Articles
- Page Path
- HOME > Restor Dent Endod > Volume 34(6); 2009 > Article
- Original Article The effects of the fluoride concentration of acidulated buffer solutions on dentine remineralization
- Won-Sub Han, Chan-Young Lee
-
2009;34(6):-536.
DOI: https://doi.org/10.5395/JKACD.2009.34.6.526
Published online: November 30, 2009
Department of Conservative Dentistry, Yonsei University, Seoul, Korea.
- Corresponding Author: Chan-Young Lee. Department of Conservative Dentistry, College of Dentistry, Yonsei University, 134, Sinchon-Dong, Seodaemun-Ku, Seoul 120-752, Korea. Tel: 82-2-2228-8700, chanyoungl@yuhs.ac
Copyright © 2009 The Korean Academy of Conservative Dentistry
- 1,138 Views
- 1 Download
- 1 Crossref
Abstract
-
The aim of this vitro-study is to evaluate the effects of fluoride on remineralization of artificial dentine caries. 10 sound permanent premolars, which were extracted for orthodontic reason within 1 week, were used for this study. Artificial dentine caries was created by using a partially saturated buffer solution for 2 days with grounded thin specimens and fractured whole-body specimens. Remineralization solutions with three different fluoride concentration (1 ppm, 2 ppm and 4 ppm) were used on demineralized-specimens for 7 days. Polarizing microscope and scanning electron microscope were used for the evaluation of the mineral distribution profile and morphology of crystallites of hydroxyapatite.The results were as follows :
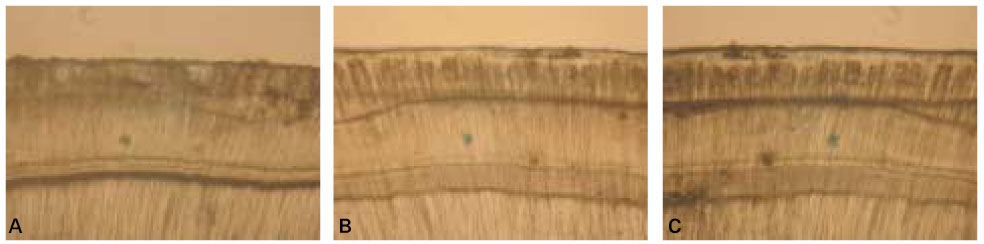
When treated with the fluoride solutions, the demineralized dentine specimens showed remineralization of the upper part and demineralization of the lower part of the lesion body simultaneously.
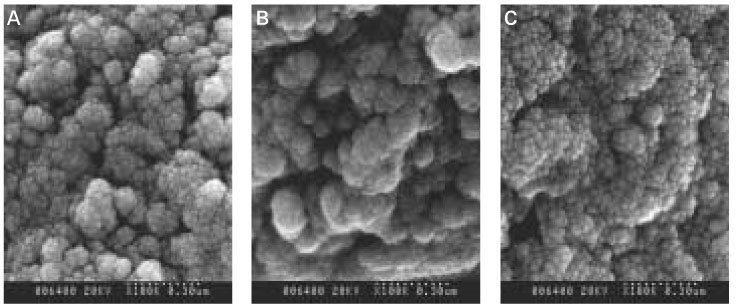
As the concentration of fluoride increased, the mineral precipitation in the caries dentine increased. The mineral precipitation mainly occurred in the surface layer in 1 and 2 ppm-specimens and in the whole lesion body in 4 ppm-specimens.
