Articles
- Page Path
- HOME > Restor Dent Endod > Volume 46(1); 2021 > Article
- Research Article Interface between calcium silicate cement and adhesive systems according to adhesive families and cement maturation
-
Nelly Pradelle-Plasse1,2,3
 , Caroline Mocquot1,2,3
, Caroline Mocquot1,2,3 , Katherine Semennikova1,2,3
, Katherine Semennikova1,2,3 , Pierre Colon1,2,3
, Pierre Colon1,2,3 , Brigitte Grosgogeat3,4,5
, Brigitte Grosgogeat3,4,5
-
Restor Dent Endod 2020;46(1):e3.
DOI: https://doi.org/10.5395/rde.2021.46.e3
Published online: December 9, 2020
1Department of Conservative Dentistry - Endodontics, Faculty of Dental Surgery, University of Paris, Paris, France.
2Rothschild Hospital, Assistance Publique Hôpitaux de Paris, Paris, France.
3Multimaterials and Interfaces Laboratory (UMR 5615), Biomaterials Team, Villeurbanne, France.
4Department of Biomaterials, Faculty of Dental Surgery, University of Lyon 1, Lyon, France.
5Hospices Civils de Lyon, Lyon, France.
- Correspondence to Nelly Pradelle-Plasse, DDS, MS, PhD. Associate Professor, Department of Conservative Dentistry - Endodontics, Faculty of Dental Surgery, University of Paris, 5 Rue Garancière, Paris 75006, France. nelly.pradelle@univ-paris-diderot.fr
Copyright © 2021. The Korean Academy of Conservative Dentistry
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Objectives This study aimed to evaluate the interface between a calcium silicate cement (CSC), Biodentine and dental adhesives in terms of sealing ability.
-
Materials and Methods Microleakage test: 160 standardized class II cavities were prepared on 80 extracted human molars. The cavities were filled with Biodentine and then divided into 2 experimental groups according to the time of restoration: composite resin obturation 15 minutes after Biodentine handling (D0); restoration after 7 days (D7). Each group was then divided into 8 subgroups (n = 5) according to the adhesive system used: etch-and-rinse adhesive (Prime & Bond); self-etch adhesive 2 steps (Optibond XTR and Clearfil SE Bond); self-etch adhesive 1 step (Xeno III, G-aenial Bond, and Clearfil Tri-S Bond); and universal used as etch-and-rinse or self-etch (ScotchBond Universal ER or SE). After thermocycling, the teeth were immersed in a silver nitrate solution, stained, longitudinally sectioned, and the Biodentine/adhesive percolation was quantified. Scanning electron microscopic observations: Biodentine/adhesive interfaces were observed.
-
Results A tendency towards less microleakage was observed when Biodentine was etched (2.47%) and when restorations were done without delay (D0: 4.31%, D7: 6.78%), but this was not significant. The adhesives containing 10-methacryloyloxydecyl dihydrogen phosphate monomer showed the most stable results at both times studied. All Biodentine/adhesive interfaces were homogeneous and regular.
-
Conclusions The good sealing of the CSC/adhesive interface is not a function of the system adhesive family used or the cement maturation before restoration. Biodentine can be used as a dentine substitute.
INTRODUCTION
MATERIALS AND METHODS
1. Materials used
Materials used in this study
2. Tooth collection
3. Artificial saliva
1. Microleakage method
1) Cavity preparation
2) Filling
3) Thermocycling
4) Dye immersion
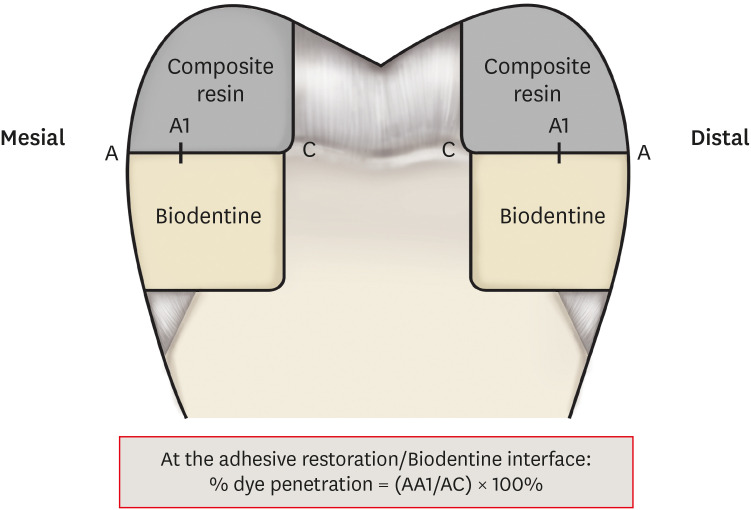
Tooth section: on the left, the arrow shows silver nitrate penetration along the interface; on the right no penetration.

