Articles
- Page Path
- HOME > Restor Dent Endod > Volume 46(3); 2021 > Article
- Review Article Silver nanoparticles in endodontics: recent developments and applications
-
Aysenur Oncu1
 , Yan Huang2
, Yan Huang2 , Gulin Amasya3
, Gulin Amasya3 , Fatma Semra Sevimay1
, Fatma Semra Sevimay1 , Kaan Orhan4
, Kaan Orhan4 , Berkan Celikten1
, Berkan Celikten1
-
Restor Dent Endod 2021;46(3):e38.
DOI: https://doi.org/10.5395/rde.2021.46.e38
Published online: July 1, 2021
1Department of Endodontics, Ankara University Faculty of Dentistry, Ankara, Turkey.
2Department of Dental Hygiene Research & Development in Health & Care, Artevelde University of Applied Sciences, Ghent, Belgium.
3Department of Pharmaceutical Technology, Ankara University Faculty of Pharmacy, Ankara, Turkey.
4Department of Dentomaxillofacial Radiology, Ankara University Faculty of Dentistry, Ankara, Turkey.
- Correspondence to Berkan Celikten, DDs, PhD. Associate Professor, Department of Endodontics, Ankara University Faculty of Dentistry, Besevler, Ankara 06560, Turkey. berkancelikten@gmail.com
Copyright © 2021. The Korean Academy of Conservative Dentistry
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
- The elimination of endodontic biofilms and the maintenance of a leak-proof canal filling are key aspects of successful root canal treatment. Several materials have been introduced to treat endodontic disease, although treatment success is limited by the features of the biomaterials used. Silver nanoparticles (AgNPs) have been increasingly considered in dental applications, especially endodontics, due to their high antimicrobial activity. For the present study, an electronic search was conducted using MEDLINE (PubMed), the Cochrane Central Register of Controlled Trials (CENTRAL), Google Scholar, and EMBASE. This review provides insights into the unique characteristics of AgNPs, including their chemical, physical, and antimicrobial properties; limitations; and potential uses. Various studies involving different application methods of AgNPs were carefully examined. Based on previous clinical studies, the synthesis, means of obtaining, usage conditions, and potential cytotoxicity of AgNPs were evaluated. The findings indicate that AgNPs are effective antimicrobial agents for the elimination of endodontic biofilms.
INTRODUCTION
REVIEW
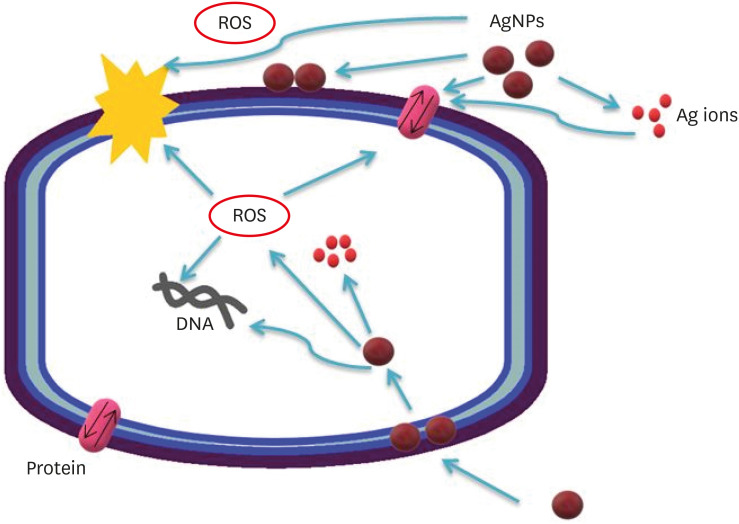
Possible antibacterial mechanisms of AgNPs. AgNPs can: 1) bind to the cell membrane, membrane proteins, and DNA bases, leading to the disruption of normal function; 2) release silver ions, affecting the membrane, DNA, and proteins; and 3) generate ROS, which may also affect DNA, the cell membrane, and membrane proteins.
