Articles
- Page Path
- HOME > Restor Dent Endod > Volume 40(3); 2015 > Article
- Research Article Do conventional glass ionomer cements release more fluoride than resin-modified glass ionomer cements?
- Maria Fernanda Costa Cabral1, Roberto Luiz de Menezes Martinho2, Manoel Valcácio Guedes-Neto1, Maria Augusta Bessa Rebelo2, Danielson Guedes Pontes2,3, Flávia Cohen-Carneiro2
-
2015;40(3):-215.
DOI: https://doi.org/10.5395/rde.2015.40.3.209
Published online: May 26, 2015
1School of Dentistry, Federal University of Amazonas, Manaus, Brazil.
2Postgraduate Program in Dentistry, School of Dentistry, Federal University of Amazonas, Manaus, Brazil.
3School of Health Sciences, State University of Amazonas, Manaus, Brazil.
- Correspondence to Flávia Cohen-Carneiro, DDS, PhD. Adjunct Professor, Postgraduate Program in Dentistry, School of Dentistry, Federal University of Amazonas, Rua Rio Mar, n. 1203/901, Nossa Senhora das Graças, Manaus, AM, Brazil 69053-120. TEL, +55-92-98855-1101; FAX, +55-92-3305-4905; flaviacohencarneiro@gmail.com
©Copyrights 2015. The Korean Academy of Conservative Dentistry.
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 3,039 Views
- 9 Download
- 17 Crossref
Abstract
-
Objectives The aim of this study was to evaluate the fluoride release of conventional glass ionomer cements (GICs) and resin-modified GICs.
-
Materials and Methods The cements were grouped as follows: G1 (Vidrion R, SS White), G2 (Vitro Fil, DFL), G3 (Vitro Molar, DFL), G4 (Bioglass R, Biodinâmica), and G5 (Ketac Fil, 3M ESPE), as conventional GICs, and G6 (Vitremer, 3M ESPE), G7 (Vitro Fil LC, DFL), and G8 (Resiglass, Biodinâmica) as resin-modified GICs. Six specimens (8.60 mm in diameter; 1.65 mm in thickness) of each material were prepared using a stainless steel mold. The specimens were immersed in a demineralizing solution (pH 4.3) for 6 hr and a remineralizing solution (pH 7.0) for 18 hr a day. The fluoride ions were measured for 15 days. Analysis of variance (ANOVA) and Tukey's test with 5% significance were applied.
-
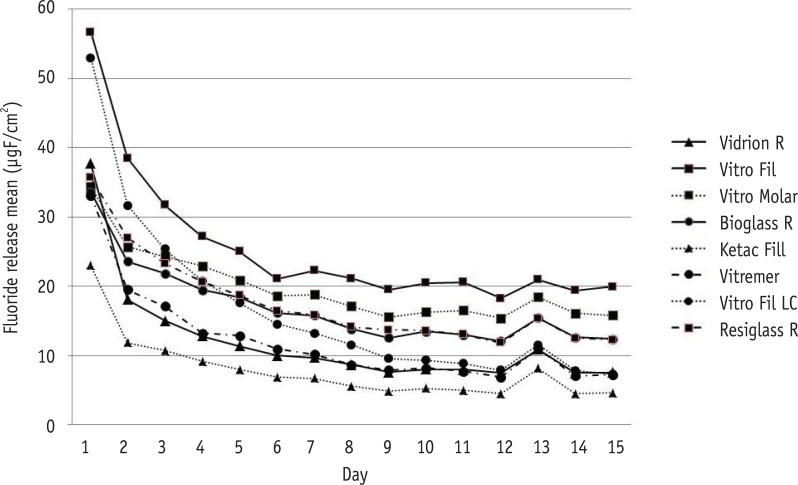
Results The highest amounts of fluoride release were found during the first 24 hr for all cements, decreasing abruptly on day 2, and reaching gradually decreasing levels on day 7. Based on these results, the decreasing scale of fluoride release was as follows: G2 > G3 > G8 = G4 = G7 > G6 = G1 > G5 (p < 0.05).
-
Conclusions There were wide variations among the materials in terms of the cumulative amount of fluoride ion released, and the amount of fluoride release could not be attributed to the category of cement, that is, conventional GICs or resin-modified GICs.