5) Statistical analysis
2. Scanning electron microscope (SEM) methodology
RESULTS
1. Experimental group D0
Percentage of percolation according to the adhesive system used for both time points
Intergroup analysis at D0
2. Experimental group D7
Intergroup analysis at D7
3. Comparison between experimental groups D0 and D7 according to the adhesive system investigated
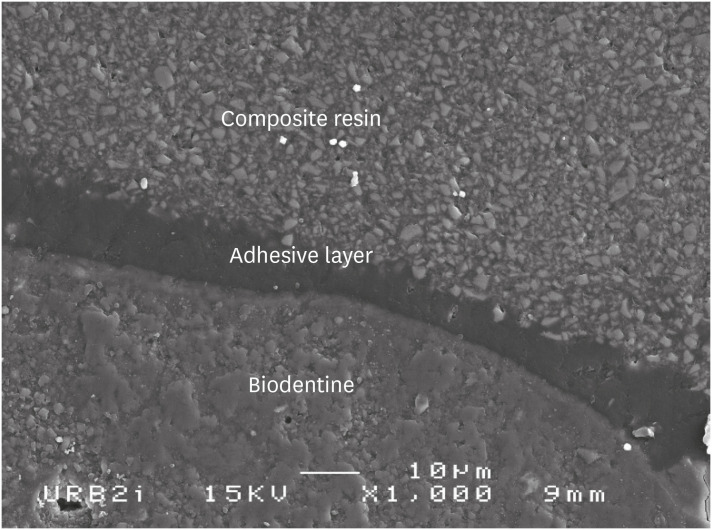
Scanning electron microscopic (SEM) observation of Biodentine/2 steps self-etch system (Clearfil SE Bond) interface (×1,000).
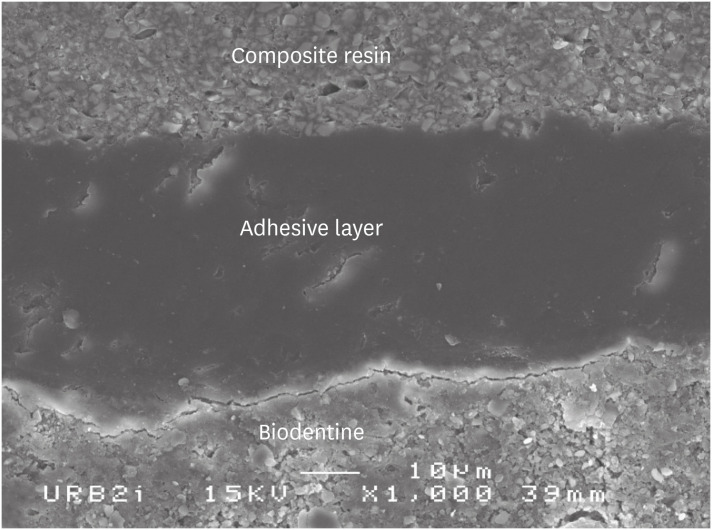
Scanning electron microscopic (SEM) observation of Biodentine/one step self-etch system (Clearfil Tri-S Bond) interface (×1,000).
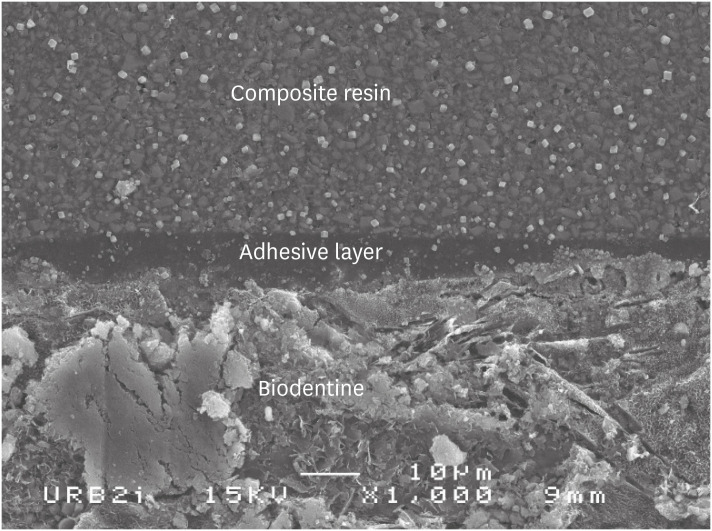
Scanning electron microscopic (SEM) observation of Biodentine/universal system used as one step self-etch system (Scotchbond Universal) interface (×1,000).
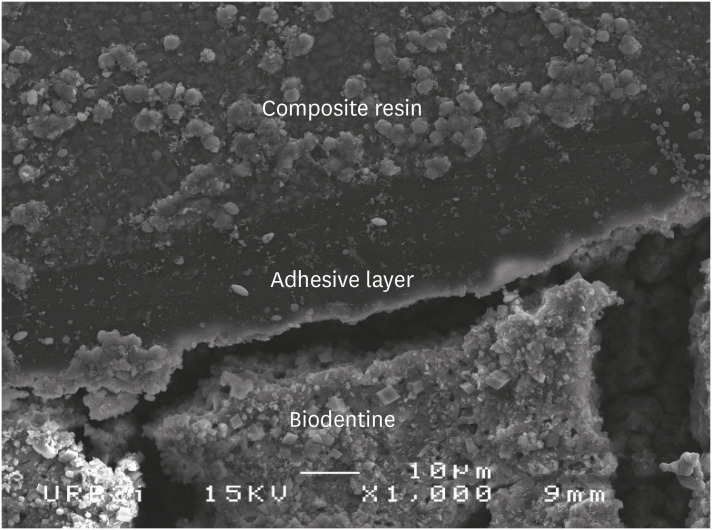
Scanning electron microscopic (SEM) observation of Biodentine/universal system used as etch-and-rinse system (Scotchbond Universal) interface (×1,000).
