Search
- Page Path
- HOME > Search
- Can silver diamine fluoride or silver nanoparticle-based anticaries agents to affect enamel bond strength?
- Jaqueline Costa Favaro, Yana Cosendey Toledo de Mello Peixoto, Omar Geha, Flaviana Alves Dias, Ricardo Danil Guiraldo, Murilo Baena Lopes, Sandrine Bittencourt Berger
- Restor Dent Endod 2021;46(1):e7. Published online January 12, 2021
- DOI: https://doi.org/10.5395/rde.2021.46.e7
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
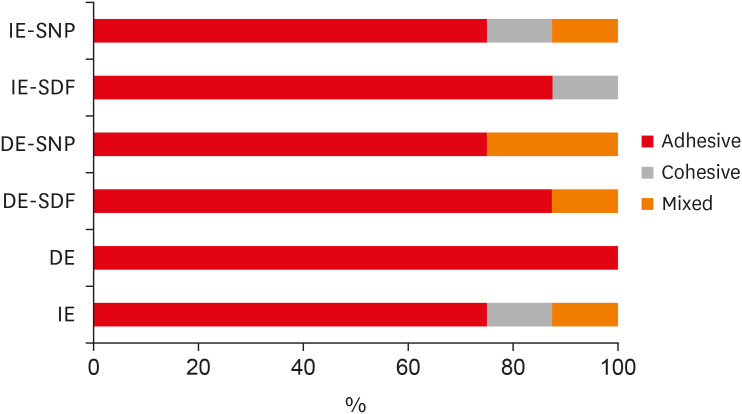
ePub Objectives The aim of the current study is to investigate the effect of different anticaries agents, such as experimental agents based on silver nanoparticles (SNPs) and silver diamine fluoride (SDF), on the micro-shear bond strength (μ-SBS) of composite resin applied to intact enamel (IE) or demineralized enamel (DE).
Materials and Methods Sixty dental enamel fragments were collected from human third molars and categorized into 6 groups (
n = 10): positive control (IE), negative control (DE), IE + SDF, DE + SDF, IE + SNP and DE + SNP. Samples from DE, DE + SDF and DE + SNP groups were subjected to pH cycling; superficial microhardness test was performed to confirm demineralization. Resin composite build-ups were applied to the samples (0.75-mm diameter and 1-mm height) after the treatments (except for IE and DE groups); μ-SBS was also evaluated. Samples were analyzed under a stereomicroscope at 40× magnification to identify failure patterns. Data were subjected to one-way analysis of variance, followed by Tukey's and Dunnett's tests (p < 0.05).Results There was no significant difference among the IE, IE + SNP, DE + SDF, and DE + SNP groups. The IE + SDF and DE groups recorded the highest and the lowest μ-SBS values, respectively. Adhesive-type failures were the most frequent for all treatments.
Conclusions Anticaries agents did not have a negative effect on the μ-SBS of composite resin when it was used on IE or DE.
-
Citations
Citations to this article as recorded by- Impact of Incorporating Nanoparticles to Adhesive Resin on the Demineralization of Enamel: A Systematic Review
Naif Almosa
Dentistry Journal.2025; 13(3): 89. CrossRef - Preventing white spot lesions around orthodontic brackets: efficacy of pre-reacted glass-ionomer barrier coat versus silver diamine fluoride: an in vitro study
Enas A. Elshenawy, Safa B. Alawy, Wafaa Yahia Alghonemy, Ahmed Ibrahime El dosoky
BDJ Open.2025;[Epub] CrossRef - Research Status of Silver Nanoparticles for Dental Applications
Yanyan Guo, Xiaomei Hou, Sanjun Fan, Chanyuan Jin
Inorganics.2025; 13(5): 168. CrossRef - The use of silver diamine fluoride to prevent/treat enamel carious lesions: a narrative review
Rasha N. AlSheikh
PeerJ.2024; 12: e17897. CrossRef - Phosphoric Acid Etch Partially Restores the Initial Bond Strength of Composite to Silver Diamine Fluoride–Treated Enamel Using Universal Adhesives
Zaher Jabbour, Mijoo Kim, Marc Hayashi, Reuben Kim
Dentistry Journal.2023; 11(7): 161. CrossRef - Efficacy of Nano Silver Fluoride and/or Diode Laser In Enhancing Enamel Anticariogenicity around orthodontic brackets