Introduction
Material and Methods
Results
Discussion
Conclusions
Acknowledgment
- 1. Mungara J, Philip J, Joseph E, Rajendran S, Elangovan A, Selvaraju G. Comparative evaluation of fluoride release and recharge of pre-reacted glass ionomer composite and nano-ionomeric glass ionomer with daily fluoride exposure: an in vitro study. J Indian Soc Pedod Prev Dent 2013;31:234-239.ArticlePubMed
- 2. Upadhyay S, Rao A, Shenoy R. Comparison of the amount of fluoride release from nanofilled resin modified glass ionomer, conventional and resin modified glass ionomer cements. J Dent (Tehran) 2013;10:134-140.PubMedPMC
- 3. Markovic DL, Petrovic BB, Peric TO. Fluoride content and recharge ability of five glass ionomer dental materials. BMC Oral Health 2008;8:21.ArticlePubMedPMCPDF
- 4. Wilson AD, Kent BE. A new translucent cement for dentistry. The glass ionomer cement. Br Dent J 1972;132:133-135.ArticlePubMedPDF
- 5. Neelakantan P, John S, Anand S, Sureshbabu N, Subbarao C. Fluoride release from a new glass-ionomer cement. Oper Dent 2011;36:80-85.ArticlePubMedPDF
- 6. Shiozawa M, Takahashi H, Iwasaki N. Fluoride release and mechanical properties after 1-year water storage of recent restorative glass ionomer cements. Clin Oral Investig 2014;18:1053-1060.ArticlePubMedPDF
- 7. McLean JW, Wilson AD. The clinical development of glass-ionomer cements III. The erosion lesion. Aust Dent J 1977;22:190-195.PubMed
- 8. Levallois B, Fovet Y, Lapeyre L, Gal JY. In vitro fluoride release from restorative materials in water versus artificial saliva medium (SAGF). Dent Mater 1998;14:441-447.ArticlePubMed
- 9. Tiwari S, Nandlal B. Comparative evaluation of fluoride release from hydroxyapatite incorporated and conventional glass ionomer cement: an in vitro study. J Indian Soc Pedod Prev Dent 2012;30:284-287.ArticlePubMed
- 10. Qvist V, Manscher E, Teglers PT. Resin-modified and conventional glass ionomer restorations in primary teeth: 8-year results. J Dent 2004;32:285-294.ArticlePubMed
- 11. Qvist V, Poulsen A, Teglers PT, Mjör IA. Fluorides leaching from restorative materials and the effect on adjacent teeth. Int Dent J 2010;60:156-160.PubMed
- 12. Hattab FN, Amin WM. Fluoride release from glass ionomer restorative materials and the effects of surface coating. Biomaterials 2001;22:1449-1458.ArticlePubMed
- 13. Diaz-Arnold AM, Holmes DC, Wistrom DW, Swift EJ Jr. Short-term fluoride release/uptake of glass ionomer restoratives. Dent Mater 1995;11:96-101.ArticlePubMed
- 14. Dionysopoulos D, Koliniotou-Koumpia E, Helvatzoglou-Antoniades M, Kotsanos N. Fluoride release and recharge abilities of contemporary fluoride-containing restorative materials and dental adhesives. Dent Mater J 2013;32:296-304.ArticlePubMed
- 15. Wiegand A, Buchalla W, Attin T. Review on fluoride-releasing restorative materials--Fluoride release and uptake characteristics, antibacterial activity and influence on caries formation. Dent Mater 2007;23:343-362.ArticlePubMed
- 16. Billington RW, Williams JA, Dorban A, Pearson GJ. Glass ionomer cement: evidence pointing to fluorine release in the form of monofluorophosphate in addition to fluoride ion. Biomaterials 2004;25:3399-3402.ArticlePubMed
- 17. Can-Karabulut DC, Batmaz I, Solak H, Taştekin M. Linear regression modeling to compare fluoride release profiles of various restorative materials. Dent Mater 2007;23:1057-1065.ArticlePubMed
- 18. Bahadure RN, Pandey RK, Kumar R, Gopal K, Singh RK. An estimation of fluoride release from various dental restorative materials at different pH: in vitro study. J Indian Soc Pedod Prev Dent 2012;30:122-126.ArticlePubMed
- 19. Moreau JL, Xu HH. Fluoride releasing restorative materials: effects of pH on mechanical properties and ion release. Dent Mater 2010;26:e227-e235.ArticlePubMedPMC