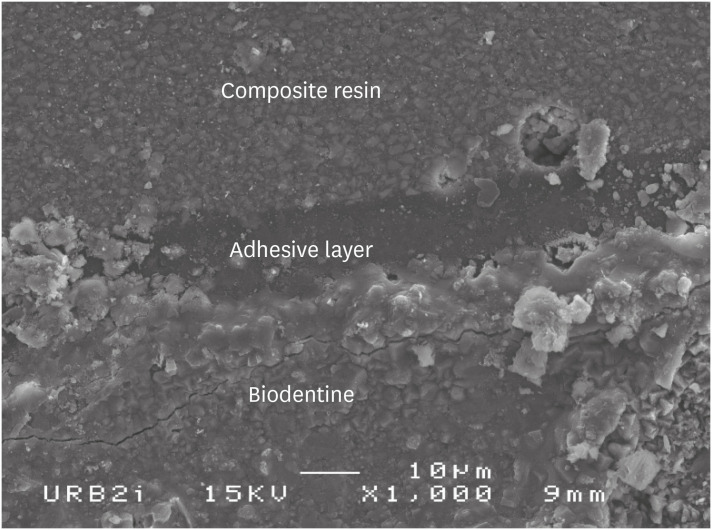
Scanning electron microscopic (SEM) observation of Biodentine/etch-and-rinse system (Prime & Bond) interface (×1,000).
DISCUSSION
CONCLUSIONS
ACKNOWLEDGEMENTS
-
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
-
Author Contributions:
Conceptualization: Pradelle-Plasse N, Mocquot C, Semennikova K, Colon P, Grosgogeat B.
Data curation: Pradelle-Plasse N, Mocquot C, Semennikova K, Colon P, Grosgogeat B.
Formal analysis: Pradelle-Plasse N, Semennikova K.
Investigation: Pradelle-Plasse N, Mocquot C, Semennikova K.
Methodology: Pradelle-Plasse N, Mocquot C, Semennikova K, Colon P, Grosgogeat B.
Project administration: Pradelle-Plasse N, Colon P, Grosgogeat B.
Resources: Pradelle-Plasse N, Mocquot C, Semennikova K.
Supervision: Pradelle-Plasse N, Colon P, Grosgogeat B.
Validation: Pradelle-Plasse N, Colon P, Grosgogeat B.
Visualization: Pradelle-Plasse N, Colon P, Grosgogeat B.
Writing - original draft: Pradelle-Plasse N.
Writing - review & editing: Pradelle-Plasse N.
- 1. Bachoo IK, Seymour D, Brunton P. A biocompatible and bioactive replacement for dentine: Is this a reality? The properties and uses of a novel calcium-based cement. Br Dent J 2013;214:E5.ArticlePubMedPDF
- 2. Torabinejad M, Hong CU, McDonald F, Pitt Ford TR. Physical and chemical properties of a new root-end filling material. J Endod 1995;21:349-353.ArticlePubMed
- 3. Schwartz RS, Mauger M, Clement DJ, Walker WA 3rd. Mineral trioxide aggregate: a new material for endodontics. J Am Dent Assoc 1999;130:967-975.ArticlePubMed
- 4. Torabinejad M, Parirokh M. Mineral trioxide aggregate: a comprehensive literature review--part II: leakage and biocompatibility investigations. J Endod 2010;36:190-202.ArticlePubMed
- 5. Sarkar NK, Caicedo R, Ritwik P, Moiseyeva R, Kawashima I. Physicochemical basis of the biologic properties of mineral trioxide aggregate. J Endod 2005;31:97-100.ArticlePubMed
- 6. Bozeman TB, Lemon RR, Eleazer PD. Elemental analysis of crystal precipitate from gray and white MTA. J Endod 2006;32:425-428.ArticlePubMed
- 7. Watson TF, Atmeh AR, Sajini S, Cook RJ, Festy F. Present and future of glass-ionomers and calcium-silicate cements as bioactive materials in dentistry: biophotonics-based interfacial analyses in health and disease. Dent Mater 2014;30:50-61.ArticlePubMedPMC
- 8. Atmeh AR, Chong EZ, Richard G, Festy F, Watson TF. Dentin-cement interfacial interaction: calcium silicates and polyalkenoates. J Dent Res 2012;91:454-459.ArticlePubMedPMCPDF
- 9. Pradelle-Plasse N, Tran X, Colon P. Biocompatibility or cytotoxic effects of dental composites. Oxfordshire: Coxmoor Publishing Company; 2009.
