Articles
- Page Path
- HOME > Restor Dent Endod > Volume 46(1); 2021 > Article
- Research Article Can silver diamine fluoride or silver nanoparticle-based anticaries agents to affect enamel bond strength?
-
Jaqueline Costa Favaro
 , Yana Cosendey Toledo de Mello Peixoto
, Yana Cosendey Toledo de Mello Peixoto , Omar Geha
, Omar Geha , Flaviana Alves Dias
, Flaviana Alves Dias , Ricardo Danil Guiraldo
, Ricardo Danil Guiraldo , Murilo Baena Lopes
, Murilo Baena Lopes , Sandrine Bittencourt Berger
, Sandrine Bittencourt Berger
-
Restor Dent Endod 2021;46(1):e7.
DOI: https://doi.org/10.5395/rde.2021.46.e7
Published online: January 12, 2021
Department of Restorative Dentistry, School of Dentistry, University of North Parana, Londrina, PR, Brazil.
- Correspondence to Sandrine Bittencourt Berger, DDS, MS, PhD. Associate Professor, Department of Restorative Dentistry, School of Dentistry, University of North Parana, Rua Marselha, 183, Londrina, PR 86041-140, Brazil. berger.sandrine@gmail.com
Copyright © 2021. The Korean Academy of Conservative Dentistry
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Objectives The aim of the current study is to investigate the effect of different anticaries agents, such as experimental agents based on silver nanoparticles (SNPs) and silver diamine fluoride (SDF), on the micro-shear bond strength (μ-SBS) of composite resin applied to intact enamel (IE) or demineralized enamel (DE).
-
Materials and Methods Sixty dental enamel fragments were collected from human third molars and categorized into 6 groups (n = 10): positive control (IE), negative control (DE), IE + SDF, DE + SDF, IE + SNP and DE + SNP. Samples from DE, DE + SDF and DE + SNP groups were subjected to pH cycling; superficial microhardness test was performed to confirm demineralization. Resin composite build-ups were applied to the samples (0.75-mm diameter and 1-mm height) after the treatments (except for IE and DE groups); μ-SBS was also evaluated. Samples were analyzed under a stereomicroscope at 40× magnification to identify failure patterns. Data were subjected to one-way analysis of variance, followed by Tukey's and Dunnett's tests (p < 0.05).
-
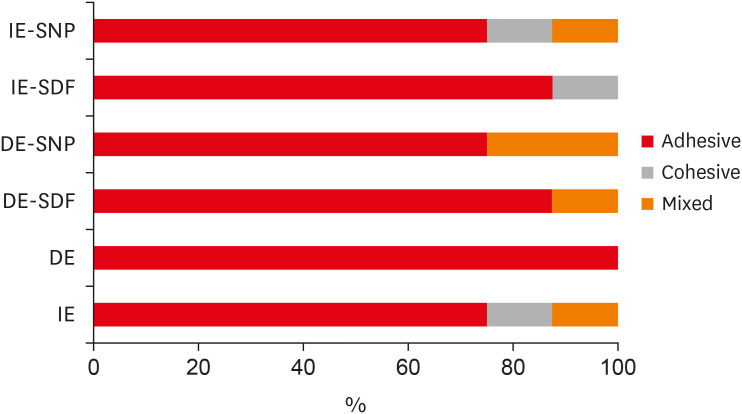
Results There was no significant difference among the IE, IE + SNP, DE + SDF, and DE + SNP groups. The IE + SDF and DE groups recorded the highest and the lowest μ-SBS values, respectively. Adhesive-type failures were the most frequent for all treatments.
-
Conclusions Anticaries agents did not have a negative effect on the μ-SBS of composite resin when it was used on IE or DE.
INTRODUCTION
MATERIALS AND METHODS
Experimental groups and anticaries agent composition
RESULTS
Mean ± standard deviation micro-shear bond strength (μ-SBS) values according to the experimental groups
| Substrate | SDF | SNP |
|---|---|---|
| IE | 47.50 ± 11.60Aa* | 42.50 ± 12.10Aa* |
| DE | 30.10 ± 9.50Ba† | 26.10 ± 8.10Ba† |
| Positive control | 33.00 ± 11.80* | |
| Negative control | 18.90 ± 2.50† | |
Details of all the fracture modes according to the experimental groups.
