Endodontic Treatments
Ilma Robo, Manola Kelmendi, Eva Habazaj, Kleves Elezi, Rialda Xhizdari, Nevila Alliu
SN Comprehensive Clinical Medicine.2025;[Epub] CrossRef - Data about application of chlorhexidine as a periodontal irrigant –
Systematic Review.
Ilma Robo, Manola Kelmendi , Eva Habazaj , Kristi Sulanjaku , Nevila Alliu
Acta Stomatologica Marisiensis Journal.2025; 8(1): 6. CrossRef - Aqueous‐Phase Surface Amidation of TEMPO‐CNF Films for Improved Adsorption of Organic Pollutants in Water
Domenico Santandrea, Cécile Sillard, Valentina Beghetto, Julien Bras
ChemPlusChem.2025;[Epub] CrossRef - Materials design of gas-releasing nanoplatforms: strategies for precision delivery in oral healthcare
Haodong Zhong, Weiming Tan, Jian Zhang, Xiongwei Huang, Haizhan Chen, Jiyuan Zou, Yuxin Ye, Tao Wang, Xuechao Yang, Jiang Li, Li Yang, Lvhua Guo, Tao Luo
Materials & Design.2025; 258: 114704. CrossRef - Pushing the limits of bacterial cellulose for biomedicine: a review
Cristina Campano, Virginia Rivero-Buceta, Ana M. Hernandez-Arriaga, Maria T. Manoli, M. Auxiliadora Prieto
International Journal of Biological Macromolecules.2025; 323: 146701. CrossRef - Prospective and applications of bacterial nanocellulose in dentistry
Yasmin Alimardani, Esmaeel Mirzakhani, Fereshteh Ansari, Hadi Pourjafar, Nadia Sadeghi
Cellulose.2024; 31(13): 7819. CrossRef - Bacterial nanocelluloses as sustainable biomaterials for advanced wound healing and dressings
Atefeh Zarepour, Bahar Gok, Yasemin Budama-Kilinc, Arezoo Khosravi, Siavash Iravani, Ali Zarrabi
Journal of Materials Chemistry B.2024; 12(48): 12489. CrossRef - Sulfated endospermic nanocellulose crystals prevent the transmission of SARS-CoV-2 and HIV-1
Enrique Javier Carvajal-Barriga, Wendy Fitzgerald, Emilios K. Dimitriadis, Leonid Margolis, R. Douglas Fields
Scientific Reports.2023;[Epub] CrossRef - A Novel Approach for the Fabrication of 3D-Printed Dental Membrane Scaffolds including Antimicrobial Pomegranate Extract
Hatice Karabulut, Songul Ulag, Basak Dalbayrak, Elif Arisan, Turgut Taskin, Mehmet Guncu, Burak Aksu, Alireza Valanezhad, Oguzhan Gunduz
Pharmaceutics.2023; 15(3): 737. CrossRef - Current advances of nanocellulose application in biomedical field
M.Y. Leong, Y.L. Kong, M.Y. Harun, C.Y. Looi, W.F. Wong