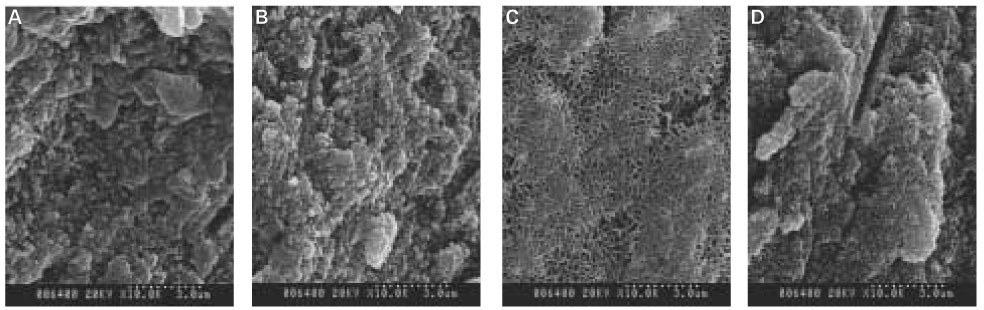
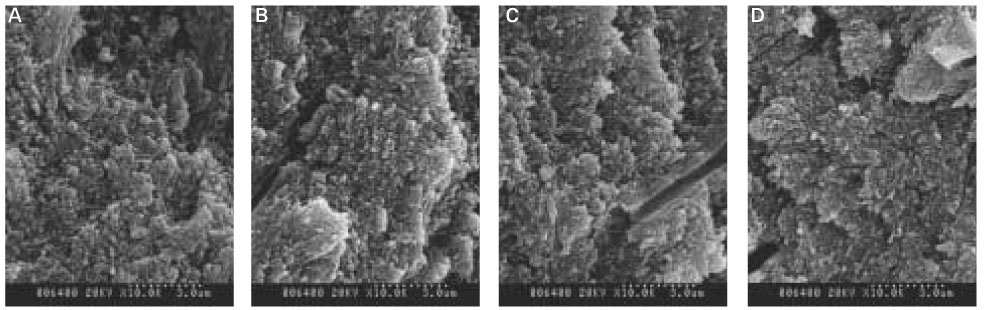
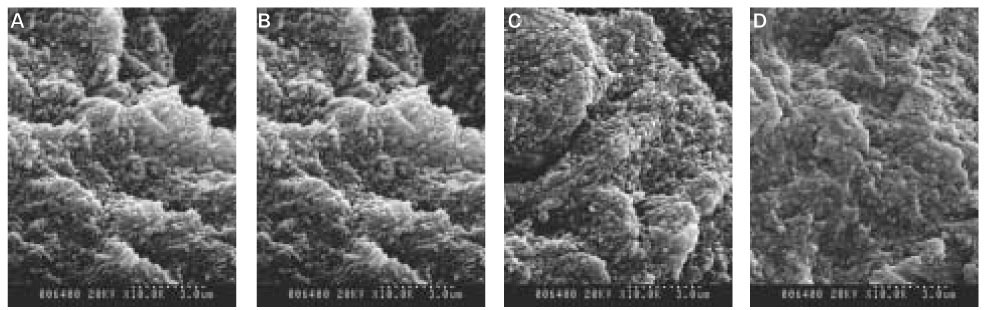
When treated with the fluoride solution, the hydroxyapatite crystals grew. This crystal growth was even observed in the lower part of the lesion body which had shown the loss of mineral.
- 1. Featherstone JD, Duncan JF, Cutress TW. A mechanism for dental caries based on chemical processes and diffusion phenomena during in-vitro caries simulation on human tooth enamel. Arch Oral Biol. 1979;24: 101-112.ArticlePubMed
- 2. Margolis HC, Moreno EC. Physicochemical perspectives on the cariostatic mechanisms of systemic and topical fluorides. J Dent Res. 1990;69(Spec No):606-613 discussion 34-36.ArticlePubMedPDF
- 3. Dean HT, Arnold FA, Elvove E. Domestic water and dental caries. V. Additional studies of the relation of fluoride in domestic waters to dental caries experience in 4,425 white children aged 12 to 14 years, of 13 cities in 4 states. Public Health Rep. 1942;57: 1155-1179.PubMedPMC
- 4. ten Cate JM, Rempt HE. Comparison of the in vivo effect of a 0 and 1,500 ppmF MFP toothpaste on fluoride uptake, acid resistance and lesion remineralization. Caries Res. 1986;20: 193-201.ArticlePubMed
- 5. Arends J, Christoffersen J, Ruben J, Jongebloed WL. Remineralization of bovine dentine in vitro. The influence of the F content in solution on mineral distribution. Caries Res. 1989;23: 309-314.ArticlePubMed
- 6. Silverstone LM, Poole DF. The effect of saliva and calcifying solutions upon the histological appearance of enamel caries. Caries Res. 1968;2: 87-93.ArticlePubMed
- 7. Varughese K, Moreno EC. Crystal growth of calcium apatites in dilute solutions containing fluoride. Calcif Tissue Int. 1981;33: 431-439.ArticlePubMedPDF
- 8. Han WS, Lee CY. The Influence of Fluoride on Remineralization of Artificial Dental Caries. J Korean Acad Conserv Dent. 1996;21: 161-173.