Aya Anwar Alsherif, Mohamed Ali Farag, Mai Badreldin Helal
BDJ Open.2023;[Epub] CrossRef - Amelioration Strategies for Silver Diamine Fluoride: Moving from Black to White
Amjad Almuqrin, Inder Preet Kaur, Laurence J. Walsh, Chaminda Jayampath Seneviratne, Sobia Zafar
Antibiotics.2023; 12(2): 298. CrossRef - The Effect of Loading Time on Color Stability of Various Restorative Materials Bonded to Silver Diamine Fluoride-Treated Demineralized Dentin
Mohammed M Aldosari, Fares S Al-Sehaibany
Clinical, Cosmetic and Investigational Dentistry.2022; Volume 14: 123. CrossRef - In vitro study of the effect of nanosilver fluoride on shear bond strength of orthodontic brackets and demineralization of enamel
Mariam H. El-Toukhy, Eman M. El-Shourbagy, Neveen M. Fakhry
Tanta Dental Journal.2022; 19(4): 281. CrossRef
- Impact of Incorporating Nanoparticles to Adhesive Resin on the Demineralization of Enamel: A Systematic Review
- 1,925 View
- 26 Download
- 10 Web of Science
- 9 Crossref

- Effect of casein phosphopeptide-amorphous calcium phosphate on fluoride release and micro-shear bond strength of resin-modified glass ionomer cement in caries-affected dentin
- Jamila Nuwayji Agob, Neven Saad Aref, Essam El Saeid Al-Wakeel
- Restor Dent Endod 2018;43(4):e45. Published online October 30, 2018
- DOI: https://doi.org/10.5395/rde.2018.43.e45
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
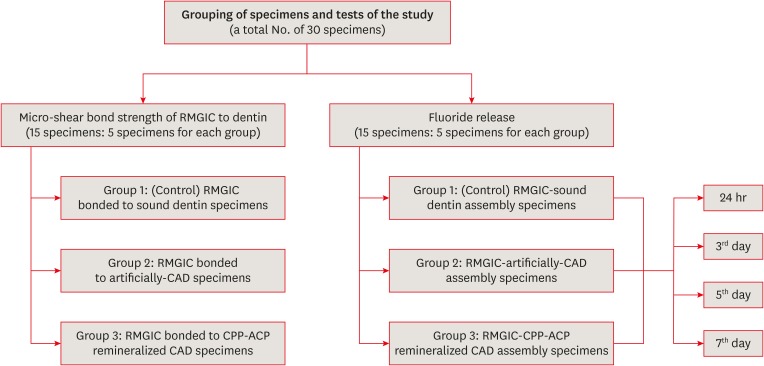
ePub Objectives This study was conducted to evaluate fluoride release and the micro-shear bond strength of resin-modified glass ionomer cement (RMGIC) in casein phosphopeptide-amorphous calcium phosphate (CPP-ACP)-remineralized caries-affected dentin (CAD).
Materials and Methods Exposed dentin surfaces of 30 human third molar teeth were divided into 2 equal groups for evaluating fluoride release and the micro-shear bond strength of RMGIC to CAD. Each group was subdivided into 3 equal subgroups: 1) control (sound dentin); 2) artificially demineralized dentin (CAD); 3) CPP-ACP remineralized dentin (remineralized CAD). To measure fluoride release, 15 disc-shaped specimens of RMGIC (4 mm in diameter and 2 mm in thickness) were bonded on one flat surface of the dentin discs of each group. Fluoride release was tested using ion chromatography at different intervals; 24 hours, 3, 5, 7 days. RMGIC micro-cylinders were built on the flat dentin surface of the 15 discs, which were prepared according to the assigned group. Micro-shear bond strength was measured after 24 hours water storage. Data were analyzed using 1- and 2-way analysis of variance and the
post hoc least significant difference test (α = 0.05).Results Fluoride detected in solutions (at all intervals) and the micro-shear bond strength of RMGIC bonded to CPP-ACP-remineralized dentin were significantly higher than those bonded to artificial CAD (
p < 0.05).Conclusions Demineralized CAD consumes more fluoride released from RMGIC into the solution for remineralization than CPP-ACP mineralized dentin does. CPP-ACP increases the micro-shear bond strength of RMGIC to CAD.