- 10. Villat C, Tran XV, Pradelle-Plasse N, Ponthiaux P, Wenger F, Grosgogeat B, Colon P. Impedance methodology: a new way to characterize the setting reaction of dental cements. Dent Mater 2010;26:1127-1132.ArticlePubMed
- 11. Koubi G, Colon P, Franquin JC, Hartmann A, Richard G, Faure MO, Lambert G. Clinical evaluation of the performance and safety of a new dentine substitute, Biodentine, in the restoration of posterior teeth - a prospective study. Clin Oral Investig 2013;17:243-249.ArticlePubMed
- 12. Bergenholtz G, Cox CF, Loesche WJ, Syed SA. Bacterial leakage around dental restorations: its effect on the dental pulp. J Oral Pathol 1982;11:439-450.ArticlePubMed
- 13. Browne RM, Tobias RS. Microbial microleakage and pulpal inflammation: a review. Endod Dent Traumatol 1986;2:177-183.ArticlePubMed
- 14. Camilleri J. Investigation of Biodentine as dentine replacement material. J Dent 2013;41:600-610.ArticlePubMed
- 15. Meraji N, Camilleri J. Bonding over dentin replacement materials. J Endod 2017;43:1343-1349.ArticlePubMed
- 16. Hashem DF, Foxton R, Manoharan A, Watson TF, Banerjee A. The physical characteristics of resin composite-calcium silicate interface as part of a layered/laminate adhesive restoration. Dent Mater 2014;30:343-349.ArticlePubMed
- 17. Cengiz E, Ulusoy N. Microshear bond strength of tri-calcium silicate-based cements to different restorative materials. J Adhes Dent 2016;18:231-237.PubMed
- 18. Çolak H, Tokay U, Uzgur R, Uzgur Z, Ercan E, Hamidi MM. The effect of different adhesives and setting times on bond strength between Biodentine and composite. J Appl Biomater Funct Mater 2016;14:e217-e222.PubMed
- 19. Odabaş ME, Bani M, Tirali RE. Shear bond strengths of different adhesive systems to Biodentine. ScientificWorldJournal 2013;2013:626103.PubMedPMC
- 20. Abraham SB, Gaintantzopoulou MD, Eliades G. Cavity adaptation of water-based restoratives placed as liners under a resin composite. Int J Dent 2017;2017:5957107.ArticlePubMedPMCPDF
- 21. Aggarwal V, Singla M, Yadav S, Yadav H, Ragini . Marginal adaptation evaluation of Biodentine and MTA plus in ‘open sandwich’ class II restorations. J Esthet Restor Dent 2015;27:167-175.ArticlePubMedPDF
- 22. Raskin A, Eschrich G, Dejou J, About I. In vitro microleakage of Biodentine as a dentin substitute compared to Fuji II LC in cervical lining restorations. J Adhes Dent 2012;14:535-542.PubMed
- 23. Besnault C, Attal JP. Simulated oral environment and microleakage of class II resin-based composite and sandwich restorations. Am J Dent 2003;16:186-190.PubMed
- 24. Gale MS, Darvell BW. Thermal cycling procedures for laboratory testing of dental restorations. J Dent 1999;27:89-99.ArticlePubMed
- 25. Cervino G, Fiorillo L, Spagnuolo G, Bramanti E, Laino L, Lauritano F, Cicciù M. Interface between MTA and dental bonding agents: scanning electron microscope evaluation. J Int Soc Prev Community Dent 2017;7:64-68.ArticlePubMedPMC
- 26. Garrault S, Behr T, Nonat A. Formation of the C-S-H layer during early hydration of tricalcium silicate grains with different sizes. J Phys Chem B 2006;110:270-275.ArticlePubMed
- 27. Rengo C, Goracci C, Juloski J, Chieffi N, Giovannetti A, Vichi A, Ferrari M. Influence of phosphoric acid etching on microleakage of a self-etch adhesive and a self-adhering composite. Aust Dent J 2012;57:220-226.ArticlePubMed
- 28. Kayahan MB, Nekoofar MH, Kazandağ M, Canpolat C, Malkondu O, Kaptan F, Dummer PM. Effect of acid-etching procedure on selected physical properties of mineral trioxide aggregate. Int Endod J 2009;42:1004-1014.ArticlePubMed
- 29. Atabek D, Sillelioğlu H, Olmez A. Bond strength of adhesive systems to mineral trioxide aggregate with different time intervals. J Endod 2012;38:1288-1292.ArticlePubMed
- 30. Silva e Souza MH Jr, Carneiro KG, Lobato MF, Silva e Souza PA, de Góes MF. Adhesive systems: important aspects related to their composition and clinical use. J Appl Oral Sci 2010;18:207-214.ArticlePubMedPMC
- 31. Karaman E, Yazici AR, Aksoy B, Karabulut E, Ozgunaltay G, Dayangac B. Effect of operator variability on microleakage with different adhesive systems. Eur J Dent 2013;7:S060-S065.ArticlePubMed
- 32. Palma PJ, Marques JA, Falacho RI, Vinagre A, Santos JM, Ramos JC. Does delayed restoration improve shear bond strength of different restorative protocols to calcium silicate-based cements? Materials (Basel) 2018;11:2216.ArticlePubMedPMC
- 33. Sultana N, Nawal RR, Chaudhry S, Sivakumar M, Talwar S. Effect of acid etching on the micro-shear bond strength of resin composite-calcium silicate interface evaluated over different time intervals of bond aging. J Conserv Dent 2018;21:194-197.ArticlePubMedPMC
- 34. Yoshioka M, Yoshida Y, Inoue S, Lambrechts P, Vanherle G, Nomura Y, Okazaki M, Shintani H, Van Meerbeek B. Adhesion/decalcification mechanisms of acid interactions with human hard tissues. J Biomed Mater Res 2002;59:56-62.ArticlePubMed
- 35. Yoshida Y, Nagakane K, Fukuda R, Nakayama Y, Okazaki M, Shintani H, Inoue S, Tagawa Y, Suzuki K, De Munck J, Van Meerbeek B. Comparative study on adhesive performance of functional monomers. J Dent Res 2004;83:454-458.ArticlePubMedPDF
- 36. Carrilho E, Cardoso M, Marques Ferreira M, Marto CM, Paula A, Coelho AS. 10-MDP based dental adhesives: adhesive interface characterization and adhesive stability-a systematic review. Materials (Basel) 2019;12:790.ArticlePubMedPMC
- 37. Yoshihara K, Yoshida Y, Nagaoka N, Hayakawa S, Okihara T, De Munck J, Maruo Y, Nishigawa G, Minagi S, Osaka A, Van Meerbeek B. Adhesive interfacial interaction affected by different carbon-chain monomers. Dent Mater 2013;29:888-897.ArticlePubMed
- 38. Chatel E, Pradelle-Plasse N, Colon P, Grosgogeat B. Degradation mechanisms of modern self–etching adhesives. Clin Oral Investig 2013;17:1035.
