Articles
- Page Path
- HOME > Restor Dent Endod > Volume 46(1); 2021 > Article
- Research Article Biocompatibility and bioactive potential of the NeoMTA Plus endodontic bioceramic-based sealer
-
Roberto Alameda Hoshino1
 , Mateus Machado Delfino1
, Mateus Machado Delfino1 , Guilherme Ferreira da Silva2
, Guilherme Ferreira da Silva2 , Juliane Maria Guerreiro-Tanomaru1
, Juliane Maria Guerreiro-Tanomaru1 , Mário Tanomaru-Filho1
, Mário Tanomaru-Filho1 , Estela Sasso-Cerri3
, Estela Sasso-Cerri3 , Paulo Sérgio Cerri3
, Paulo Sérgio Cerri3
-
Restor Dent Endod 2020;46(1):e4.
DOI: https://doi.org/10.5395/rde.2021.46.e4
Published online: December 17, 2020
1Department of Restorative Dentistry, Dental School, São Paulo State University (UNESP), Araraquara, SP, Brazil.
2Pro-Rectory of Research and Post-graduation, School of Dentistry, Universidade Sagrado Coração (USC), Bauru, SP, Brazil.
3Department of Morphology, Genetics, Orthodontics and Pediatric Dentistry, Laboratory of Histology and Embryology, Dental School, São Paulo State University (UNESP), Araraquara, SP, Brazil.
- Correspondence to Paulo Sérgio Cerri, DDS, MS, PhD. Professor, Laboratory of Histology and Embryology, Dental School, São Paulo State University (UNESP), Rua Humaitá, 1680, Araraquara, SP 14.801-903, Brazil. paulo.cerri@unesp.br
Copyright © 2021. The Korean Academy of Conservative Dentistry
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Objectives This study evaluated the biocompatibility and bioactive potential of NeoMTA Plus mixed as a root canal sealer in comparison with MTA Fillapex.
-
Materials and Methods Polyethylene tubes filled with NeoMTA Plus (n = 20), MTA Fillapex (n = 20), or nothing (control group, CG; n = 20) were inserted into the connective tissue in the dorsal subcutaneous layer of rats. After 7, 15, 30 and 60 days, the specimens were processed for paraffin embedding. The capsule thickness, collagen content, and number of inflammatory cells (ICs) and interleukin-6 (IL-6) immunolabeled cells were measured. von Kossa-positive structures were evaluated and unstained sections were analyzed under polarized light. Two-way analysis of variance was performed, followed by the post hoc Tukey test (p ≤ 0.05).
-
Results At 7 days, the capsules around NeoMTA Plus and MTA Fillapex had more ICs and IL-6-immunostained cells than the CG. However, at 60 days, there was no significant difference in the IC number between NeoMTA Plus and the CG (p = 0.1137) or the MTA Fillapex group (p = 0.4062), although a greater number of IL-6-immunostained cells was observed in the MTA Fillapex group (p = 0.0353). From 7 to 60 days, the capsule thickness of the NeoMTA Plus and MTA Fillapex specimens significantly decreased, concomitantly with an increase in the collagen content. The capsules around root canal sealers showed positivity to the von Kossa stain and birefringent structures.
-
Conclusions The NeoMTA Plus root canal sealer is biocompatible and exhibits bioactive potential.
INTRODUCTION
MATERIALS AND METHODS
Manufacturers, chemical components, and proportions of the root canal sealers
1. Capsule thickness
2. IL-6 detection by immunohistochemistry
3. Collagen measurement
4. von Kossa histochemical reaction and unstained sections analyzed under polarized light
RESULTS
Light micrographs of sections of capsules at 7 days (A-F) and 15 days (G-L). (A-C and G-I) Photomicrographs at a low magnification show well-defined capsules around the implants (bars = 500 µm). (D-F and J-L) Higher magnification of (A-C and G-I), showing inflammatory cells (arrows) and Fb (bars = 20 µm). In MTA Fillapex (K), several material particles are seen.
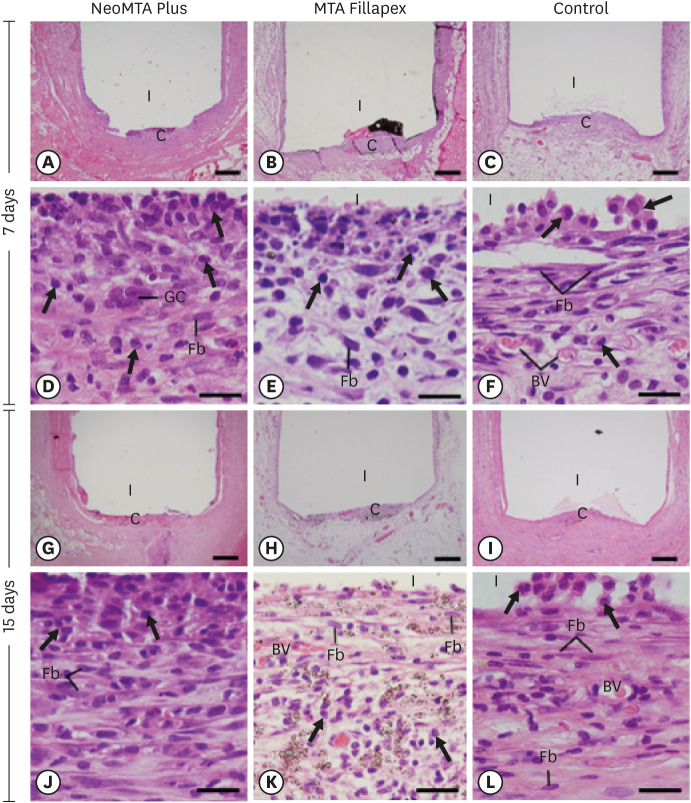
Light micrographs of sections of capsules at 30 days (A-F) and 60 days (G-L). (A-C and G-I) Photomicrographs show a general view of the capsules juxtaposed to the opening of the tubes. All groups exhibited thinner capsules at 60 days than at 30 days (bars = 500 µm). (D-F and J-L) Inflammatory cells (arrows) and Fb (bars = 20 µm). Note that in the control group (F and L), the capsules exhibited Fb dispersed among CF and few inflammatory cells (arrows). In MTA Fillapex (K), material particles were observed throughout the capsule. Graphs show data on the numerical density of inflammatory cells (M) and the capsule thickness (N) of the NeoMTA Plus, MTA Fillapex, and control groups at 7, 15, 30 and 60 days. Superscript letters indicate comparisons among the groups; different letters denote significant differences. Superscript numbers indicate the analysis of each group over time; different numbers denote significant differences. Tukey's test (p ≤ 0.05).