-
Citations
Citations to this article as recorded by- Synergistic effect of nanosilver fluoride with L-arginine on remineralization of early carious lesions
Ahmad S. Albahoth, Mi-Jeong Jeon, Jeong-Won Park
Scientific Reports.2025;[Epub] CrossRef - Evaluation of the bond strength of glass ionomer cement modified with fluoride-loaded chitosan nanoparticles to caries-affected dentin
Hanife Altınışık, Merve Nezir, Hülya Erten Can, Necibe Başaran Mutlu Ağardan, Aysel Berkkan
BMC Oral Health.2025;[Epub] CrossRef - Non-collagenous protein analog-induced biomimetic mineralization strategy to restore the dentin interface
Ruhua Chen, Yimeng Xie, Liang Ma, Bing Li, Wei Yao
Biomedical Physics & Engineering Express.2024; 10(6): 062004. CrossRef - A Critical Review on the Factors Affecting the Bond Strength of Direct Restorative Material Alternatives to Amalgam
Zeynep Batu Eken, Nicoleta Ilie
Materials.2024; 17(19): 4853. CrossRef - ÇOCUK DİŞ HEKİMLİĞİNDE GÜMÜŞ DİAMİN FLORÜR KULLANIMI
Zeynep UÇAR, Bahar Melis AKYILDIZ
Selcuk Dental Journal.2022; 9(2): 652. CrossRef - Microshear Bond Strength of Nanoparticle-Incorporated Conventional and Resin-Modified Glass Ionomer to Caries-Affected Dentin
Zahra Fattah, Zahra Jowkar, Safoora Rezaeian, Lucas da Fonseca Roberti Garcia
International Journal of Dentistry.2021; 2021: 1. CrossRef
- Synergistic effect of nanosilver fluoride with L-arginine on remineralization of early carious lesions
- 1,950 View
- 12 Download
- 6 Crossref

- The effect of different fluoride application methods on the remineralization of initial carious lesions
- Seon Mi Byeon, Min Ho Lee, Tae Sung Bae
- Restor Dent Endod 2016;41(2):121-129. Published online May 10, 2016
- DOI: https://doi.org/10.5395/rde.2016.41.2.121
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub Objectives The purpose of this study was to assess the effect of single and combined applications of fluoride on the amount of fluoride release, and the remineralization and physical properties of enamel.
Materials and Methods Each of four fluoride varnish and gel products (Fluor Protector, FP, Ivoclar Vivadent; Tooth Mousse Plus, TM, GC; 60 Second Gel, A, Germiphene; CavityShield, CS, 3M ESPE) and two fluoride solutions (2% sodium fluoride, N; 8% tin(ii) fluoride, S) were applied on bovine teeth using single and combined methods (10 per group), and then the amount of fluoride release was measured for 4 wk. The electron probe microanalysis and the Vickers microhardness measurements were conducted to assess the effect of fluoride application on the surface properties of bovine teeth.
Results The amount of fluoride release was higher in combined applications than in single application (
p < 0.05). Microhardness values were higher after combined applications of N with FP, TM, and CS than single application of them, and these values were also higher after combined applications of S than single application of A (p < 0.05). Ca and P values were higher in combined applications of N with TM and CS than single application of them (p < 0.05). They were also increased after combined applications of the S with A than after single application (p < 0.05).Conclusions Combined applications of fluoride could be used as a basis to design more effective methods of fluoride application to provide enhanced remineralization.
-
Citations
Citations to this article as recorded by- Effect of Different Topical Fluorides on the Microhardness of Bleached Enamel: In Vitro Study
Soumyashri Das, Mansi Jain, HP Suma Sogi, Sonali Sukesh K, Apurva Gambhir, FNU Gagandeep
International Journal of Clinical Pediatric Dentistry.2025; 18(11): 1365. CrossRef - Therapeutic effect of ozone gel on the initial carious lesions
Maha A. Alsharqawy, Wedad M Etman, Mirvat M Salama, Reda G. Saleh
Tanta Dental Journal.2023; 20(3): 203. CrossRef - Evaluation of Remineralization Potential of Natural Substances on Artificially Induced Carious Lesions in Primary Teeth: An In Vitro Study
Kavitha Ramar, Pooja V Ravi, Rajakumar Sekar
International Journal of Clinical Pediatric Dentistry.2023; 16(2): 244. CrossRef - Upaya Preventif Kesehatan Gigi dan Mulut dengan Aplikasi Fluor pada Gigi Siswa SMPN 77 Jakarta