DISCUSSION
CONCLUSIONS
ACKNOWLEDGEMENTS
-
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
-
Author Contributions:
Conceptualization: Berger SB.
Data curation: Favaro JC, Geha O.
Formal analysis: Favaro JC, Geha O, Guiraldo RD, Lopes MB, Berger SB.
Investigation: Favaro JC, Mello-Peixoto YCT, Geha O, Guiraldo RD.
Methodology: Favaro JC, Geha O, Guiraldo RD, Lopes MB.
Project administration: Berger SB.
Resources: Guiraldo RD.
Supervision: Berger SB.
Validation: Favaro JC, Geha O, Dias FA.
Visualization: Favaro JC, Geha O.
Writing - original draft: Favaro JC, Dias FA.
Writing - review & editing: Favaro JC, Geha O, Guiraldo RD, Lopes MB, Dias FA, Berger SB.
- 1. Yamaga R, Nishino M, Yoshida S, Yokomizo I. Diammine silver fluoride and its clinical application. J Osaka Univ Dent Sch 1972;12:1-20.PubMed
- 2. Lou YL, Botelho MG, Darvell BW. Reaction of silver diamine fluoride with hydroxyapatite and protein. J Dent 2011;39:612-618.ArticlePubMed
- 3. Shah S, Bhaskar V, Venkatraghavan K, Choudhary P, Ganesh M, Trivedi K. Silver diamine fluoride: a review and current applications. J Adv Oral Res 2018;5:25-35.ArticlePDF
- 4. Rosenblatt A, Stamford TC, Niederman R. Silver diamine fluoride: a caries “silver-fluoride bullet”. J Dent Res 2009;88:116-125.ArticlePubMedPDF
- 5. Burgess JO, Vaghela PM. Silver diamine fluoride: a successful anticarious solution with limits. Adv Dent Res 2018;29:131-134.ArticlePubMedPDF
- 6. Santos VE Jr, Vasconcelos Filho A, Targino AG, Flores MA, Galembeck A, Caldas AF Jr, Rosenblatt A. A new “silver-bullet” to treat caries in children--nano silver fluoride: a randomised clinical trial. J Dent 2014;42:945-951.ArticlePubMed
- 7. Targino AG, Flores MA, dos Santos Junior VE, de Godoy Bené Bezerra F, de Luna Freire H, Galembeck A, Rosenblatt A. An innovative approach to treating dental decay in children. A new anti-caries agent. J Mater Sci Mater Med 2014;25:2041-2047.ArticlePubMedPDF
- 8. Mei ML, Nudelman F, Marzec B, Walker JM, Lo EC, Walls AW, Chu CH. Formation of fluorohydroxyapatite with silver diamine fluoride. J Dent Res 2017;96:1122-1128.ArticlePubMedPMCPDF
- 9. Nozari A, Ajami S, Rafiei A, Niazi E. Impact of nano hydroxyapatite, nano silver fluoride and sodium fluoride varnish on primary teeth enamel remineralization: an in vitro study. J Clin Diagn Res 2017;11:ZC97-ZC100.