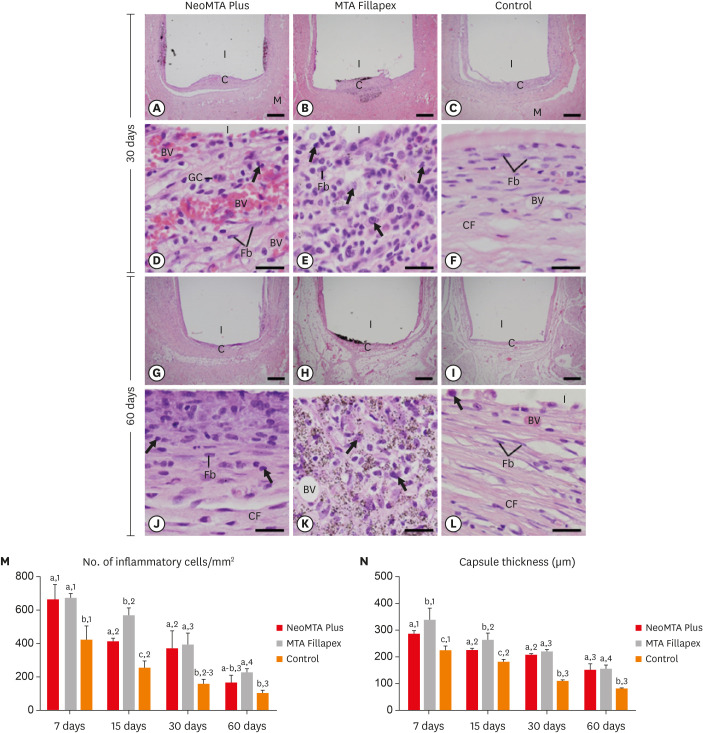
Light micrographs show portions of capsules juxtaposed with the implant tubes. (A-L) Immunohistochemistry was used to detect IL-6 with hematoxylin counterstaining. The immunostaining (brown-yellow color) is seen in inflammatory cells (arrows) and Fb in the capsules at all periods (bars = 20 µm). (M) Graph showing the numerical density of IL-6-immunostained cells in the NeoMTA Plus, MTA Fillapex, and control groups at 7, 15, 30, and 60 days. Superscript letters indicate comparisons among the groups; different letters denote significant differences. Superscript numbers indicate the analysis of each group over time; different numbers denote significant differences. Tukey's test (p ≤ 0.05).
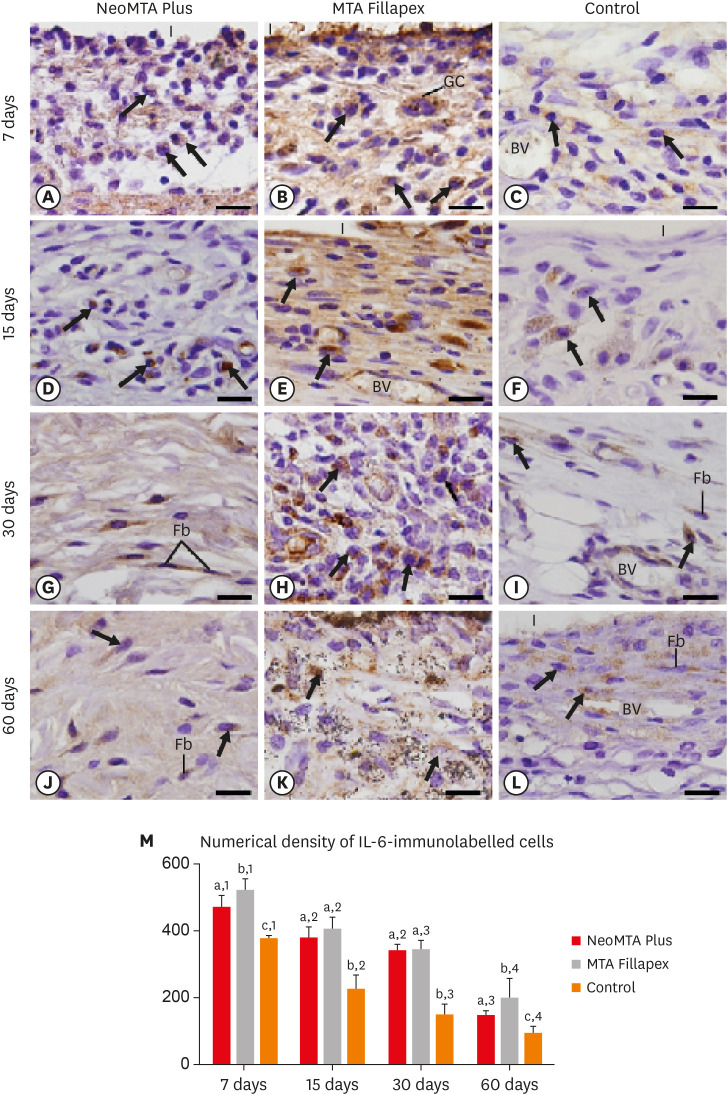
Light micrographs of portions of capsules juxtaposed with the implant tubes. The sections were stained with picrosirius red and photographed with a polarization microscope. At 7 (A-C) and 15 (D-F) days, the capsules contained few birefringent fibers (orange/red colors). At 30 (G-I) and 60 (J-L) days, the capsules contained thick bundles of collagen exhibiting strong birefringence (bars = 20 µm). (M) Graph illustrating the birefringent collagen content (in percentage) in NeoMTA Plus, MTA Fillapex, and control groups at 7, 15, 30, and 60 days. Superscript asterisks denote significant between-group differences. Superscript numbers indicate the analysis of each group over time; different numbers denote significant differences. Tukey's test (p ≤ 0.05).