Agus Ardinansyah, Mochammad Atmaji Windrianto, Nur Hidayati Nosi Prastiyani
Info Abdi Cendekia.2023; 6(2): 74. CrossRef - Evaluation of the antibacterial activity of Enamelast® and Fluor defender® fluoride varnishes against Streptococcus mutans biofilm: an in vitro study in primary teeth
M. A. Matar, S. S. Darwish, R. S. Salma, W. A. Lotfy
European Archives of Paediatric Dentistry.2023; 24(5): 549. CrossRef - In-vitro evaluation of the anti-cariogenic effect of a hybrid coating associated with encapsulated sodium fluoride and stannous chloride in nanoclays on enamel
Sávio José Cardoso BEZERRA, Ítallo Emídio Lira VIANA, Idalina Vieira AOKI, Simone DUARTE, Anderson Takeo HARA, Taís SCARAMUCCI
Journal of Applied Oral Science.2022;[Epub] CrossRef - Comparative Evaluation of Salivary Fluoride Concentration after Topical Application of Silver Diamine Fluoride and Sodium Fluoride: A Randomized Controlled Trial
Nidhi Agarwal, V Vishnu Priya, Zohra Jabin, Iffat Nasim
International Journal of Clinical Pediatric Dentistry.2022; 15(3): 371. CrossRef - Release and Recharge of Fluoride Ions from Acrylic Resin Modified with Bioactive Glass
Zbigniew Raszewski, Danuta Nowakowska, Wlodzimierz Wieckiewicz, Agnieszka Nowakowska-Toporowska
Polymers.2021; 13(7): 1054. CrossRef - Enamel remineralisation-inducing materials for caries prevention
Sri Kunarti, Widya Saraswati, Dur Muhammad Lashari, Nadhifa Salma, Tasya Nafatila
Dental Journal.2021; 54(3): 165. CrossRef - Fluoride Concentration in Saliva following Professional Topical Application of 2% Sodium Fluoride Solution
Manjit Talwar, Amrit Tewari, H. S. Chawla, Vinod Sachdev, Suresh Sharma
Contemporary Clinical Dentistry.2019; 10(3): 423. CrossRef - Clinical and laboratory evaluation of the Elgydium Protection caries toothpaste effectiveness in patients with high intensity of dental caries
O. A. Zorina, N. B. Petruhina, A. Z. M, O. A. Boriskina, A. A. Tupicin, V. A. Prohodnaja
Stomatologiya.2019; 98(3): 21. CrossRef - Bleaching of simulated stained-remineralized caries lesions in vitro
Sarah S. Al-Angari, Frank Lippert, Jeffrey A. Platt, George J. Eckert, Carlos González-Cabezas, Yiming Li, Anderson T. Hara
Clinical Oral Investigations.2019; 23(4): 1785. CrossRef - Short-Time Antibacterial Effects of Dimethylaminododecyl Methacrylate on Oral Multispecies Biofilm In Vitro
Yujie Zhou, Suping Wang, Xuedong Zhou, Yiran Zou, Mingyun Li, Xian Peng, Biao Ren, Hockin H. K. Xu, Michael D. Weir, Lei Cheng, Yu Chen, Qi Han
BioMed Research International.2019; 2019: 1. CrossRef - Comparison of the Application of Different Fluoride Supplements on Enamel Demineralization Adjacent to Orthodontic Brackets: An In Vitro Study
Arman Mohammadi Shayan, Monireh Rassouli, Soodabeh Kimyai, Hadi Valizadeh, Mohammad Hossein Ahangar Atashi, Sahand Rikhtegaran
Iranian Journal of Orthodontics.2019;[Epub] CrossRef - Effects of nicomethanol hydrofluoride on dental enamel and synthetic apatites: a role for anti-caries protection
N. Sharkov
European Archives of Paediatric Dentistry.2017; 18(6): 411. CrossRef - Intérêt prophylactique et thérapeutique des chewing-gums sans sucre en orthodontie. Une étude menée auprès de professionnels de santé et de patients
Pauline Ferney, François Clauss, Damien Offner, Delphine Wagner
L'Orthodontie Française.2017; 88(3): 275. CrossRef - Silver Diamine Fluoride Has Efficacy in Controlling Caries Progression in Primary Teeth: A Systematic Review and Meta-Analysis
Ana Cláudia Chibinski, Letícia Maíra Wambier, Juliana Feltrin, Alessandro Dourado Loguercio, Denise Stadler Wambier, Alessandra Reis
Caries Research.2017; 51(5): 527. CrossRef - Dental Caries Management of a Patient with a High Caries Risk Based on the Caries Risk Assessment: a Case Peport
Dong-Hyun Lee, Sung-Ok Hong, Seok-Ryun Lee
Korean Journal of Dental Materials.2016; 43(3): 231. CrossRef
- Effect of Different Topical Fluorides on the Microhardness of Bleached Enamel: In Vitro Study
- 2,250 View
- 13 Download
- 18 Crossref

- Do conventional glass ionomer cements release more fluoride than resin-modified glass ionomer cements?