- 20. Jeong YN, Yang SY, Park BJ, Park YJ, Hwang YC, Hwang IN, Oh WM. Physical and chemical properties of experimental mixture of mineral trioxide aggregate and glass ionomer cement. J Korean Acad Conserv Dent 2010;35:344-352.Article
- 21. Seppä L, Forss H, Ogaard B. The effect of fluoride application on fluoride release and the antibacterial action of glass ionomers. J Dent Res 1993;72:1310-1314.ArticlePubMedPDF
- 22. Grobler SR, Rossouw RJ, Van Wyk Kotze TJ. A comparison of fluoride release from various dental materials. J Dent 1998;26:259-265.ArticlePubMed
- 23. Carvalho AS, Cury JA. Fluoride release from some dental materials in different solutions. Oper Dent 1999;24:14-19.PubMed
- 24. Williams JA, Billington RW, Pearson GJ. The influence of sample dimensions on fluoride ion release from a glass ionomer restorative cement. Biomaterials 1999;20:1327-1337.ArticlePubMed
- 25. Dionysopoulos P, Kotsanos N, Pataridou A. Fluoride release and uptake by four new fluoride releasing materials. J Oral Rehabil 2003;30:866-872.PubMed
- 26. Xu X, Burgess JO. Compressive strength, fluoride release and recharge of fluoride-releasing materials. Biomaterials 2003;24:2451-2461.ArticlePubMed
- 27. Hayacibara MF, Ambrosano GM, Cury JA. Simultaneous release of fluoride and aluminum from dental materials in various immersion media. Oper Dent 2004;29:16-22.PubMed
- 28. Hsu HM, Huang GF, Chang HH, Wang YL, Guo MK. A continuous flow system for assessing fluoride release/uptake of fluoride-containing restorative materials. Dent Mater 2004;20:740-749.ArticlePubMed
- 29. Chan WD, Yang L, Wan W, Rizkalla AS. Fluoride release from dental cements and composites: a mechanistic study. Dent Mater 2006;22:366-373.ArticlePubMed
- 30. Gandolfi MG, Chersoni S, Acquaviva GL, Piana G, Prati C, Mongiorgi R. Fluoride release and absorption at different pH from glass-ionomer cements. Dent Mater 2006;22:441-449.ArticlePubMed
- 31. Selimović-Dragaš M, Hasić-Branković L, Korać F, Ðapo N, Huseinbegović A, Kobašlija S, Lekić M, Hatibović-Kofman Š. In vitro fluoride release from a different kind of conventional and resin modified glass-ionomer cements. Bosn J Basic Med Sci 2013;13:197-202.ArticlePubMedPMCPDF
- 32. Mousavinasab SM, Meyers I. Fluoride release by glass ionomer cements, compomer and giomer. Dent Res J (Isfahan) 2009;6:75-81.PubMedPMC
- 33. McKenzie MA, Linden RW, Nicholson JW. The physical properties of conventional and resin-modified glass-ionomer dental cements stored in saliva, proprietary acidic beverages, saline and water. Biomaterials 2003;24:4063-4069.ArticlePubMed
- 34. Rothwell M, Anstice HM, Pearson GJ. The uptake and release of fluoride by ion-leaching cements after exposure to toothpaste. J Dent 1998;26:591-597.ArticlePubMed
- 35. Gao W, Smales RJ. Fluoride release/uptake of conventional and resin-modified glass ionomers, and compomers. J Dent 2001;29:301-306.ArticlePubMed
- 36. Rodrigues JA, Marchi GM, Serra MC, Hara AT. Visual evaluation of in vitro cariostatic effect of restorative materials associated with dentifrices. Braz Dent J 2005;16:112-118.ArticlePubMed
- 37. Dijkman GE, de Vries J, Lodding A, Arends J. Long-term fluoride release of visible light-activated composites in vitro: a correlation with in situ demineralization data. Caries Res 1993;27:117-123.ArticlePubMed
REFERENCES
Daily mean fluoride release of conventional and resin-modified glass ionomer cements (GICs) in demineralization-remineralization solutions over 15 days. Vidrion R, Group 1; Vitro Fil, Group 2; Vitro Molar, Group 3; Bioglass R, Group 4; Ketac Fil, Group 5; Vitremer, Group 6; Vitro Fil LC, Group 7; Resiglass, Group 8.