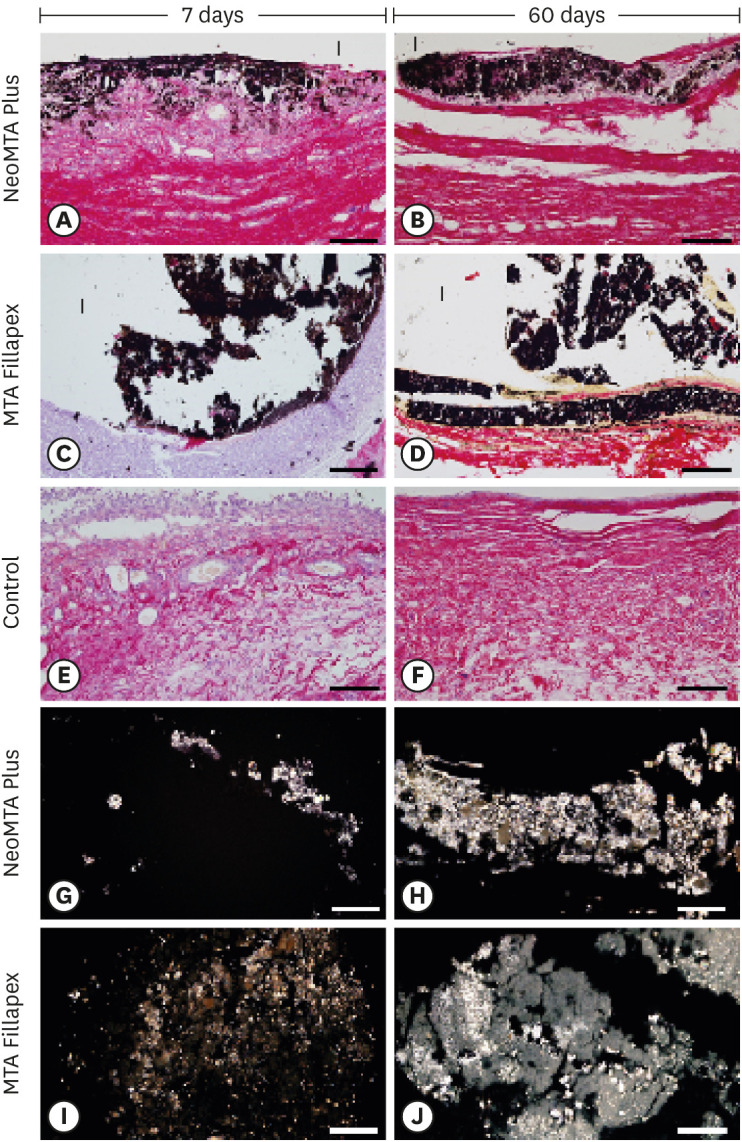
Light micrographs of portions of capsules juxtaposed with the implant tubes. (A-F) von Kossa histochemical reaction (black) and counterstaining with picrosirius red (red). The capsules in the NeoMTA Plus and MTA Fillapex groups exhibited reactivity to the von Kossa method (structures shown in black). In the control group (E and F), von Kossa-positive structures are absent. (G-J) Unstained sections analyzed under polarized light. Fine granular material exhibiting birefringence is observed in the capsules of NeoMTA Plus and MTA Fillapex (bars = 20 µm).
DISCUSSION
CONCLUSIONS
ACKNOWLEDGEMENTS
-
Funding: Financial support provided by FAPESP (2016/09264-9) and CNPq (Brazil).
-
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
-
Author Contributions:
Conceptualization: Cerri PS, Tanomaru-Filho M.
Formal analysis: Hoshino RA, Delfino MM, Cerri PS, Sasso-Cerri E.
Funding acquisition: Cerri PS.
Investigation: Hoshino RA, Silva GF, Guerreiro-Tanomaru J.
Methodology: Hoshino RA, Delfino MM.
Supervision: Cerri PS.
Writing - original draft: Hoshino RA, Delfino MM, Sasso-Cerri E, Cerri PS.
Writing - review & editing: Hoshino RA, Delfino MM, Silva GF, Guerreiro-Tanomaru JM, Tanomaru-Filho M, Sasso-Cerri E, Cerri PS.