- Maria Fernanda Costa Cabral, Roberto Luiz de Menezes Martinho, Manoel Valcácio Guedes-Neto, Maria Augusta Bessa Rebelo, Danielson Guedes Pontes, Flávia Cohen-Carneiro
- Restor Dent Endod 2015;40(3):209-215. Published online May 26, 2015
- DOI: https://doi.org/10.5395/rde.2015.40.3.209
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub Objectives The aim of this study was to evaluate the fluoride release of conventional glass ionomer cements (GICs) and resin-modified GICs.
Materials and Methods The cements were grouped as follows: G1 (Vidrion R, SS White), G2 (Vitro Fil, DFL), G3 (Vitro Molar, DFL), G4 (Bioglass R, Biodinâmica), and G5 (Ketac Fil, 3M ESPE), as conventional GICs, and G6 (Vitremer, 3M ESPE), G7 (Vitro Fil LC, DFL), and G8 (Resiglass, Biodinâmica) as resin-modified GICs. Six specimens (8.60 mm in diameter; 1.65 mm in thickness) of each material were prepared using a stainless steel mold. The specimens were immersed in a demineralizing solution (pH 4.3) for 6 hr and a remineralizing solution (pH 7.0) for 18 hr a day. The fluoride ions were measured for 15 days. Analysis of variance (ANOVA) and Tukey's test with 5% significance were applied.
Results The highest amounts of fluoride release were found during the first 24 hr for all cements, decreasing abruptly on day 2, and reaching gradually decreasing levels on day 7. Based on these results, the decreasing scale of fluoride release was as follows: G2 > G3 > G8 = G4 = G7 > G6 = G1 > G5 (
p < 0.05).Conclusions There were wide variations among the materials in terms of the cumulative amount of fluoride ion released, and the amount of fluoride release could not be attributed to the category of cement, that is, conventional GICs or resin-modified GICs.
-
Citations
Citations to this article as recorded by-
Impact of biofilm model of
Streptococcus mutans
on the pH, ions release, and sorption/solubility of glass ionomer cements enriched with 45S5 bioglass
Fábia Regina Vieira de Oliveira Roma, Mayron Guedes Silva, Tarcisio Jorge Leitão de Oliveira, José Bauer, Leily Macedo Firoozmand
Biofouling.2026; 42(1): 42. CrossRef - Fluoride Uptake and Surface Characteristics of Ion-Releasing Restoratives After Brushing with Fluoride Toothpastes
Llubitza Slaviza Banic Vidal, Ivan Šalinović, Nikolina Nika Veček, Anja Ivica, Ivana Miletić, Silvana Jukić Krmek
Materials.2025; 18(9): 2152. CrossRef - Strategic approaches for enhancing the bioactivity of glass ionomer cement: A mechanistic and clinical perspective in terms of structural and surface modifications
Ali Saatchifard, Nader Nezafati, Saeed Hesaraki
Journal of Dentistry.2025; 163: 106126. CrossRef - Antibacterial effects of bioactive restorative dental materials on Streptococcus mutans: An in vitro study using the direct contact test
Sirirat Boondireke, Onsasi Kitrueangphatchara, Charnsak Sukajintanakarn, Sirichan Chiaraputt
The Saudi Dental Journal.2025;[Epub] CrossRef - Ion release of the glass ionomer restoration with silver diamine fluoride dentin pretreatment
Kelsey Xingyun Ge, Ryan Quock, Feng Yan, Walter Yu-Hang Lam, Chun-Hung Chu, Ollie Yiru Yu
Journal of Dentistry.2024; 148: 105247. CrossRef - Dual function of anti-biofilm and modulating biofilm equilibrium of orthodontic cement containing quaternary ammonium salt
Wenqi YU, Chaochao REN, Ning ZHANG, Li CAO, Michael D. WEIR, Kai YANG, Hockin H. K. XU, Yuxing BAI
Dental Materials Journal.2023; 42(2): 149. CrossRef - Fluoride exchange by glass-ionomer dental cements and its clinical effects: a review
John W. Nicholson, Sharanbir K. Sidhu, Beata Czarnecka
Biomaterial Investigations in Dentistry.2023;[Epub] CrossRef - Effects of Protective Surface Coating on Fluoride Release and Recharge of Recent Uncoated High-Viscosity Glass Ionomer Cement
Nantawan Krajangta, Chayanee Dulsamphan, Tongjai Chotitanmapong