- 9. Kawasaki K, Ruben J, Tsuda H, Huysmans MC, Takagi O. Relationship between mineral distributions in dentine lesions and subsequent remineralization in vitro. Caries Res. 2000;34: 395-403.ArticlePubMedPDF
- 10. Levine RS, Rowles SL. Further studies on the remineralization of human carious dentine in vitro. Arch Oral Biol. 1973;18: 1351-1356.ArticlePubMed
- 11. Groeneveld A, Jongebloed W, Arends J. The mineral content of decalcified surface enamel. A combined microprobe- quantitative microradiography study. Caries Res. 1974;8: 267-274.ArticlePubMed
- 12. Aoba T, Okazaki M, Takahashi J, Moriwaki Y. X-ray diffraction study on remineralization using synthetic hydroxyapatite pellets. Caries Res. 1978;12: 223-230.PubMed
- 13. Darling AI. Some observations on amelogenesis imperfecta and calcification of the dental enamel. Proc R Soc Med. 1956;49: 759-765.ArticlePubMedPMCPDF
- 14. Featherstone JD, ten Cate JM, Shariati M, Arends J. Comparison of artificial caries-like lesions by quantitative microradiography and microhardness profiles. Caries Res. 1983;17: 385-391.ArticlePubMed
- 15. Haikel Y, Frank RM, Voegel JC. Scanning electron microscopy of the human enamel surface layer of incipient carious lesions. Caries Res. 1983;17: 1-13.ArticlePubMed
- 16. Vizioli MR. Dichroism and birefringence in human carious dentine. Ann Histochim. 1970;15: 131-140.PubMed
- 17. Dietz W, Kraft U, Hoyer I, Klingberg G. Influence of cementum on the demineralization and remineralization processes of root surface caries in vitro. Acta Odontol Scand. 2002;60: 241-247.ArticlePubMed
- 18. Arends J, Ruben J, Jongebloed WL. Dentine caries in vivo. Combined scanning electron microscopic and microradiographic investigation. Caries Res. 1989;23: 36-41.ArticlePubMed
- 19. Takuma S. Demineralization and Remineralization of tooth substance- an ultrastructural basis for caries prevention. J Dent Res. 1980;2146-2156.
- 20. LeGeros RZ. Formation and Stability of Synthetic Apatites. Calcium Phosphates in Oral Biology and Medicine. 1991;Karger; 82-107.
- 21. Holmen L, Thylstrup A, Featherstone JD, Fredebo L, Shariati M. A scanning electron microscopic study of surface changes during development of artificial caries. Caries Res. 1985;19: 11-21.ArticlePubMed
- 22. Hayashi Y. High resolution electron microscopy of the interface between dental calculus and denture resin. Scanning Microsc. 1995;9: 419-425 discussion 425-27.PubMed
- 23. Park JW, Lee CY. The Effects of the Degree of Saturation of Acidulated Buffer Solutions in Enamel and Dentin Remineralization and AFM Observation of Hydroxyapatite Crystals. J Korean Acad Conserv Dent. 2000;25: 459-473.