Current analysis methods used for silver nanoparticle characterization
| Methods | Abbreviation | Uniqueness of the method | Reference |
|---|---|---|---|
| Scanning electron microscopy | SEM | SEM can be used to completely differentiate particle sizes, size distributions, nanomaterial shapes, and surface morphologies of synthesized particles in microscale and nanoscale. Additionally, a histogram can be derived from the images by manually measuring and counting particles or using specific software. | Fissan et al., 2014 [59] |
| Transmission electron microscopy | TEM | While TEM has advantages that include good spatial resolution and additional analytical measurements, sample preparation is time-consuming. | Zhang et al., 2016 [44] |
| Scanning electrochemical microscopy | SECM | SECM is a noninvasive method developed to measure load/mass transport rates across surface film using electrodes. | Blanchard et al., 2016 [60] |
| Atomic force microscopy | AFM | AFM can also be used to characterize the real-time interaction of nanomaterials with supported lipid layers. However, a major disadvantage is that the lateral dimensions of the samples are overestimated. | Zhang et al., 2016 [44] and Eaton and Batziou, 2019 [61] |
| Dynamic light scattering | DLS | DLS, a method that depends on the interaction of light with particles, is used to characterize the particle size and dimension distribution in aqueous or physiological solutions. | Leung et al., 2006 [62] |
| Ultraviolet-visible spectroscopy | UVS | UV-Vis spectroscopy is quick, simple, precise, and selective for nanoparticles. Additionally, it requires only a short time for measurement, and calibration is not required for particle characterization of colloidal suspensions. | Zhang et al., 2016 [44] and Das et al., 2009 [63] |
| Confocal laser scanning microscopy | CLSM | Rapid visualization of dynamic processes in fixed and live cells enables the detailed morphological analysis of tissues and automatic collection of 3-dimensional data. | Paddock and Eliceiri, 2014 [64] |
Recent applications of silver nanoparticles in endodontics
| Related area | Application procedure | Main results | Reference |
|---|---|---|---|
| Irrigation solution | The antimicrobial effects of 6 solutions were compared: 0.85% saline (control), 2% CHX, 5% NaOCl, 1% NaOCl, 1% AgNP, and 26% ZnONP. | The 1% AgNP and 26% ZnONP solutions were similarly effective against E. faecalis biofilm relative to conventional endodontic irrigants. | De Almeida et al., 2018 [74] |
| Irrigation solution | Equal amounts of 2% CHX and 15 µg/mL AgNPs were mixed homogeneously and compared with the solutions used individually. | The CHX-AgNP combined solution exhibited higher efficacy than the individual solutions. | Charannya et al., 2018 [69] |
| Root canal medicaments solution and gel form | The antibacterial efficacy of silver nanoparticles as an irrigant (0.1% AgNP) or medicament (0.02% and 0.01% AgNP) against E. faecalis biofilms was evaluated. | As a medication, 0.02% AgNP gel significantly impaired the structural integrity of the biofilm and resulted in the fewest viable E. faecalis cells remaining after treatment. | Wu et al., 2014 [68] |
| Development of bioactive material | The antibacterial activities of NanoAg and NanoAg-MTA against 4 types of anaerobic pathogens were tested in vitro. Each gram of MTA powder was mixed with 350 µL of 25 ppm, 12.5 ppm, and 6.25 ppm preparations of NanoAg solution on sterile glass slabs using a sterile spatula. | AgNPs can effectively enhance the antibacterial activity of MTA against anaerobic periodontal/endodontic pathogens. | Bahador et al., 2015 [72] |
| Root canal sealer | Methacrylate-resin dual-cured root canal sealer contained 5% dimethylaminohexadecyl methacrylate (DMAHDM), 0.15% AgNP, and nanoparticles of amorphous calcium phosphate (NACP) at 10%, 20%, and 30% mass fractions. Antibacterial properties against E. faecalis were measured. | The novel therapeutic root canal sealer with triple bioactive agents of DMAHDM, AgNP, and NACP neutralized acid and raised the pH, regenerated dentin minerals, and increased root dentin hardness. | Baras et al., 2019 [73] |
| Regenerative endodontic procedures | In regenerative endodontics, the antibacterial effectiveness of double antibiotic paste (1 mg/mL DAP), silver nanoparticle (0.02% AgNP) gel, and tailored amorphous multiporous bioactive glass (100 mg/mL TAMP-BG) against 3 weeks of E. faecalis biofilms were evaluated. | These medicaments can function as potent intracanal drugs for regenerative endodontic procedures. However, complete elimination of E. faecalis biofilms occurred only at recommended concentrations and was made possible with AgNPs. | Athanassiads et al., 2007 [76] |
| Fiber post cementation | The effect of AgNP solution on the mechanical properties of resin cements used for fiber post bonding was investigated. | The results indicate that the AgNP solution can be used as an irrigation protocol before glass fiber post cementation. | Suzuki et al., 2019 [77] |
CONCLUSIONS
-
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
-
Author Contributions:
Conceptualization: Celikten B.
Data curation: Amasya G.
Investigation: Oncu A, Amasya G.
Methodology: Oncu A.
Project administration: Celikten B.
Resources: Sevimay FS.
Supervision: Huang Y.
Validation: Orhan K.
Visualization: Sevimay FS.
Writing - original draft: Oncu A, Amasya G.