- 1. Tomás-Catalá CJ, Collado-González M, García-Bernal D, Oñate-Sánchez RE, Forner L, Llena C, Lozano A, Moraleda JM, Rodríguez-Lozano FJ. Biocompatibility of new pulp-capping materials NeoMTA Plus, MTA Repair HP, and biodentine on human dental pulp stem cells. J Endod 2018;44:126-132.ArticlePubMed
- 2. Cintra LTA, Benetti F, de Azevedo Queiroz ÍO, de Araújo Lopes JM, Penha de Oliveira SH, Sivieri Araújo G, Gomes-Filho JE. Cytotoxicity, biocompatibility, and biomineralization of the new high-plasticity MTA Material. J Endod 2017;43:774-778.ArticlePubMed
- 3. Mondelli JAS, Hoshino RA, Weckwerth PH, Cerri PS, Leonardo RT, Guerreiro-Tanomaru JM, Tanomaru-Filho M, da Silva GF. Biocompatibility of mineral trioxide aggregate flow and biodentine. Int Endod J 2019;52:193-200.ArticlePubMedPDF
- 4. Parirokh M, Torabinejad M, Dummer PMH. Mineral trioxide aggregate and other bioactive endodontic cements: an updated overview - Part I: Vital pulp therapy. Int Endod J 2018;51:177-205.ArticlePubMedPDF
- 5. Siboni F, Taddei P, Prati C, Gandolfi MG. Properties of NeoMTA Plus and MTA Plus cements for endodontics. Int Endod J 2017;50(Supplement 2):e83-e94.PubMed
- 6. Slompo C, Peres-Buzalaf C, Gasque KC, Damante CA, Ordinola-Zapata R, Duarte MA, de Oliveira RC. Experimental calcium silicate-based cement with and without zirconium oxide modulates fibroblasts viability. Braz Dent J 2015;26:587-591.ArticlePubMed
- 7. Bozeman TB, Lemon RR, Eleazer PD. Elemental analysis of crystal precipitate from gray and white MTA. J Endod 2006;32:425-428.ArticlePubMed
- 8. Torabinejad M, Parirokh M. Mineral trioxide aggregate: a comprehensive literature review - Part II: Leakage and biocompatibility investigations. J Endod 2010;36:190-202.ArticlePubMed
- 9. McMichael GE, Primus CM, Opperman LA. Dentinal tubule penetration of tricalcium silicate sealers. J Endod 2016;42:632-636.ArticlePubMedPMC
- 10. Siboni F, Taddei P, Zamparini F, Prati C, Gandolfi MG. Properties of BioRoot RCS, a tricalcium silicate endodontic sealer modified with povidone and polycarboxylate. Int Endod J 2017;50(Supplement 2):e120-e136.ArticlePubMedPDF
- 11. Quintana RM, Jardine AP, Grechi TR, Grazziotin-Soares R, Ardenghi DM, Scarparo RK, Grecca FS, Kopper PMP. Bone tissue reaction, setting time, solubility, and pH of root repair materials. Clin Oral Investig 2019;23:1359-1366.ArticlePubMedPDF
- 12. Tanomaru-Filho M, Andrade AS, Rodrigues EM, Viola KS, Faria G, Camilleri J, Guerreiro-Tanomaru JM. Biocompatibility and mineralized nodule formation of NeoMTA Plus and an experimental tricalcium silicate cement containing tantalum oxide. Int Endod J 2017;50(Supplement 2):e31-e39.PubMed
- 13. Prüllage RK, Urban K, Schäfer E, Dammaschke T. Material properties of a tricalcium silicate-containing, a mineral trioxide aggregate-containing, and an epoxy resin-based root canal sealer. J Endod 2016;42:1784-1788.ArticlePubMed
- 14. Collado-González M, García-Bernal D, Oñate-Sánchez RE, Ortolani-Seltenerich PS, Lozano A, Forner L, Llena C, Rodríguez-Lozano FJ. Biocompatibility of three new calcium silicate-based endodontic sealers on human periodontal ligament stem cells. Int Endod J 2017;50:875-884.ArticlePubMedPDF
- 15. Camilleri J, Montesin FE, Papaioannou S, McDonald F, Pitt Ford TR. Biocompatibility of two commercial forms of mineral trioxide aggregate. Int Endod J 2004;37:699-704.ArticlePubMed
- 16. Tanomaru-Filho M, Cristine Prado M, Torres FFE, Viapiana R, Pivoto-João MMB, Guerreiro-Tanomaru JM. Physicochemical properties and bioactive potential of a new epoxy resin-based root canal sealer. Braz Dent J 2019;30:563-568.ArticlePubMed
- 17. Delfino MM, Guerreiro-Tanomaru JM, Tanomaru-Filho M, Sasso-Cerri E, Cerri PS. Immunoinflammatory response and bioactive potential of GuttaFlow bioseal and MTA Fillapex in the rat subcutaneous tissue. Sci Rep 2020;10:7173.ArticlePubMedPMCPDF
- 18. Silva GF, Tanomaru-Filho M, Bernardi MI, Guerreiro-Tanomaru JM, Cerri PS. Niobium pentoxide as radiopacifying agent of calcium silicate-based material: evaluation of physicochemical and biological properties. Clin Oral Investig 2015;19:2015-2025.ArticlePubMedPDF
- 19. da Fonseca TS, da Silva GF, Tanomaru-Filho M, Sasso-Cerri E, Guerreiro-Tanomaru JM, Cerri PS. In vivo evaluation of the inflammatory response and IL-6 immunoexpression promoted by biodentine and MTA Angelus. Int Endod J 2016;49:145-153.PubMed
- 20. Saraiva JA, da Fonseca TS, da Silva GF, Sasso-Cerri E, Guerreiro-Tanomaru JM, Tanomaru-Filho M, Cerri PS. Reduced interleukin-6 immunoexpression and birefringent collagen formation indicate that MTA Plus and MTA Fillapex are biocompatible. Biomed Mater 2018;13:035002.ArticlePubMedPDF
- 21. de Pizzol Júnior JP, Sasso-Cerri E, Cerri PS. Matrix metalloproteinase-1 and acid phosphatase in the degradation of the lamina propria of eruptive pathway of rat molars. Cells 2018;7:e206.