- 39. Koubi S, Elmerini H, Koubi G, Tassery H, Camps J. Quantitative evaluation by glucose diffusion of microleakage in aged calcium silicate-based open-sandwich restorations. Inter J Dent 2012;2012:105863.ArticlePDF
- 40. Aksel H, Küçükkaya Eren S, Askerbeyli Õrs S, Karaismailoğlu E. Surface and vertical dimensional changes of mineral trioxide aggregate and Biodentine in different environmental conditions. J Appl Oral Sci 2018;27:e20180093.ArticlePubMedPMC
- 41. Agrafioti A, Tzimpoulas N, Chatzitheodoridis E, Kontakiotis EG. Comparative evaluation of sealing ability and microstructure of MTA and Biodentine after exposure to different environments. Clin Oral Investig 2016;20:1535-1540.ArticlePubMedPDF
REFERENCES
Tables & Figures
REFERENCES
Citations

- Comparison of compressive strength, surface microhardness, and surface microstructure of different types of bioceramics following varying surface treatments
Zeynep Hale Keleş, Vasfiye Işık, Soner Sismanoglu
Journal of the Australian Ceramic Society.2025;[Epub] CrossRef - Effect of Er Cr YSGG laser etching procedure on the bond strength of different calcium silicate cements
Yesim Sesen Uslu, Hakan Yasin Gönder, Pinar Sesen, Gizem Gunduz Bektaş
Lasers in Dental Science.2024;[Epub] CrossRef - Managing Cracked Teeth with Root Extension: A Prospective Preliminary Study Using Biodentine™ Material
Kênia Maria Soares de Toubes, Isabella Sousa Corrêa, Regina Célia Lopes Valadares, Stephanie Quadros Tonelli, Fábio Fernandes Borém Bruzinga, Frank Ferreira Silveira, Dr Karthikeyan Ramalingam
International Journal of Dentistry.2024;[Epub] CrossRef - In Vitro Resistance of Natural Molars vs. Additive-Manufactured Simulators Treated with Pulpotomy and Endocrown
Marie-Laure Munoz-Sanchez, Alexis Gravier, Olivier Francois, Emmanuel Nicolas, Martine Hennequin, Nicolas Decerle
Journal of Functional Biomaterials.2023; 14(9): 444. CrossRef - Characterisation of the calcium silicate‐based cement–composite interface and the bonding strength with total‐etch or single/two‐stage self‐etch adhesive systems
Abidin Talha Mutluay, Merve Mutluay
Australian Endodontic Journal.2022; 48(3): 501. CrossRef - Bond Strength of Adhesive Systems to Calcium Silicate-Based Materials: A Systematic Review and Meta-Analysis of In Vitro Studies
Louis Hardan, Davide Mancino, Rim Bourgi, Alejandra Alvarado-Orozco, Laura Emma Rodríguez-Vilchis, Abigailt Flores-Ledesma, Carlos Enrique Cuevas-Suárez, Monika Lukomska-Szymanska, Ammar Eid, Maya-Line Danhache, Maryline Minoux, Youssef Haïkel, Naji Kharo
Gels.2022; 8(5): 311. CrossRef
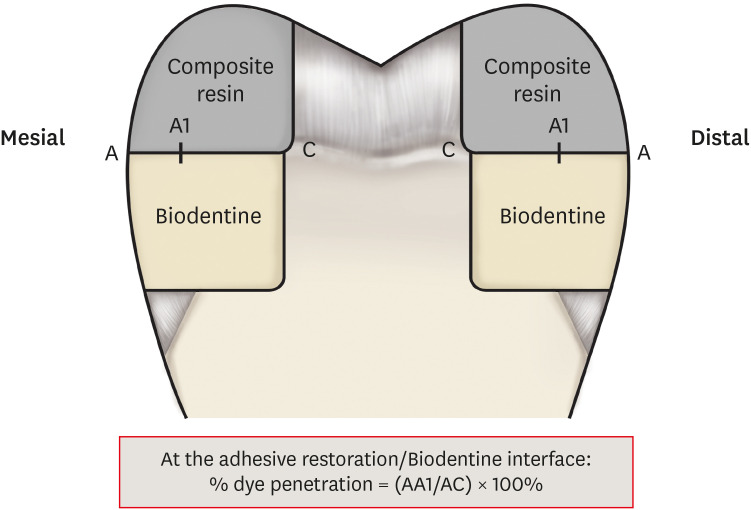

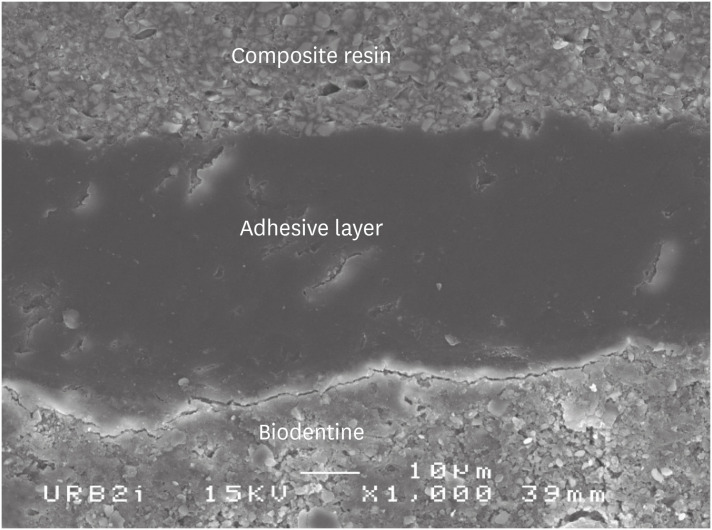
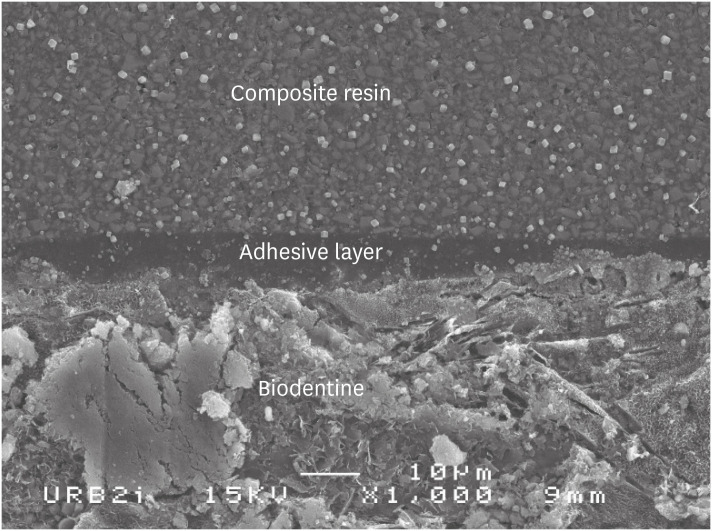
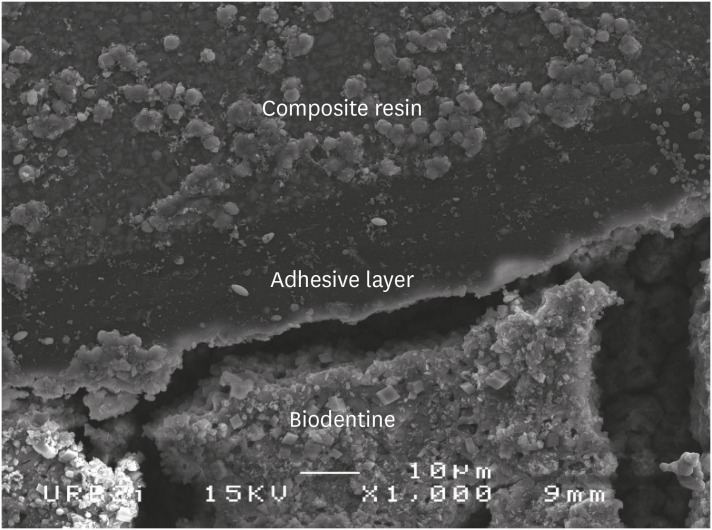
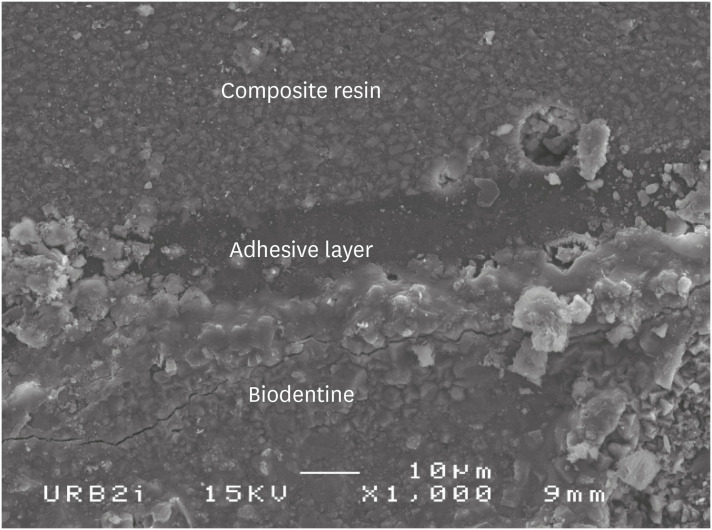
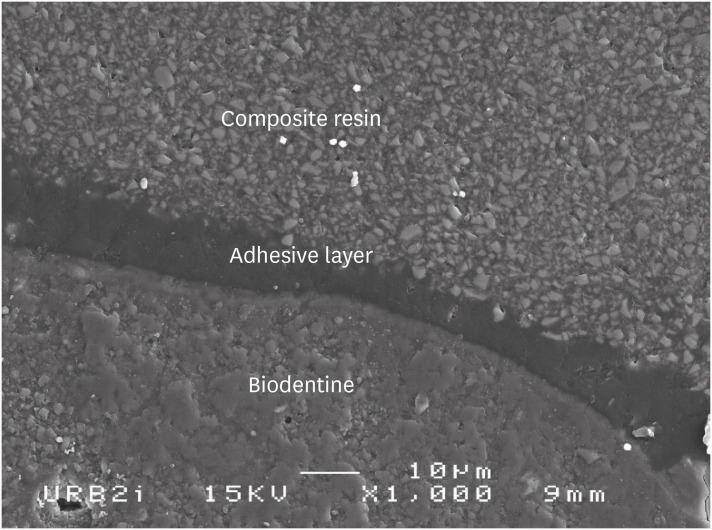