Carbohydrate Research.2023; 532: 108899. CrossRef - Bacterial cellulose as a potential biopolymer in biomedical applications: a state-of-the-art review
Prachi Shrivastav, Sheersha Pramanik, Gayatri Vaidya, Mohamed A. Abdelgawad, Mohammed M. Ghoneim, Ajeet Singh, Bassam M. Abualsoud, Larissa Souza Amaral, Mohammed A. S. Abourehab
Journal of Materials Chemistry B.2022; 10(17): 3199. CrossRef - Nanocelluloses as new generation materials: natural resources, structure-related properties, engineering nanostructures, and technical challenges
Ahmed Barhoum, Vibhore K. Rastogi, Bhupender K. Mahur, Amit Rastogi, Fatehy M. Abdel-Haleem, Pieter Samyn
Materials Today Chemistry.2022; 26: 101247. CrossRef - The current natural/chemical materials and innovative technologies in periodontal diseases therapy and regeneration: A narrative review
Peyman Esmaeili Fard Barzegar, Reza Ranjbar, Mohsen Yazdanian, Elahe Tahmasebi, Mostafa Alam, Kamyar Abbasi, Hamid Tebyaniyan, Keyvan Esmaeili Fard Barzegar
Materials Today Communications.2022; 32: 104099. CrossRef
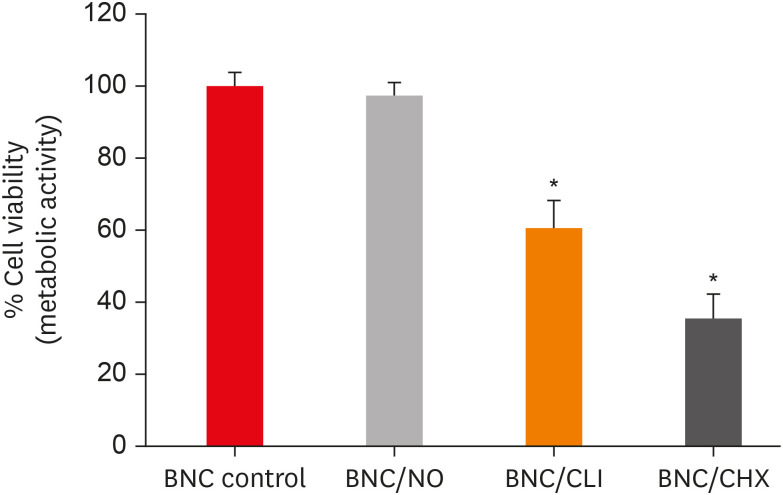
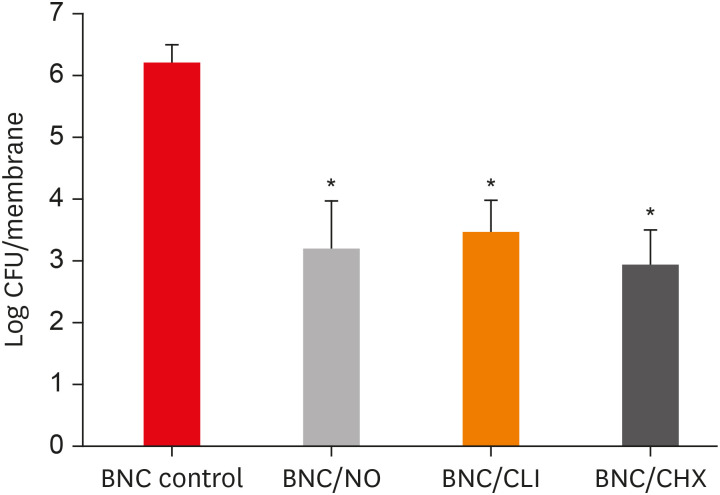
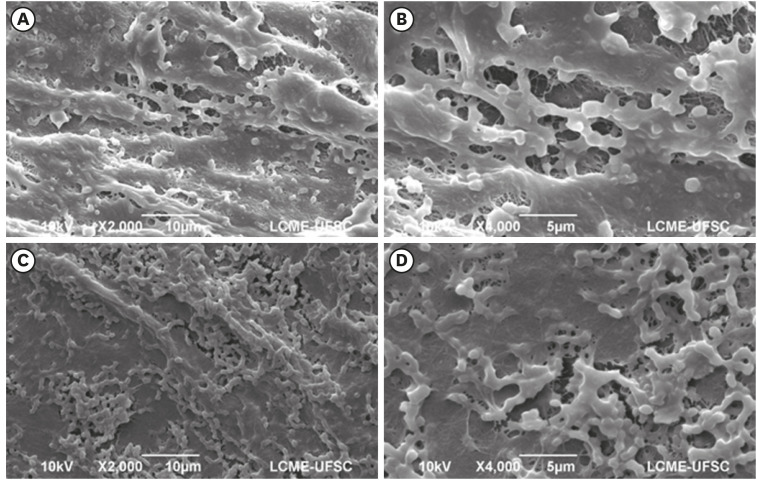
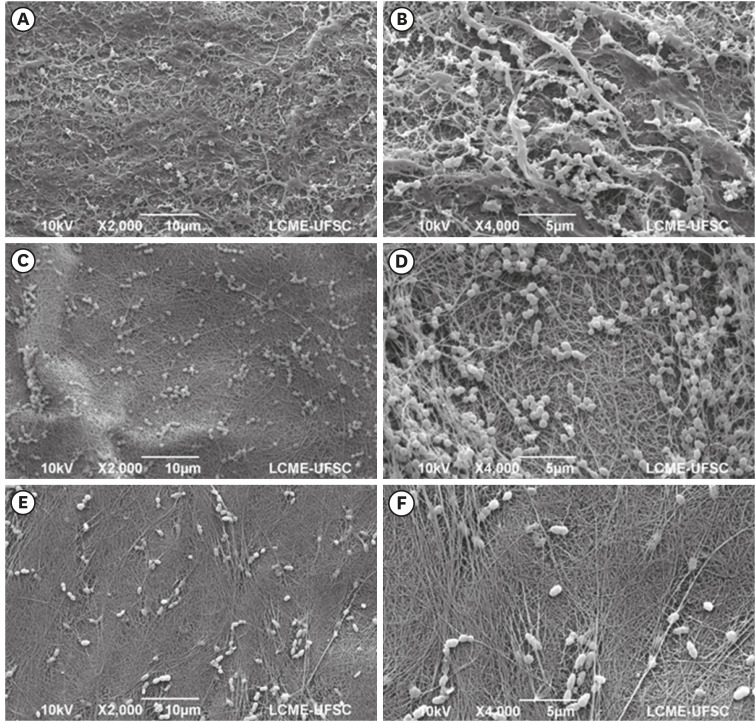
Figure 1
Figure 2
Figure 3
Figure 4
The inhibition halos value (mm) formed after the contact of the different bacterial nanocelluloses (BNCs) with Enterococcus faecalis (E. faecalis), Streptococcus sanguinis (S. sanguinis), and Actinomyces naeslundii (A. naeslundii)
| Groups | Bacterial species | ||
|---|---|---|---|
| E. faecalis | S. sanguinis | A. naeslundii | |
| BNC/CHX | 13.44 ± 2.74a,c | 11.88 ± 1.45a | 13.87 ± 2.85a,c |
| BNC/NO | 11.00 ± 2.28b | 12.16 ± 2.71a | 9.33 ± 1.96c |
| BNC/CLI | 21.77 ± 7.32a | 19.66 ± 3.32b | 20.66 ± 7.98a |
| BNC (negative control) | 0b | 0c | 0b,c |
| CHX (positive control) | 9.22 ± 2.16b,c | 8.22 ± 2.16a,c | 9.22 ± 1.71b,c |
Data are shown as mean ± standard deviation. Different lower-case letters in the same column indicate a significant difference between groups (p < 0.05).
CHX, chlorhexidine; NO, nitric oxide; CLI, clindamycin.
Data are shown as mean ± standard deviation. Different lower-case letters in the same column indicate a significant difference between groups (
CHX, chlorhexidine; NO, nitric oxide; CLI, clindamycin.

 KACD
KACD
 ePub Link
ePub Link Cite
Cite