Dentistry Journal.2022; 10(12): 233. CrossRef - Reinforcing an immature tooth model using three different restorative materials
Pooja Misar, Hemalatha Hiremath, Chhaya Harinkhere, ShailendraS Sonawane, Vinay Sharma, KuldeepSingh Rana
Dental Research Journal.2022; 19(1): 28. CrossRef - Fluoride release from two types of fluoride-containing orthodontic adhesives: Conventional versus resin-modified glass ionomer cements—An in vitro study
Yasemin Dziuk, Sachin Chhatwani, Stephan C. Möhlhenrich, Sabrina Tulka, Ella A. Naumova, Gholamreza Danesh, Richard Johannes Wierichs
PLOS ONE.2021; 16(2): e0247716. CrossRef - Phosphate Ion Release and Alkalizing Potential of Three Bioactive Dental Materials in Comparison with Composite Resin
Shahin Kasraei, Sahebeh Haghi, Sara Valizadeh, Narges Panahandeh, Sogol Nejadkarimi, Shinn Jyh Ding
International Journal of Dentistry.2021; 2021: 1. CrossRef - The effect of the polishing procedure and surface sealant application on the fluoride release of different restorative materials
Muhittin Ugurlu, Hikmet Orhan
Journal of Conservative Dentistry.2021; 24(2): 135. CrossRef - Mechanical and antimicrobial property of different surface treated glass ionomer cements under desiccated condition
Hemalatha Hiremath, Chhaya Harinkhere, Pooja Misar, Kshitij Sabley, Trupti Bajpai
Dental Research Journal.2021; 18(1): 64. CrossRef - Dental Restorative Materials for Elderly Populations
Yuyao Huang, Bingqing Song, Xuedong Zhou, Hui Chen, Haohao Wang, Lei Cheng
Polymers.2021; 13(5): 828. CrossRef - Monomer conversion, dimensional stability, biaxial flexural strength, and fluoride release of resin-based restorative material containing alkaline fillers
Piyaphong PANPISUT, Arnit TONELUCK
Dental Materials Journal.2020; 39(4): 608. CrossRef - Factors influencing fluoride release in atraumatic restorative treatment (ART) materials: A review
P.Divya Kumari, Shahnawaz Khijmatgar, Avidyuti Chowdhury, Edward Lynch, Chitta R. Chowdhury
Journal of Oral Biology and Craniofacial Research.2019; 9(4): 315. CrossRef - Incorporation of chlorhexidine and nano-sized sodium trimetaphosphate into a glass-ionomer cement: Effect on mechanical and microbiological properties and inhibition of enamel demineralization
Márjully Eduardo Rodrigues da Silva, Marcelle Danelon, José Antonio Santos Souza, Dinah Fressato Silva, Jesse Augusto Pereira, Denise Pedrini, Emerson Rodrigues de Camargo, Alberto Carlos Botazzo Delbem, Cristiane Duque
Journal of Dentistry.2019; 84: 81. CrossRef
-
Impact of biofilm model of
Streptococcus mutans
on the pH, ions release, and sorption/solubility of glass ionomer cements enriched with 45S5 bioglass
- 3,042 View
- 9 Download
- 17 Crossref

- Effect of fluoride concentration in pH 4.3 and pH 7.0 supersaturated solutions on the crystal growth of hydroxyapatite
- Haneol Shin, Sung-Ho Park, Jeong-Won Park, Chan-Young Lee
- Restor Dent Endod 2012;37(1):16-23. Published online March 2, 2012
- DOI: https://doi.org/10.5395/rde.2012.37.1.16
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub Objectives Present study was undertaken to investigate the crystal growth onto synthetic hydroxyapatite (HA) seeds in pH 4.3 and pH 7.0 supersaturated solutions with different fluoride concentrations.
Materials and Methods 8 groups of pH 4.3 and 7.0 calcium phosphate supersaturated solutions were prepared with different fluoride concentrations (0, 1, 2 and 4 ppm). Calcium phosphate precipitates yield crystal growth onto the HA seed surface while solutions flow. For evaluation of crystallizing process, the changes of Ca2+, PO43-, F- concentrations of the inlet and outlet solutions were determined. The recovered solid samples were weighed to assess the amount of minerals precipitated, and finally determined their composition to deduce characteristics of crystals.