- 22. Silva GF, Guerreiro-Tanomaru JM, da Fonseca TS, Bernardi MIB, Sasso-Cerri E, Tanomaru-Filho M, Cerri PS. Zirconium oxide and niobium oxide used as radiopacifiers in a calcium silicate-based material stimulate fibroblast proliferation and collagen formation. Int Endod J 2017;50(Supplement 2):e95-e108.ArticlePubMedPDF
- 23. Hoshino RA, Silva GF, Delfino MM, Guerreiro-Tanomaru JM, Tanomaru-Filho M, Sasso-Cerri E, Filho IB, Cerri PS. Physical properties, antimicrobial activity and in vivo tissue response to Apexit Plus. Materials (Basel) 2020;13:E1171.Article
- 24. Holland R, de Souza V, Nery MJ, Otoboni Filho JA, Bernabé PF, Dezan Júnior E. Reaction of rat connective tissue to implanted dentin tubes filled with mineral trioxide aggregate or calcium hydroxide. J Endod 1999;25:161-166.ArticlePubMed
- 25. Gomes-Filho JE, Watanabe S, Bernabé PF, de Moraes Costa MT. A mineral trioxide aggregate sealer stimulated mineralization. J Endod 2009;35:256-260.ArticlePubMed
- 26. Arias-Moliz MT, Camilleri J. The effect of the final irrigant on the antimicrobial activity of root canal sealers. J Dent 2016;52:30-36.ArticlePubMed
- 27. Li Y, Chi L, Stechschulte DJ, Dileepan KN. Histamine-induced production of interleukin-6 and interleukin-8 by human coronary artery endothelial cells is enhanced by endotoxin and tumor necrosis factor-alpha. Microvasc Res 2001;61:253-262.PubMed
- 28. de Oliveira PA, de Pizzol-Júnior JP, Longhini R, Sasso-Cerri E, Cerri PS. Cimetidine reduces interleukin-6, matrix metalloproteinases-1 and -9 immunoexpression in the gingival mucosa of rat molars with induced periodontal disease. J Periodontol 2017;88:100-111.ArticlePubMed
- 29. Kang S, Tanaka T, Narazaki M, Kishimoto T. Targeting interleukin-6 signaling in clinic. Immunity 2019;50:1007-1023.ArticlePubMed
- 30. Hashizume M, Mihara M. The roles of interleukin-6 in the pathogenesis of rheumatoid arthritis. Arthritis (Egypt) 2011;2011:765624.ArticlePubMedPMCPDF
- 31. Silva EJ, Rosa TP, Herrera DR, Jacinto RC, Gomes BP, Zaia AA. Evaluation of cytotoxicity and physicochemical properties of calcium silicate-based endodontic sealer MTA Fillapex. J Endod 2013;39:274-277.ArticlePubMed
- 32. James SK, Oldgren J, Lindbäck J, Johnston N, Siegbahn A, Wallentin L. An acute inflammatory reaction induced by myocardial damage is superimposed on a chronic inflammation in unstable coronary artery disease. Am Heart J 2005;149:619-626.ArticlePubMed