- 10. Ortiz-Ruiz AJ, Muñoz-Gómez IJ, Pérez-Pardo A, Germán-Cecilia C, Martínez-Beneyto Y, Vicente A. Influence of fluoride varnish on shear bond strength of a universal adhesive on intact and demineralized enamel. Odontology 2018;106:460-468.ArticlePubMedPDF
- 11. Scarpelli BB, Punhagui MF, Hoeppner MG, Almeida RS, Juliani FA, Guiraldo RD, Berger SB. In vitro evaluation of the remineralizing potential and antimicrobial activity of a cariostatic agent with silver nanoparticles. Braz Dent J 2017;28:738-743.ArticlePubMed
- 12. Akin M, Baka ZM, Ileri Z, Basciftci FA. Can demineralized enamel surfaces be bonded safely? Acta Odontol Scand 2014;72:283-289.ArticlePubMed
- 13. Kucukyilmaz E, Savas S, Akcay M, Bolukbasi B. Effect of silver diamine fluoride and ammonium hexafluorosilicate applications with and without Er:YAG laser irradiation on the microtensile bond strength in sound and caries-affected dentin. Lasers Surg Med 2016;48:62-69.ArticlePubMedPDF
- 14. William V, Burrow MF, Palamara JE, Messer LB. Microshear bond strength of resin composite to teeth affected by molar hypomineralization using 2 adhesive systems. Pediatr Dent 2006;28:233-241.PubMed
- 15. Puwanawiroj A, Trairatvorakul C, Dasanayake AP, Auychai P. Microtensile bond strength between glass ionomer cement and silver diamine fluoride-treated carious primary dentin. Pediatr Dent 2018;40:291-295.PubMed
- 16. Shimaoka AM, de Andrade AP, Cardoso MV, de Carvalho RC. The importance of adhesive area delimitation in a microshear bond strength experimental design. J Adhes Dent 2011;13:307-314.PubMed
- 17. Quock RL, Barros JA, Yang SW, Patel SA. Effect of silver diamine fluoride on microtensile bond strength to dentin. Oper Dent 2012;37:610-616.ArticlePubMedPDF
- 18. Selvaraj K, Sampath V, Sujatha V, Mahalaxmi S. Evaluation of microshear bond strength and nanoleakage of etch-and-rinse and self-etch adhesives to dentin pretreated with silver diamine fluoride/potassium iodide: an in vitro study. Indian J Dent Res 2016;27:421-425.ArticlePubMed
- 19. Noronha MS, Romão DA, Cury JA, Tabchoury CP. Effect of fluoride concentration on reduction of enamel demineralization according to the cariogenic challenge. Braz Dent J 2016;27:393-398.ArticlePubMed
- 20. Cheng YL, Musonda J, Cheng H, Attin T, Zheng M, Yu H. Effect of surface removal following bleaching on the bond strength of enamel. BMC Oral Health 2019;19:50.ArticlePubMedPMCPDF
- 21. De Munck J, Van Landuyt K, Peumans M, Poitevin A, Lambrechts P, Braem M, Van Meerbeek B. A critical review of the durability of adhesion to tooth tissue: methods and results. J Dent Res 2005;84:118-132.ArticlePubMedPDF
- 22. Pérez-Hernández J, Aguilar-Díaz FC, Venegas-Lancón RD, Gayosso CA, Villanueva-Vilchis MC, de la Fuente-Hernández J. Effect of silver diamine fluoride on adhesion and microleakage of a pit and fissure sealant to tooth enamel: in vitro trial. Eur Arch Paediatr Dent 2018;19:411-416.ArticlePubMedPDF
- 23. Swift EJ Jr, Perdigão J, Heymann HO. Bonding to enamel and dentin: a brief history and state of the art, 1995. Quintessence Int 1995;26:95-110.PubMed
- 24. Espíndola-Castro LF, Rosenblatt A, Galembeck A, Monteiro G. Dentin staining caused by nano-silver fluoride: a comparative study. Oper Dent 2020;45:435-441.ArticlePubMedPDF
REFERENCES
Tables & Figures
REFERENCES
Citations

- Impact of Incorporating Nanoparticles to Adhesive Resin on the Demineralization of Enamel: A Systematic Review
Naif Almosa
Dentistry Journal.2025; 13(3): 89. CrossRef - Preventing white spot lesions around orthodontic brackets: efficacy of pre-reacted glass-ionomer barrier coat versus silver diamine fluoride: an in vitro study
Enas A. Elshenawy, Safa B. Alawy, Wafaa Yahia Alghonemy, Ahmed Ibrahime El dosoky