Figure 1
Figure 2
Figure 3
Figure 4
Figure 5
Figure 6
Figure 7
Materials used in this study
| Family of material | Material | Components | pH | Protocol | ||
|---|---|---|---|---|---|---|
| Calcium silicate | Biodentine (Septodont, St. Maur-des-Fossés, France) | Solid: tricalcium silicate (> 80%), calcium carbonate, zirconium oxide | 12.5 | 1. Incorporate 5 drops of liquid in the powder. | ||
| Liquid: water, CaCl2, partially modified polycarboxylate | 2. Close the capsule and put it 30 sec in the oscillator. | |||||
| Adhesive system | ||||||
| Self-etch 2 steps | Optibond XTR (Kerr, Orange, CA, USA; batch: LE03355) | Primer: GPDM, HEMA, water, acetone, ethanol, camphorquinone | Primer: 2.4 | 1. Apply primer to enamel/dentin using scrubbing motion (20 sec). | ||
| Bonding: HEMA, MEHQ, ethanol, camphorquinone, loads of silica | Bond: 3.3 | 2. Air thin with medium air pressure (5 sec). | ||||
| 3. Shake adhesive briefly, and apply to enamel/dentin surface using light brushing motion (15 sec). | ||||||
| 4. Air thin with medium air pressure, and then strong air for at least 5 sec. | ||||||
| 5. Light-cure (10 sec). | ||||||
| Clearfil SE Bond (Kuraray, Okayama, Japan; batch: 041838) | Primer: 10-MDP, HEMA, dimethacrylate aliphatque absorbent, camphorquinone, N-diethanol-p-toluidine, water | 2.0 | 1. Apply primer and leave for 20 sec. | |||
| Bonding:10-MDP, Bis-GMA, HEMA, dimethacrylate aliphatic hydrophobic subject, camphoroquinone, N-diethanol-p-toluidine, loads of silica | 2. Do not rinse and dry with mild air flow. | |||||
| 3. Apply bond and distribute evenly with mild air flow. | ||||||
| 4. Light-cure for 10 sec. | ||||||
| Self-etch 1 step | Xeno III (Dentsply/Caulk, Mildford, DE, USA; batch: 1212000562) | Liquid A: HEMA, water purified, ethanol, BHT, silicon dioxide | < 1.0 | 1. Apply generous amounts of self-etch adhesive to wet all cavity surfaces thoroughly. | ||
| Liquid B: Pyro-EMA, PEM-F, urethan dimethacrylate, BHT, camphorquinone, ethyl-4-Dimethylaminobenzoate | 2. Leave undisturbed for at least 20 sec. | |||||
| 3. Uniformly spread the adhesive using a gentle stream of oil free air for at least 2 sec. | ||||||
| 4. Cure the adhesive with a light-curing unit for at least 10 sec. | ||||||
| G-aenial Bond (GC, Tokyo, Japan; batch: 1301161) | 4-META, acetone, water, triethyleneglycol dimethacrylate, phosphoric acid ester monomer, silica loads, photo-initiators | 2.0 | 1. Apply to the prepared enamel and dentin surfaces using the disposable applicator. | |||
| 2. Leave undisturbed for 10 sec after applying. | ||||||
| 3. Then, dry thoroughly for 5 sec with oil-free air under maximum air pressure. | ||||||
| 4. Light-cure for 10 sec. | ||||||