- 33. Narazaki M, Tanaka T, Kishimoto T. The role and therapeutic targeting of IL-6 in rheumatoid arthritis. Expert Rev Clin Immunol 2017;13:535-551.ArticlePubMed
- 34. Noh MK, Jung M, Kim SH, Lee SR, Park KH, Kim DH, Kim HH, Park YG. Assessment of IL-6, IL-8 and TNF-α levels in the gingival tissue of patients with periodontitis. Exp Ther Med 2013;6:847-851.ArticlePubMedPMC
- 35. Poggio C, Riva P, Chiesa M, Colombo M, Pietrocola G. Comparative cytotoxicity evaluation of eight root canal sealers. J Clin Exp Dent 2017;9:e574-e578.ArticlePubMedPMC
- 36. Vitti RP, Prati C, Silva EJ, Sinhoreti MA, Zanchi CH, de Souza e Silva MG, Ogliari FA, Piva E, Gandolfi MG. Physical properties of MTA Fillapex sealer. J Endod 2013;39:915-918.ArticlePubMed
- 37. Yaltirik M, Ozbas H, Bilgic B, Issever H. Reactions of connective tissue to mineral trioxide aggregate and amalgam. J Endod 2004;30:95-99.ArticlePubMed
- 38. Cintra LT, Ribeiro TA, Gomes-Filho JE, Bernabé PF, Watanabe S, Facundo AC, Samuel RO, Dezan-Júnior E. Biocompatibility and biomineralization assessment of a new root canal sealer and root-end filling material. Dent Traumatol 2013;29:145-150.ArticlePubMed
- 39. Bueno CR, Valentim D, Marques VA, Gomes-Filho JE, Cintra LT, Jacinto RC, Dezan-Junior E. Biocompatibility and biomineralization assessment of bioceramic-, epoxy-, and calcium hydroxide-based sealers. Braz Oral Res 2016;30:e81.Article
REFERENCES
Tables & Figures
REFERENCES
Citations

- Retrievability of NeoMTA 2 vs AH Plus Sealer from Retreated Mesial Canals of Mandibular First Molars: A Microcomputed Tomography Ex Vivo Study
Mey A Al-Habib, Mona Alsulaiman
The Journal of Contemporary Dental Practice.2025; 26(5): 493. CrossRef - Effect of calcium silicate-based repair sealers on bone healing in rat skull defects: histological and histomorphometric study
J. M. Sauer, C. E. S. Bueno, R. A. Pelegrine, C. E. Fontana, E. F. Martinez, P. G. Montagner, W. M. Nascimento, A. G. S. Limoeiro, D. G. P. Rocha, M. F. V. Marceliano-Alves, M. P. W. Galhardi, M. Klymus, A. S. Martin
Endodontics Today.2025; 23(3): 433. CrossRef - Biocompatibility and bioactivity of bioceramic endodontic sealer: NeoSealer Flo
Evelin Carine Alves SILVA, Jéssica Arielli PRADELLI, Guilherme Ferreira da SILVA, Paulo Sérgio CERRI, Mario TANOMARU-FILHO, Juliane Maria GUERREIRO-TANOMARU