Results During the seeded crystal growth, there were significantly more consumption of Ca2+, PO43-, F- in pH 4.3 solutions than pH 7.0 (
p < 0.05). As fluoride concentration increased in pH 4.3 solution, Ca2+, PO43-, F- consumption in experimental solutions, weight increment of HA seed, and fluoride ratio in crystallized samples were increased. There were significant differences among the groups (p < 0.05). But in pH 7.0 solution, these phenomena were not significant. In pH 7.0 solutions, analyses of crystallized samples showed higher Ca/P ratio in higher fluoride concentration. There were significant differences among the groups (p < 0.05). But in pH 4.3 solution, there were not significant differences in Ca/P ratio.Conclusions Crystal growth in pH 4.3 solutions was superior to that in pH 7.0 solutions. In pH 4.3 solutions, crystal growth increased with showed in higher fluoride concentration up to 4 ppm.
-
Citations
Citations to this article as recorded by- Qualitative analysis on crystal growth of synthetic hydroxyapatite influenced by fluoride concentration
Sumi Kang, Jeong Taeg Seo, Sung-Ho Park, Il Young Jung, Chan Young Lee, Jeong-Won Park
Archives of Oral Biology.2019; 104: 52. CrossRef
- Qualitative analysis on crystal growth of synthetic hydroxyapatite influenced by fluoride concentration
- 1,228 View
- 2 Download
- 1 Crossref

- Elemental analysis of the fluoride varnish effects on root caries initiation
- Se-Eun Park, Keewook Yi, Hae-Young Kim, Ho-Hyun Son, Juhea Chang
- J Korean Acad Conserv Dent 2011;36(4):290-299. Published online July 31, 2011
- DOI: https://doi.org/10.5395/JKACD.2011.36.4.290
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub Objectives The usage of fluoride varnish for a moderate to low caries-risk group has not been well validated. This study aimed to evaluate the preventive and therapeutic efficacies of fluoride varnish on the initiated root caries.
Materials and Methods Ten premolars were sectioned into quarters, further divided into two windows, one of which was painted with Fluor Protector (1,000 ppm fluoride, Ivoclar Vivadent). An initial lesion with a well-preserved surface layer was produced by pH cycling. Scanned line analysis using energy dispersive spectrometry determined the weight percentages of Ca and P in the demineralized layer. Scanning Electron microscopy and confocal laser scanning microscopy (CLSM) evaluated the varnish-applied root surfaces.
Results The mean lesion depth (SD) was 12.3 (2.6) µm (single cycling) and 19.6 (3.8) µm (double cycling). Double cycling extended the lesion depth, but induced no more mineral loss than single cycling (
p < 0.05). The mean weight percentages of Ca and P between groups with and without varnish were not significantly different (p < 0.05). A CLSM showed varnish remained within 15 µm of the surface layer.Conclusions When a mild acid challenge initiated root tissue demineralization, the application of low-concentration fluoride varnish did not influence the lesion depth or the mineral composition of the subsurface lesion.
-
Citations
Citations to this article as recorded by- The combined occluding effect of sodium fluoride varnish and Nd:YAG laser irradiation on dentinal tubules—A CLSM and SEM study
Samet Tosun, Emre Culha, Ugur Aydin, Abdul Semih Ozsevik
Scanning.2016; 38(6): 619. CrossRef - How to designin situstudies: an evaluation of experimental protocols
Young-Hye Sung, Hae-Young Kim, Ho-Hyun Son, Juhea Chang
Restorative Dentistry & Endodontics.2014; 39(3): 164. CrossRef - Evaluation of release of fluoride from dental varnishes marketed in Korea
Han-Na Kim, Myung-Su Jeong, Se-Yeon Kim, Jin-Bom Kim, Seung-Hwa Jeong
Journal of Korean Academy of Oral Health.2014; 38(3): 131. CrossRef
- The combined occluding effect of sodium fluoride varnish and Nd:YAG laser irradiation on dentinal tubules—A CLSM and SEM study
- 1,674 View
- 5 Download
- 3 Crossref

- The effects of the fluoride concentration of acidulated buffer solutions on dentine remineralization
- Won-Sub Han, Chan-Young Lee
- J Korean Acad Conserv Dent 2009;34(6):526-536. Published online November 30, 2009
- DOI: https://doi.org/10.5395/JKACD.2009.34.6.526
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub The aim of this vitro-study is to evaluate the effects of fluoride on remineralization of artificial dentine caries. 10 sound permanent premolars, which were extracted for orthodontic reason within 1 week, were used for this study. Artificial dentine caries was created by using a partially saturated buffer solution for 2 days with grounded thin specimens and fractured whole-body specimens. Remineralization solutions with three different fluoride concentration (1 ppm, 2 ppm and 4 ppm) were used on demineralized-specimens for 7 days. Polarizing microscope and scanning electron microscope were used for the evaluation of the mineral distribution profile and morphology of crystallites of hydroxyapatite.