Materials used in the study
Total amount of fluoride released by each tested group (µgF/cm2) during the 15 day study period
Daily mean of fluoride released (µgF/cm2) during the study period for each tested material and the corresponding significance levels
Tables & Figures
REFERENCES
Citations

-
Impact of biofilm model of
Streptococcus mutans
on the pH, ions release, and sorption/solubility of glass ionomer cements enriched with 45S5 bioglass
Fábia Regina Vieira de Oliveira Roma, Mayron Guedes Silva, Tarcisio Jorge Leitão de Oliveira, José Bauer, Leily Macedo Firoozmand
Biofouling.2026; 42(1): 42. CrossRef - Fluoride Uptake and Surface Characteristics of Ion-Releasing Restoratives After Brushing with Fluoride Toothpastes
Llubitza Slaviza Banic Vidal, Ivan Šalinović, Nikolina Nika Veček, Anja Ivica, Ivana Miletić, Silvana Jukić Krmek
Materials.2025; 18(9): 2152. CrossRef - Strategic approaches for enhancing the bioactivity of glass ionomer cement: A mechanistic and clinical perspective in terms of structural and surface modifications
Ali Saatchifard, Nader Nezafati, Saeed Hesaraki
Journal of Dentistry.2025; 163: 106126. CrossRef - Antibacterial effects of bioactive restorative dental materials on Streptococcus mutans: An in vitro study using the direct contact test
Sirirat Boondireke, Onsasi Kitrueangphatchara, Charnsak Sukajintanakarn, Sirichan Chiaraputt
The Saudi Dental Journal.2025;[Epub] CrossRef - Ion release of the glass ionomer restoration with silver diamine fluoride dentin pretreatment
Kelsey Xingyun Ge, Ryan Quock, Feng Yan, Walter Yu-Hang Lam, Chun-Hung Chu, Ollie Yiru Yu
Journal of Dentistry.2024; 148: 105247. CrossRef - Dual function of anti-biofilm and modulating biofilm equilibrium of orthodontic cement containing quaternary ammonium salt
Wenqi YU, Chaochao REN, Ning ZHANG, Li CAO, Michael D. WEIR, Kai YANG, Hockin H. K. XU, Yuxing BAI
Dental Materials Journal.2023; 42(2): 149. CrossRef - Fluoride exchange by glass-ionomer dental cements and its clinical effects: a review
John W. Nicholson, Sharanbir K. Sidhu, Beata Czarnecka
Biomaterial Investigations in Dentistry.2023;[Epub] CrossRef - Effects of Protective Surface Coating on Fluoride Release and Recharge of Recent Uncoated High-Viscosity Glass Ionomer Cement
Nantawan Krajangta, Chayanee Dulsamphan, Tongjai Chotitanmapong
Dentistry Journal.2022; 10(12): 233. CrossRef - Reinforcing an immature tooth model using three different restorative materials
Pooja Misar, Hemalatha Hiremath, Chhaya Harinkhere, ShailendraS Sonawane, Vinay Sharma, KuldeepSingh Rana
Dental Research Journal.2022; 19(1): 28. CrossRef - Fluoride release from two types of fluoride-containing orthodontic adhesives: Conventional versus resin-modified glass ionomer cements—An in vitro study
Yasemin Dziuk, Sachin Chhatwani, Stephan C. Möhlhenrich, Sabrina Tulka, Ella A. Naumova, Gholamreza Danesh, Richard Johannes Wierichs