Journal of Applied Oral Science.2025;[Epub] CrossRef - The osteoinductive potential of different root-end filling materials in a rat femur model
Seçkin Aksu, Ebru Delikan, Ayşe Özcan Küçük, Zehra Demiray Asoğlu, Şakir Necat Yılmaz
Scientific Reports.2024;[Epub] CrossRef - Clinical outcomes of nonsurgical root canal treatment using NeoSealer Flo and Endosequence BC Sealer: A retrospective analysis with short-term follow-up
Christian Lepure, Ryan M. Walsh, Sayeed Attar, Casey L. Turner, Joshua Crawford, Poorya Jalali
Clinical Oral Investigations.2024;[Epub] CrossRef - Biocompatibility and bioactive potential of NeoPUTTY calcium silicate‐based cement: An in vivo study in rats
Evelin Carine Alves Silva, Jéssica Arielli Pradelli, Guilherme Ferreira da Silva, Paulo Sérgio Cerri, Mario Tanomaru‐Filho, Juliane Maria Guerreiro‐Tanomaru
International Endodontic Journal.2024; 57(6): 713. CrossRef - Carbon Nanotubes Induce Mineralization of Human Cementoblasts
Ting-Hsuan Wang, Kiyoko Watanabe, Koichiro Muromachi, Nobushiro Hamada, Nobuyuki Tani-Ishii
Journal of Endodontics.2024; 50(8): 1117. CrossRef - Tissue repair capacity of bioceramic endodontic sealers in rat subcutaneous tissue
George Sampaio Bonates dos Santos, Ceci Nunes Carvalho, Rudys Rodolfo de Jesus Tavares, Paulo Goberlânio de Barros Silva, George Táccio de Miranda Candeiro, Etevaldo Matos Maia Filho
Brazilian Dental Journal.2023; 34(3): 25. CrossRef - Participation of fibroblast growth factor‐1 and interleukin‐10 in connective tissue repair following subcutaneous implantation of bioceramic materials in rats
Mateus Machado Delfino, José Leandro de Abreu Jampani, Camila Soares Lopes, Juliane Maria Guerreiro‐Tanomaru, Mário Tanomaru‐Filho, Estela Sasso‐Cerri, Paulo Sérgio Cerri
International Endodontic Journal.2023; 56(3): 385. CrossRef - Biocompatibility and bioactive potential of an experimental tricalcium silicate‐based cement in comparison with Bio‐C repair and MTA Repair HP materials
Marcela Borsatto Queiroz, Rafaela N. H. Inada, José Leandro de Abreu Jampani, Juliane Maria Guerreiro‐Tanomaru, Estela Sasso‐Cerri, Mário Tanomaru‐Filho, Paulo Sérgio Cerri
International Endodontic Journal.2023; 56(2): 259. CrossRef - Calcium Silicate-Based Sealer Dentinal Tubule Penetration—A Systematic Review of In Vitro Studies