BDJ Open.2025;[Epub] CrossRef - Research Status of Silver Nanoparticles for Dental Applications
Yanyan Guo, Xiaomei Hou, Sanjun Fan, Chanyuan Jin
Inorganics.2025; 13(5): 168. CrossRef - The use of silver diamine fluoride to prevent/treat enamel carious lesions: a narrative review
Rasha N. AlSheikh
PeerJ.2024; 12: e17897. CrossRef - Phosphoric Acid Etch Partially Restores the Initial Bond Strength of Composite to Silver Diamine Fluoride–Treated Enamel Using Universal Adhesives
Zaher Jabbour, Mijoo Kim, Marc Hayashi, Reuben Kim
Dentistry Journal.2023; 11(7): 161. CrossRef - Efficacy of Nano Silver Fluoride and/or Diode Laser In Enhancing Enamel Anticariogenicity around orthodontic brackets
Aya Anwar Alsherif, Mohamed Ali Farag, Mai Badreldin Helal
BDJ Open.2023;[Epub] CrossRef - Amelioration Strategies for Silver Diamine Fluoride: Moving from Black to White
Amjad Almuqrin, Inder Preet Kaur, Laurence J. Walsh, Chaminda Jayampath Seneviratne, Sobia Zafar
Antibiotics.2023; 12(2): 298. CrossRef - The Effect of Loading Time on Color Stability of Various Restorative Materials Bonded to Silver Diamine Fluoride-Treated Demineralized Dentin
Mohammed M Aldosari, Fares S Al-Sehaibany
Clinical, Cosmetic and Investigational Dentistry.2022; Volume 14: 123. CrossRef - In vitro study of the effect of nanosilver fluoride on shear bond strength of orthodontic brackets and demineralization of enamel
Mariam H. El-Toukhy, Eman M. El-Shourbagy, Neveen M. Fakhry
Tanta Dental Journal.2022; 19(4): 281. CrossRef
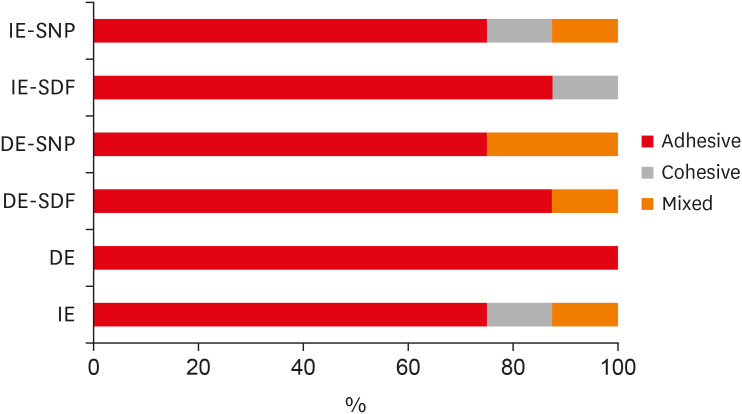
Figure 1
Experimental groups and anticaries agent composition
| Experimental group | Anticaries agent composition |
|---|---|
| Positive control: IE | NA |
| Negative control: DE | NA |
| IE + 30% SDF* | Silver nitrate, ammonium hydroxide, fluoroboric acid, deionized water |
| DE + SDF* | Silver nitrate, ammonium hydroxide, fluoroboric acid, deionized water |
| IE + experimental solution with SNP† | SNPs |
| DE + SNP† | SNPs |
IE, intact enamel; DE, demineralized enamel; SDF, silver diamine fluoride; SNP, silver nanoparticle; NA, not available.
*Cariestop, Biodiâmica Química e Farmacêutica LTDA, Londrina, PR, Brazil; †Experimental solution, not commercially available.
Mean ± standard deviation micro-shear bond strength (μ-SBS) values according to the experimental groups
| Substrate | SDF | SNP |
|---|---|---|
| IE | 47.50 ± 11.60Aa* | 42.50 ± 12.10Aa* |
| DE | 30.10 ± 9.50Ba† | 26.10 ± 8.10Ba† |
| Positive control | 33.00 ± 11.80* | |
| Negative control | 18.90 ± 2.50† | |
IE, intact enamel; DE, demineralized enamel; SDF, silver diamine fluoride; SNP, silver nanoparticle.
Mean values followed by different upper case letters, in columns, comparing the treatments, are statistically different according to Tukey's test (p < 0.05). Mean values followed by lower case letters, in rows, comparing the enamel surface, are statistically different according to Tukey's test (p < 0.05).
*Statistically different from the negative control group according to Dunnett's test (p < 0.05); †Statistically different from the positive control group according to Dunnett's test (p < 0.05).
IE, intact enamel; DE, demineralized enamel; SDF, silver diamine fluoride; SNP, silver nanoparticle; NA, not available.
*Cariestop, Biodiâmica Química e Farmacêutica LTDA, Londrina, PR, Brazil; †Experimental solution, not commercially available.
IE, intact enamel; DE, demineralized enamel; SDF, silver diamine fluoride; SNP, silver nanoparticle.
Mean values followed by different upper case letters, in columns, comparing the treatments, are statistically different according to Tukey's test (
*Statistically different from the negative control group according to Dunnett's test (

 KACD
KACD
 ePub Link
ePub Link Cite
Cite