Writing - review & editing: Huang Y.
- 1. Vestby LK, Grønseth T, Simm R, Nesse LL. Bacterial biofilm and its role in the pathogenesis of disease. Antibiotics (Basel) 2020;9:59.ArticlePubMedPMC
- 2. Neelakantan P, Romero M, Vera J, Daood U, Khan AU, Yan A, Cheung GS. Biofilms in endodontics-current status and future directions. Int J Mol Sci 2017;18:1748.ArticlePubMedPMC
- 3. Mensi M, Scotti E, Sordillo A, Agosti R, Calza S. Plaque disclosing agent as a guide for professional biofilm removal: a randomized controlled clinical trial. Int J Dent Hyg 2020;18:285-294.ArticlePubMedPDF
- 4. Tolker-Nielsen T. Biofilm development. Microbiol Spectr 2015;3:MB-0001-MB-2014.ArticlePDF
- 5. Abusrewil S, Alshanta OA, Albashaireh K, Alqahtani S, Nile CJ, Scott JA, McLean W. Detection, treatment and prevention of endodontic biofilm infections: what's new in 2020? Crit Rev Microbiol 2020;46:194-212.ArticlePubMed
- 6. Haapasalo M, Shen Y, Wang Z, Gao Y. Irrigation in endodontics. Br Dent J 2014;216:299-303.ArticlePubMedPDF
- 7. García-Guerrero C, Delgado-Rodríguez CE, Molano-González N, Pineda-Velandia GA, Marín-Zuluaga DJ, Leal-Fernandez MC, Gutmann JL. Predicting the outcome of initial non-surgical endodontic procedures by periapical status and quality of root canal filling: a cohort study. Odontology 2020;108:697-703.ArticlePubMedPDF
- 8. Schmalz G, Hickel R, van Landuyt KL, Reichl FX. Nanoparticles in dentistry. Dent Mater 2017;33:1298-1314.ArticlePubMed
- 9. Kaur P, Luthra R. Silver nanoparticles in dentistry: an emerging trend. SRM J Res Dent Sci 2016;7:162.Article
- 10. Abdal Dayem A, Hossain MK, Lee SB, Kim K, Saha SK, Yang GM, Choi HY, Cho SG. The role of reactive oxygen species (ROS) in the biological activities of metallic nanoparticles. Int J Mol Sci 2017;18:120.ArticlePubMedPMC
- 11. Tang S, Zheng J. Antibacterial activity of silver nanoparticles: structural effects. Adv Healthc Mater 2018;7:e1701503.ArticlePubMedPDF
- 12. Prabhu S, Poulose EK. Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int Nano Lett 2012;2:32.ArticlePDF
- 13. Stohs SJ, Bagchi D. Oxidative mechanisms in the toxicity of metal ions. Free Radic Biol Med 1995;18:321-336.ArticlePubMed
- 14. Samiei M, Farjami A, Dizaj SM, Lotfipour F. Nanoparticles for antimicrobial purposes in endodontics: a systematic review of in vitro studies. Mater Sci Eng C 2016;58:1269-1278.Article
- 15. Salata O. Applications of nanoparticles in biology and medicine. J Nanobiotechnology 2004;2:3.ArticlePubMedPMCPDF
- 16. Du Q, Fu M, Zhou Y, Cao Y, Guo T, Zhou Z, Li M, Peng X, Zheng X, Li Y, Xu X, He J, Zhou X. Sucrose promotes caries progression by disrupting the microecological balance in oral biofilms: an in vitro study. Sci Rep 2020;10:2961.ArticlePubMedPMCPDF
- 17. Pamp SJ, Gjermansen M, Tolker-Nielsen T. The biofilm mode of life: mechanisms and adaptation. Biosci Horiz 2007;16:37-69.
- 18. Teves A, Blanco D, Casaretto M, Torres J, Alvarado D, Jaramillo DE. Effectiveness of different disinfection techniques of the root canal in the elimination of a multi-species biofilm. J Clin Exp Dent 2019;11:e978-e983.ArticlePubMedPMC
- 19. Nwodo UU, Green E, Okoh AI. Bacterial exopolysaccharides: functionality and prospects. Int J Mol Sci 2012;13:14002-14015.ArticlePubMedPMC
- 20. Sutherland I. Biofilm exopolysaccharides: a strong and sticky framework. Microbiology (Reading) 2001;147:3-9.ArticlePubMed
- 21. Lynch DJ, Fountain TL, Mazurkiewicz JE, Banas JA. Glucan-binding proteins are essential for shaping Streptococcus mutans biofilm architecture. FEMS Microbiol Lett 2007;268:158-165.ArticlePubMed
- 22. In: Lemos J, Palmer S, Zeng L, Wen Z, Kajfasz J, Freires I, Abranches J, Brady L, editors. The biology of Streptococcus mutans. 3rd ed. Gram-Positive Pathogens; 2019. p. 435-448.