The results were as follows :
When treated with the fluoride solutions, the demineralized dentine specimens showed remineralization of the upper part and demineralization of the lower part of the lesion body simultaneously.
As the concentration of fluoride increased, the mineral precipitation in the caries dentine increased. The mineral precipitation mainly occurred in the surface layer in 1 and 2 ppm-specimens and in the whole lesion body in 4 ppm-specimens.
When treated with the fluoride solution, the hydroxyapatite crystals grew. This crystal growth was even observed in the lower part of the lesion body which had shown the loss of mineral.
-
Citations
Citations to this article as recorded by- Infant Oral Health Care Concerning Education of Mothers – Part 2
Lehya Mounica Kadali, Viddyasagar Mopagar, Shilpa Shetty, Shridhar Shetty, Venkatesh Kodgi, Shantanu Chaudhari
Journal of Evolution of Medical and Dental Sciences.2021; 10(31): 2538. CrossRef
- Infant Oral Health Care Concerning Education of Mothers – Part 2
- 1,140 View
- 1 Download
- 1 Crossref

- Influence of microhardness and fluoride content of tooth structure by fluoride-containing restorative materials
- Su-Jong Lee, Young-Gon Cho, Jong-Uk Kim, Byung-Cheul Park
- J Korean Acad Conserv Dent 2004;29(1):36-43. Published online January 31, 2004
- DOI: https://doi.org/10.5395/JKACD.2004.29.1.036
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub The purpose of this study was to compare the microhardness and the fluoride content of enamel and dentin around fluoride- or non fluoride-containing restorations. Forty extracted human teeth were used and prepared cervical cavities on proximal surface. Experimental teeth were divided into five groups. Group 1 : Prime & Bond NT and Z100, Group 2 : Prime & Bond NT and F2000, Group 3 : Scotchbond Multi-Purpose and Z100, Group 4 : Scothcbond Multi-purpose and F2000, Group 5 : Fuji II LC. The cavities were filled with dentin adhesives and restorative materials. After each tooth was bisected, one half was tested microhardness and the other half was analyzed the fluoride at the enamel and dentin by an EPMA-WDX device. The results were as follows:
1. There was no statistical difference among the microhardness of enamel surface in all group.
2. The microhardness at dentin of 100 µm point in Group 2 and 20 µm point in Group 4 was lower than that of normal dentin (p>0.05).
3. There was no statistical difference among the fluoride content of enamel surface in all group.
4. The fluoride content at the dentin of 30 µm point in Group 2 and 5 were higher than those at 100 µm and 200 µm point in Group 2 and normal dentin (p<0.05).
5. At the dentin of 30 µm point, Group 2 showed higher fluoride content than Group 1 and 3, and Group 5 showed higher fluoride content than other groups.
- 865 View
- 1 Download

- ANTICARIOGENCI EFFECT OF COMPOMER AND RMGIC
- Sung-Ho Park
- J Korean Acad Conserv Dent 2002;27(1):12-15. Published online January 14, 2002
- DOI: https://doi.org/10.5395/JKACD.2002.27.1.012
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub ABSTRACT The first purpose of present study was to compare the anticariogenic effect of compomer, resin modified glass ionomer cement and composite (RMGIC).
The second purpose was to evaluate the recently introduced methods, which use confocal scanning microscope, in detecting initial caries around restoration.
2×4×1.5mm cavities were prepared from the recently extracted 50 human teeth on the buccal or lingual surface. The prepared teeth were randomly devided into 5 groups and restored with each filling material. Group 1: Dyract AP, Group 2: compoglass F, Group 3: F2000, Group 4: Z100, Group 5:Fuji Ⅱ LC. The teeth were stored for 30 days in the distilled water, then stored in the buffer solution for artificial caries development; pH 4.3, lactic acid 100 mM, calcium 16 mM, phosphate 8mM, sodium azide 3mM. Then, the samples were sectioned longitudinally and examined with confical scanning microscope. The results showed that the use of compomer and resin modified glass ionomer cement showed caries inhibition zone whereas the composite did not. There was no difference in the width of caries inhibition zone between compomers and RMGIC. The confocal scanning microscope was useful in detecting initial caries around restoration.
- 1,117 View
- 2 Download


 KACD
KACD

 First
First Prev
Prev