| Clearfil Tri-S Bond (Kuraray, Okayama, Japan; batch: 1M004) | MDP, Bis-GMA, HEMA, Absorbent aliphatic dimethacrylate, hydrophobic aliphatic methacrylate, silica colloidal, sodium fluoride, di-camphorquinone, accelerators, initiators, ethanol, water | 2.7 | 1. Apply bond to the entire cavity wall with the applicator brush. Leave it in place for 10 sec. | |||
| 2. Dry the entire cavity wall sufficiently by blowing mild air for more than 5 sec. | ||||||
| 3. Light-cure for 10 sec. | ||||||
| Universal | ScotchBond Universal (3M ESPE, Monrovia, CA, USA; batch: 70201139014) | MDP, resin of dimethacrylate, HEMA, Vitrebond copolymer, loads, ethanol, water, silane | 2.7 | Protocol 1: self-etch 1-step | ||
| 1. Apply the adhesive to the prepared tooth and rub it in for 20 sec. | ||||||
| 2. Gently air dry the adhesive for approximately 5 sec to evaporate the solvent. | ||||||
| 3. Light-cure for 10 sec. | ||||||
| Protocol 2: etch-and-rinse 2-steps | ||||||
| 1. Apply orthophosphoric acid for 15 sec. | ||||||
| 2. Apply the adhesive to the prepared tooth and rub it in for 20 sec. | ||||||
| 3. Gently air dry the adhesive for approximately 5 sec to evaporate the solvent. | ||||||
| 4. Light-cure for 10 sec. | ||||||
| Etch-and-rinse 2 steps | Prime & Bond (Dentsply DeTrey, Konstanz, Germany; batch: 1206001168) | Resin di- and trimethacrylate, silica, PENTA, photo-initiators, stabilizing, cetylamine hydrofluoride, acetone | 2.0 | 1. Application of orthophosphoric acid 37% for at least 15 sec. | ||
| 2. Rinsing and blot drying for at least 10 sec. | ||||||
| 3. Application of Prime & Bond for 20 sec. | ||||||
| 4. Remove excess solvent by gently drying for at least 5 sec. | ||||||
| 5. Cure Prime & Bond adhesive for 10 sec. | ||||||
| Acid | Orthophosphoric acid 37% (Dentsply DeTrey, Konstanz, Germany; batch: 155245) | Phosphoric acid, water, thickener, methylen Blue benzalkonium chloride | 0.4 | |||
| Composite resin | Ceram X Mono (Dentsply, York, PA, USA; batch: 1107000M97) | Methacrylate modified polysiloxane, dimethacrylate resin, fluorescence pigment, UV stabilizer, stabilizer, camphorquinone, ethyl-4(dimethylamino)benzoate, barium-aluminium-borosilicate glass, methacrylate functionalised silicon dioxide nano filler, iron oxide pigments and titanium oxide aluminium sulfo silicate pigments | ND | The composite resin material is incrementally placed and light-cured for 20 sec. | ||
CaCl2, calcium chloride; GPDM, glycero-phosphate dimethacrylate; HEMA, hydroxyethylmethacrylate; MEHQ, hydroquinone monomethyl ether; MDP, methacryloyloxydecyl dihydrogen phosphate; Bis-GMA, bisphenol A-glycidyl methacrylate; BHT, butylated hydroxytoluene; Pyro-EMA, tetramethacryloxyethyl pyrophosphate; PEM-F, pentamethacryloxyethyl cyclophosphazen mono fluoride; META, methacryloyloxyethy trimellitate anhydride; PENTA, dipentaerythritol penta acrylate monophosphate; UV, ultraviolet; ND, not designated.