Israa Ashkar, José Luis Sanz, Leopoldo Forner, María Melo
Materials.2023; 16(7): 2734. CrossRef - Bioactivity Potential of Bioceramic-Based Root Canal Sealers: A Scoping Review
Mauro Schmitz Estivalet, Lucas Peixoto de Araújo, Felipe Immich, Adriana Fernandes da Silva, Nadia de Souza Ferreira, Wellington Luiz de Oliveira da Rosa, Evandro Piva
Life.2022; 12(11): 1853. CrossRef - Tricalcium silicate cement sealers
Anita Aminoshariae, Carolyn Primus, James C. Kulild
The Journal of the American Dental Association.2022; 153(8): 750. CrossRef - Bioactive potential of Bio‐C Pulpo is evidenced by presence of birefringent calcite and osteocalcin immunoexpression in the rat subcutaneous tissue
Marcela Borsatto Queiroz, Rafaela Nanami Handa Inada, Camila Soares Lopes, Juliane Maria Guerreiro‐Tanomaru, Estela Sasso‐Cerri, Mário Tanomaru‐Filho, Paulo Sérgio Cerri
Journal of Biomedical Materials Research Part B: Applied Biomaterials.2022; 110(10): 2369. CrossRef - An Updated Review on Properties and Indications of Calcium Silicate‐Based Cements in Endodontic Therapy
Fateme Eskandari, Alireza Razavian, Rozhina Hamidi, Khadije Yousefi, Susan Borzou, Zohaib Khurshid
International Journal of Dentistry.2022;[Epub] CrossRef
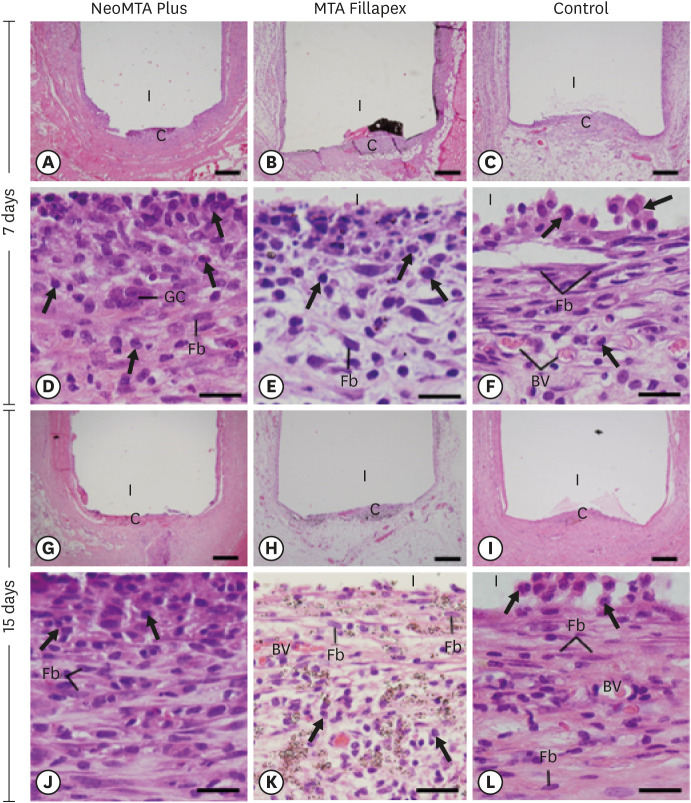
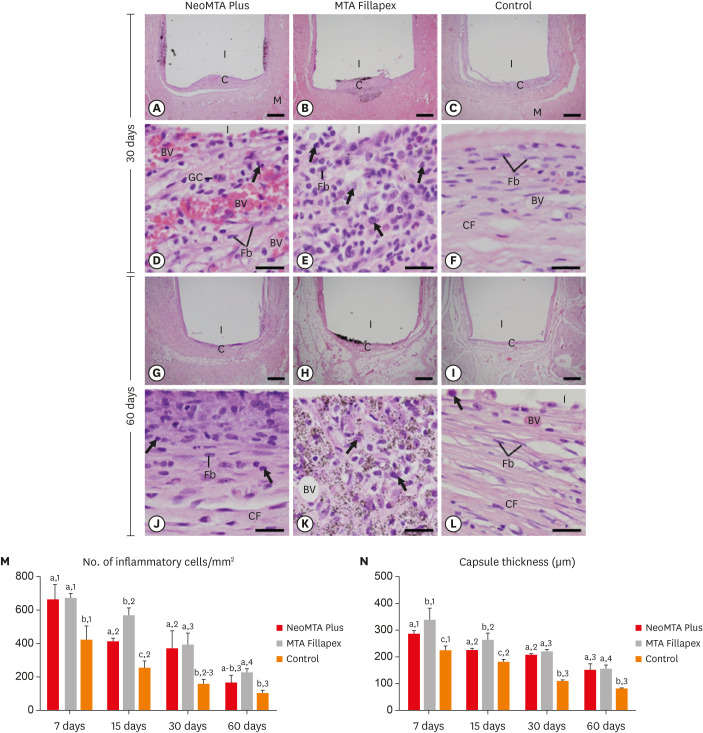
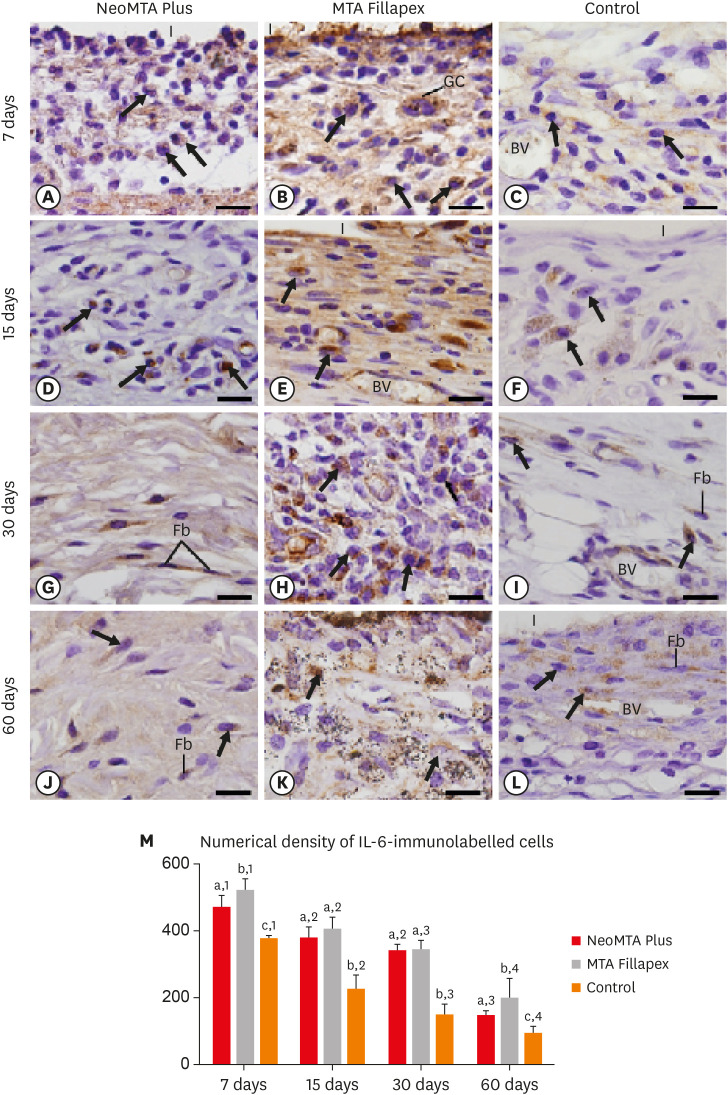
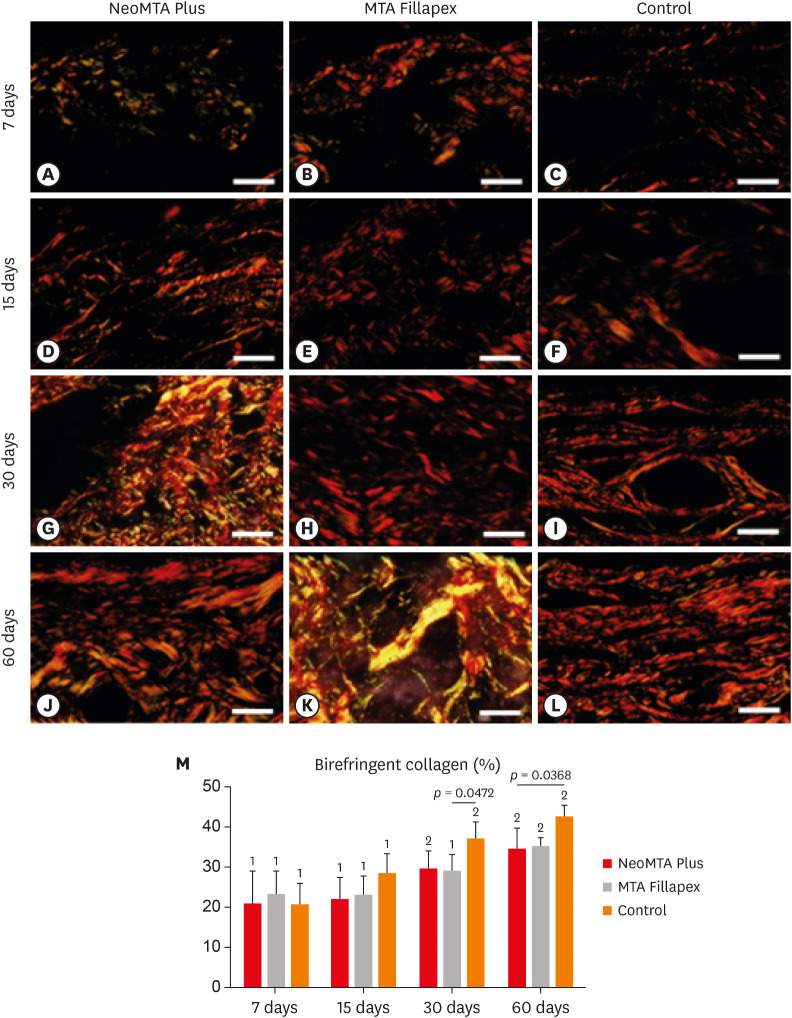
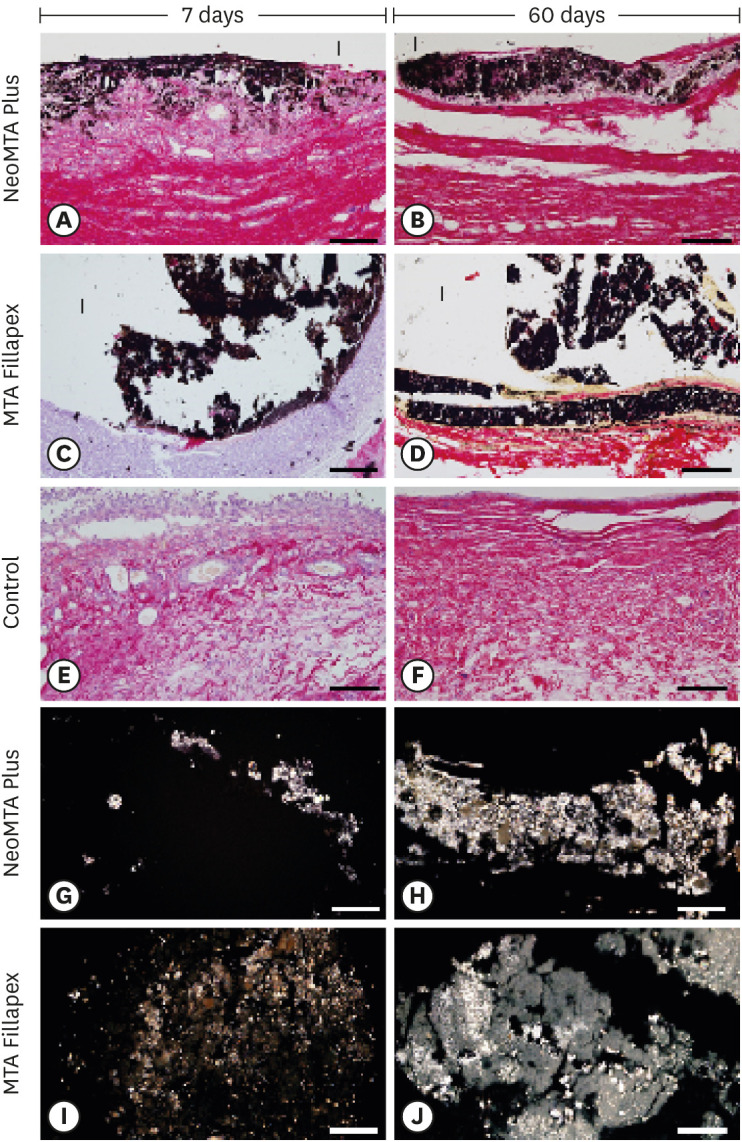
Figure 1
Figure 2
Figure 3
Figure 4
Figure 5
Manufacturers, chemical components, and proportions of the root canal sealers
| Root canal sealers | Manufacturer and chemical composition | Powder-liquid ratio |
|---|---|---|
| NeoMTA Plus | • Avalon Biomed Inc., Bradenton, FL, USA. | One scoop of powder (1 g) and 3 drops of water-based gel (350 µL) |
| • Powder: Tricalcium silicate, dicalcium silicate, tantalum oxide, tricalcium aluminate and calcium sulphate. | ||
| • Liquid: water-based gel with thickener agents and water soluble polymers. | ||
| MTA Fillapex | • Angelus Dental Industry S/A, Lindóia, SP, Brazil. | Two pastes mixed with same proportion |
| • Paste: salicylate resin, diluting resin, natural resin, calcium tungstate, nanoparticulated silica, MTA, pigments. |
MTA, mineral trioxide aggregate.
MTA, mineral trioxide aggregate.

 KACD
KACD
 ePub Link
ePub Link Cite
Cite