PLOS ONE.2021; 16(2): e0247716. CrossRef - Phosphate Ion Release and Alkalizing Potential of Three Bioactive Dental Materials in Comparison with Composite Resin
Shahin Kasraei, Sahebeh Haghi, Sara Valizadeh, Narges Panahandeh, Sogol Nejadkarimi, Shinn Jyh Ding
International Journal of Dentistry.2021; 2021: 1. CrossRef - The effect of the polishing procedure and surface sealant application on the fluoride release of different restorative materials
Muhittin Ugurlu, Hikmet Orhan
Journal of Conservative Dentistry.2021; 24(2): 135. CrossRef - Mechanical and antimicrobial property of different surface treated glass ionomer cements under desiccated condition
Hemalatha Hiremath, Chhaya Harinkhere, Pooja Misar, Kshitij Sabley, Trupti Bajpai
Dental Research Journal.2021; 18(1): 64. CrossRef - Dental Restorative Materials for Elderly Populations
Yuyao Huang, Bingqing Song, Xuedong Zhou, Hui Chen, Haohao Wang, Lei Cheng
Polymers.2021; 13(5): 828. CrossRef - Monomer conversion, dimensional stability, biaxial flexural strength, and fluoride release of resin-based restorative material containing alkaline fillers
Piyaphong PANPISUT, Arnit TONELUCK
Dental Materials Journal.2020; 39(4): 608. CrossRef - Factors influencing fluoride release in atraumatic restorative treatment (ART) materials: A review
P.Divya Kumari, Shahnawaz Khijmatgar, Avidyuti Chowdhury, Edward Lynch, Chitta R. Chowdhury
Journal of Oral Biology and Craniofacial Research.2019; 9(4): 315. CrossRef - Incorporation of chlorhexidine and nano-sized sodium trimetaphosphate into a glass-ionomer cement: Effect on mechanical and microbiological properties and inhibition of enamel demineralization
Márjully Eduardo Rodrigues da Silva, Marcelle Danelon, José Antonio Santos Souza, Dinah Fressato Silva, Jesse Augusto Pereira, Denise Pedrini, Emerson Rodrigues de Camargo, Alberto Carlos Botazzo Delbem, Cristiane Duque
Journal of Dentistry.2019; 84: 81. CrossRef
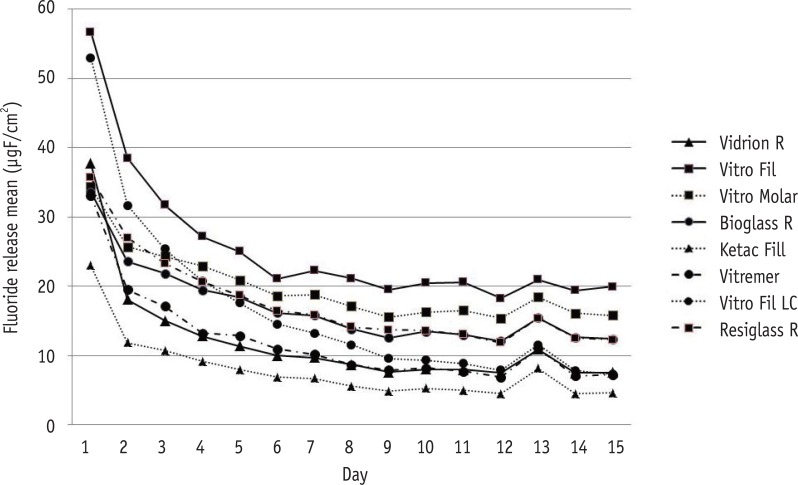
Figure 1
Materials used in the study
| Group | Material | Type | Composition | Manufacturer |
|---|---|---|---|---|
| G1 | Vidrion R | GIC | Powder: sodium, calcium, aluminum fluorosilicate, barium sulfate, polyacrylic acid, and pigments | S.S. WHITE Artigos Dentários Ltda, Rio de Janeiro, Brazil |
| Liquid: tartaric acid, distilled water | ||||
| G2 | Vitro Fil | GIC | Powder: strontium, aluminum silicate, dehydrated polyacrylic acid, and iron oxide | DFL Indústria e Comércio S.A., Rio de Janeiro, Brazil |
| Liquid: polyacrylic acid, tartaric acid, and distilled water | ||||
| G3 | Vitro Molar | GIC | Powder: barium, aluminum silicate, dehydrated polyacrylic acid, and iron oxide | DFL Indústria e Comércio S.A., Rio de Janeiro, Brazil |
| Liquid: polyacrylic acid, tartaric acid, and distilled water | ||||
| G4 | Bioglass R | GIC | Powder: calcium, barium, aluminum fluorosilicate, polyacylic acid, and inorganic fillers | BIODINÂMICA Química e Farmacê utica Ltda, Paraná, Brazil |