- 24. Takuma S, Sunohara H, Watanabe H, Yama K. Some structural aspects of carious lesions in human dentine. Bull Tokyo dent Coll. 1969;10: 173-181.PubMed
- 25. LeGeros RZ. Chemical and crystallographic events in the caries process. J Dent Res. 1990;69(Spec No):567-574 discussion 634-636.ArticlePubMedPDF
- 26. Watson ML, Avery JK. The development of the hamster lower incisor as observed by electron microscopy. Am J Anat. 1954;95: 109.ArticlePubMed
- 27. Levine RS. Remineralization of human carious dentine in vitro. Arch Oral Biol. 1972;17: 1005-1008.ArticlePubMed
- 28. Klont B, ten Cate JM. Remineralization of bovine incisor root lesions in vitro: the role of the collagenous matrix. Caries Res. 1991;25: 39-45.ArticlePubMed
- 29. Scott DB, Simmelink JW, Nygaard V. Structural aspects of dental caries. J Dent Res. 1974;53: 165-178.ArticlePubMedPDF
REFERENCES



Tables & Figures
REFERENCES
Citations

- Infant Oral Health Care Concerning Education of Mothers – Part 2
Lehya Mounica Kadali, Viddyasagar Mopagar, Shilpa Shetty, Shridhar Shetty, Venkatesh Kodgi, Shantanu Chaudhari
Journal of Evolution of Medical and Dental Sciences.2021; 10(31): 2538. CrossRef


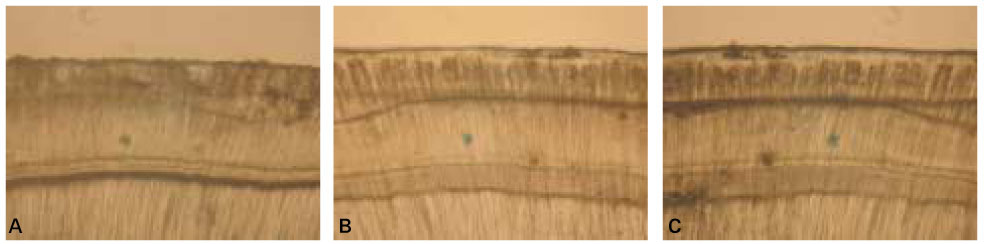
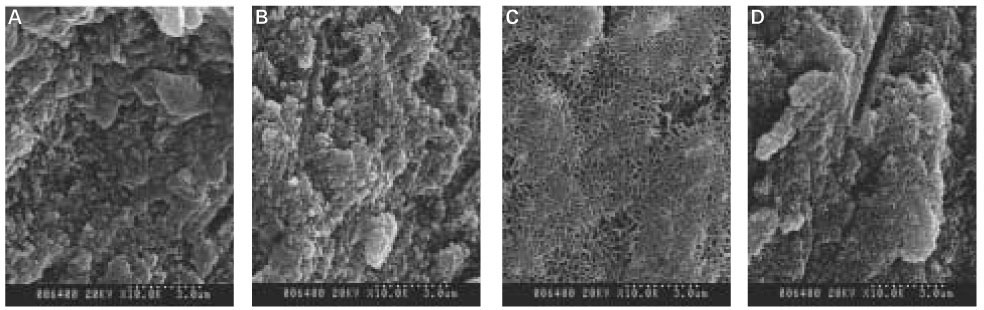
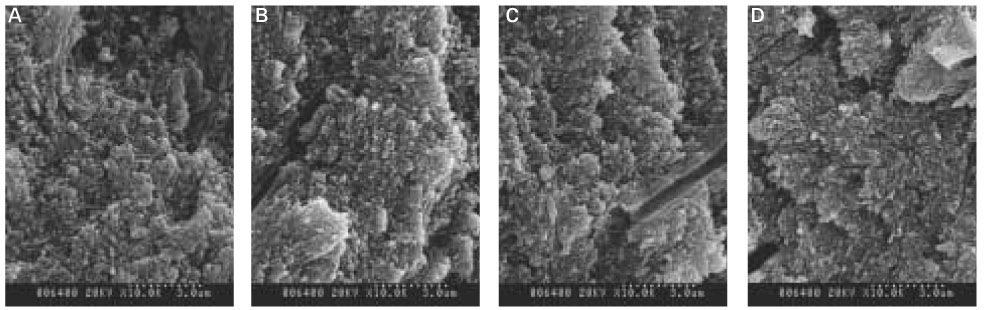
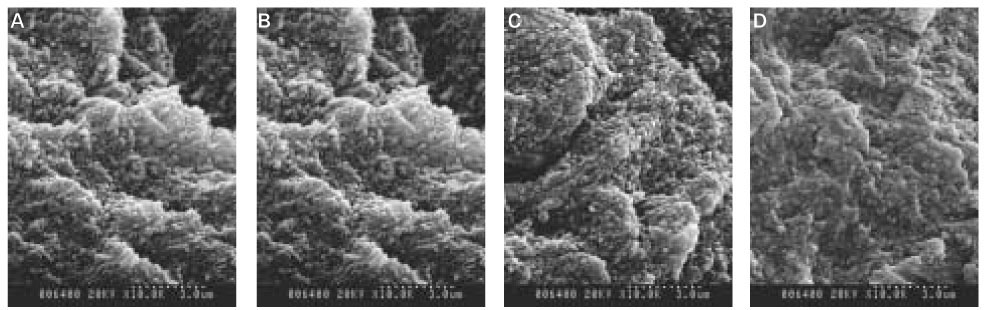
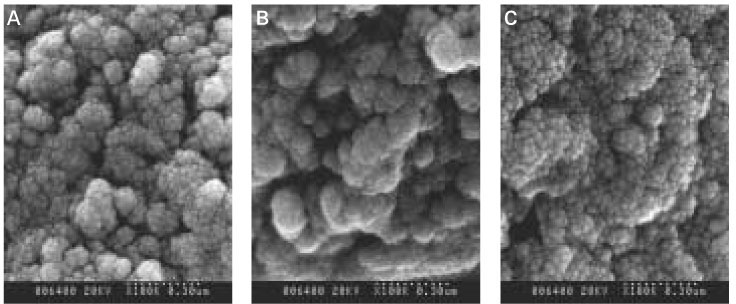

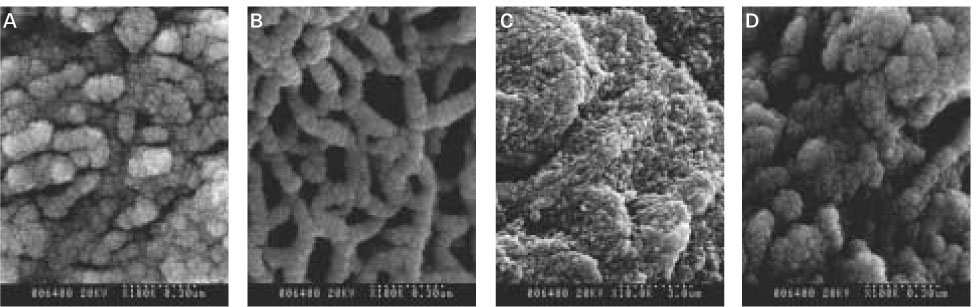
Figure 1
Figure 2
Figure 3
Figure 4
Figure 5
Figure 6
Figure 7
Figure 8
Figure 9
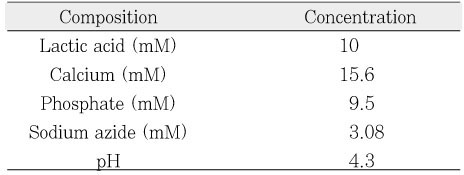
Composition of decalcification solution.
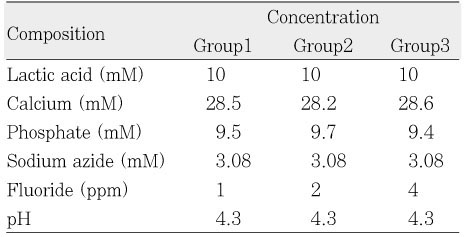
Composition of remineralinzation solution.

 KACD
KACD
 ePub Link
ePub Link Cite
Cite