Percentage of percolation according to the adhesive system used for both time points
| Adhesive family | Adhesive system | % of percolation at D0 | % of percolation at D7 |
|---|---|---|---|
| Etch & rinse | Prime & Bond NT | 2.01% (1.072) | 0.38% (0.38) |
| Scotchbond Universal ER | 2.19% (0.611)* | 5.3% (1.328)* | |
| Self-etch 1 step | G-aenial Bond | 2.32% (0.821) | 4.95% (1.642) |
| Scotchbond Universal SE | 2.58% (1.084)* | 12.58% (2.177)* | |
| Xeno III | 4.33% (0.930)* | 18.49% (2.652)* | |
| Clearfil Tri-S Bond | 5.37% (2.274) | 5.7% (1.715) | |
| Self-etch 2 steps | Clearfil SE Bond | 5.95% (2.723) | 5.85% (1.303) |
| Optibond XTR | 9.73% (1.832)* | 0.97% (0.464)* |
Data are presented as mean % (standard error).
D0, immediate restoration; D7, delayed restoration (7 days).
*Represents significant differences for each adhesive system at D0 and D7 (p < 0.05).
Intergroup analysis at D0
| Prime & Bond NT | Scotchbond Universal ER | G-aenial Bond | Scotchbond Universal SE | Xeno III | Clearfil Tri-S Bond | Clearfil SE Bond | Optibond XTR | |
|---|---|---|---|---|---|---|---|---|
| Prime & Bond NT | NS | NS | NS | NS | NS | NS | S | |
| Scotchbond Universal ER | NS | NS | NS | NS | NS | NS | S | |
| G-aenial Bond | NS | NS | NS | NS | NS | NS | S | |
| Scotchbond Universal SE | NS | NS | NS | NS | NS | NS | S | |
| Xeno III | NS | NS | NS | NS | NS | NS | S | |
| Clearfil Tri-S Bond | NS | NS | NS | NS | NS | NS | NS | |
| Clearfil SE Bond | NS | NS | NS | NS | NS | NS | NS | |
| Optibond XTR | S | S | S | S | S | NS | NS |
NS, no significant difference; S, significant difference (p < 0.05).
Intergroup analysis at D7
| Prime & Bond NT | Scotchbond Universal ER | G-aenial Bond | Scotchbond Universal SE | Xeno III | Clearfil Tri-S Bond | Clearfil SE Bond | Optibond XTR | |
|---|---|---|---|---|---|---|---|---|
| Prime & Bond NT | NS | NS | S | S | S | S | NS | |
| Scotchbond Universal ER | S | NS | S | S | NS | NS | NS | |
| G-aenial Bond | NS | NS | S | S | NS | NS | NS | |
| Scotchbond Universal SE | S | S | S | S | S | S | S | |
| Xeno III | S | S | S | S | S | S | S | |
| Clearfil Tri-S Bond | S | NS | NS | S | S | NS | NS | |
| Clearfil SE Bond | S | NS | NS | S | S | NS | NS | |
| Optibond XTR | NS | NS | NS | S | S | NS | NS |
NS, no significant difference; S, significant difference (p < 0.05).
CaCl2, calcium chloride; GPDM, glycero-phosphate dimethacrylate; HEMA, hydroxyethylmethacrylate; MEHQ, hydroquinone monomethyl ether; MDP, methacryloyloxydecyl dihydrogen phosphate; Bis-GMA, bisphenol A-glycidyl methacrylate; BHT, butylated hydroxytoluene; Pyro-EMA, tetramethacryloxyethyl pyrophosphate; PEM-F, pentamethacryloxyethyl cyclophosphazen mono fluoride; META, methacryloyloxyethy trimellitate anhydride; PENTA, dipentaerythritol penta acrylate monophosphate; UV, ultraviolet; ND, not designated.
Data are presented as mean % (standard error).
D0, immediate restoration; D7, delayed restoration (7 days).
*Represents significant differences for each adhesive system at D0 and D7 (
NS, no significant difference; S, significant difference (
NS, no significant difference; S, significant difference (

 KACD
KACD
 ePub Link
ePub Link Cite
Cite