| Liquid: polyacrylic acid, tartaric acid, and deionized water | ||||
| G5 | Ketac Fil | GIC | Powder: lanthanum, aluminum, strontium fluorosilicate glass, and pigments | 3M ESPE Dental Products, Minnesota, USA |
| Liquid: tartaric acid and water | ||||
| G6 | Vitremer | RMGIC | Powder/liquid: methacrylate polyacids, water, aluminum fluorosilicate glass, methacrylate monomers, and initiators | 3M ESPE Dental Products, Minnesota, USA |
| G7 | Vitro Fil LC | RMGIC | Powder: strontium, aluminum silicate, excipients, activators, and iron oxide | DFL Indústria e Comércio S.A., Rio de Janeiro, Brazil |
| Liquid: 2-hydroxyethyl methacrylate polyacids, stabilizer, catalyzer, and ethyl alcohol | ||||
| G8 | Resiglass R Restore | RMGIC | Powder: calcium, barium, aluminum fluorosilicate, polyacrylic acid, and inorganic filers | BIODINÂMICA Química e Farmacêutica Ltda, Paraná, Brazil |
| Liquid: dimethacrylate groups, deionized water, and catalyst |
GIC, glass ionomer cement; RMGIC, resin-modified glass ionomer cement.
Total amount of fluoride released by each tested group (µgF/cm2) during the 15 day study period
| Group/cement | Fluoride released | Cumulative fluoride | |||
|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 7 | Day 15 | ||
| G1: Vidrion R | 37.75 ± 1.28 | 18.08 ± 1.25 | 9.69 ± 0.38 | 7.54 ± 0.36 | 180.45 ± 7.76 |
| G2: Vitro Fil | 56.72 ± 0.73 | 38.49 ± 1.12 | 22.28 ± 0.49 | 19.98 ± 1.57 | 382.77 ± 10.21 |
| G3: Vitro Molar | 34.39 ± 2.13 | 25.69 ± 2.16 | 18.81 ± 1.07 | 15.78 ± 0.84 | 296.97 ± 5.22 |
| G4: Bioglass R | 33.58 ± 3.52 | 23.58 ± 4.39 | 15.81 ± 3.24 | 12.38 ± 2.07 | 254.26 ± 5.84 |
| G5: Ketac Fil | 22.98 ± 1.69 | 11.84 ± 1.21 | 6.68 ± 0.86 | 4.59 ± 0.59 | 118.56 ± 4.78 |
| G6: Vitremer | 33.09 ± 4.35 | 19.53 ± 5.37 | 10.14 ± 2.29 | 7.23 ± 1.30 | 181.73 ± 6.91 |
| G7: Vitro Fil LC | 52.98 ± 3.80 | 31.69 ± 6.32 | 13.23 ± 3.08 | 7.29 ± 1.78 | 250.22 ± 12.29 |
| G8: Resiglass | 35.71 ± 0.81 | 26.99 ± 2.53 | 15.84 ± 1.72 | 12.91 ± 1.47 | 264.15 ± 6.65 |
Daily mean of fluoride released (µgF/cm2) during the study period for each tested material and the corresponding significance levels
| Group | Material | Daily fluoride release* |
|---|---|---|
| G2 | Vitro Fil | 25.52 ± 10.21a |
| G3 | Vitro Molar | 19.80 ± 5.22b |
| G8 | Resiglass | 17.61 ± 6.65c |
| G4 | Bioglass R | 16.95 ± 5.84c |
| G7 | Vitro Fil Lc | 16.68 ± 12.29c |
| G6 | Vitremer | 12.12 ± 6.91d |
| G1 | Vidrion R | 12.03 ± 7.76d |
| G5 | Ketac Fil | 7.90 ± 4.78e |
*Different letters represent statistically significant differences (p < 0.05) according to analysis of variance (ANOVA) and Tukey's test.
GIC, glass ionomer cement; RMGIC, resin-modified glass ionomer cement.
*Different letters represent statistically significant differences (

 KACD
KACD
 ePub Link
ePub Link Cite
Cite