- 23. Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 2002;15:167-193.ArticlePubMedPMCPDF
- 24. Jhajharia K, Parolia A, Shetty KV, Mehta LK. Biofilm in endodontics: a review. J Int Soc Prev Community Dent 2015;5:1-12.ArticlePubMedPMC
- 25. Abisado RG, Benomar S, Klaus JR, Dandekar AA, Chandler JR. Bacterial quorum sensing and microbial community interactions. MBio 2018;9:e02331-17.ArticlePubMedPMCPDF
- 26. Schluter J, Schoech AP, Foster KR, Mitri S. The evolution of quorum sensing as a mechanism to infer kinship. PLOS Comput Biol 2016;12:e1004848.ArticlePubMedPMC
- 27. Koo H, Allan RN, Howlin RP, Stoodley P, Hall-Stoodley L. Targeting microbial biofilms: current and prospective therapeutic strategies. Nat Rev Microbiol 2017;15:740-755.ArticlePubMedPMCPDF
- 28. Stuart CH, Schwartz SA, Beeson TJ, Owatz CB. Enterococcus faecalis: its role in root canal treatment failure and current concepts in retreatment. J Endod 2006;32:93-98.ArticlePubMed
- 29. Saatchi M, Shokraneh A, Navaei H, Maracy MR, Shojaei H. Antibacterial effect of calcium hydroxide combined with chlorhexidine on Enterococcus faecalis: a systematic review and meta-analysis. J Appl Oral Sci 2014;22:356-365.ArticlePubMedPMC
- 30. Yu MK, Kim MA, Rosa V, Hwang YC, Del Fabbro M, Sohn WJ, Min KS. Role of extracellular DNA in Enterococcus faecalis biofilm formation and its susceptibility to sodium hypochlorite. J Appl Oral Sci 2019;27:e20180699.ArticlePubMedPMC
- 31. Barnes AM, Ballering KS, Leibman RS, Wells CL, Dunny GM. Enterococcus faecalis produces abundant extracellular structures containing DNA in the absence of cell lysis during early biofilm formation. MBio 2012;3:e00193-e12.ArticlePubMedPMCPDF
- 32. Chang JD, Wallace AG, Foster EE, Kim SJ. Peptidoglycan compositional analysis of Enterococcus faecalis biofilm by stable isotope labeling by amino acids in a bacterial culture. Biochemistry 2018;57:1274-1283.ArticlePubMedPMC
- 33. Bulacio ML, Galván LR, Gaudioso C, Cangemi R, Erimbaue MI. Enterococcus Faecalis biofilm. Formation and development in vitro observed by scanning electron microscopy. Acta Odontol Latinoam 2015;28:210-214.PubMed
- 34. Kuang X, Chen V, Xu X. Novel approaches to the control of oral microbial biofilms. BioMed Res Int 2018;2018:6498932.ArticlePubMedPMCPDF
- 35. Rabin N, Zheng Y, Opoku-Temeng C, Du Y, Bonsu E, Sintim HO. Agents that inhibit bacterial biofilm formation. Future Med Chem 2015;7:647-671.ArticlePubMed
- 36. Veerapandian M, Yun K. Functionalization of biomolecules on nanoparticles: specialized for antibacterial applications. Appl Microbiol Biotechnol 2011;90:1655-1667.ArticlePubMedPDF
- 37. Shrestha A, Kishen A. Antibacterial nanoparticles in endodontics: a review. J Endod 2016;42:1417-1426.ArticlePubMed
- 38. Khezerlou A, Alizadeh-Sani M, Azizi-Lalabadi M, Ehsani A. Nanoparticles and their antimicrobial properties against pathogens including bacteria, fungi, parasites and viruses. Microb Pathog 2018;123:505-526.ArticlePubMed
- 39. Cao W, Zhang Y, Wang X, Li Q, Xiao Y, Li P, Wang L, Ye Z, Xing X. Novel resin-based dental material with anti-biofilm activity and improved mechanical property by incorporating hydrophilic cationic copolymer functionalized nanodiamond. J Mater Sci Mater Med 2018;29:162.ArticlePubMedPDF
- 40. Saafan A, Zaazou MH, Sallam MK, Mosallam O, El Danaf HA. Assessment of photodynamic therapy and nanoparticles effects on caries models. Open Access Maced J Med Sci 2018;6:1289-1295.ArticlePubMedPMCPDF
- 41. Bukhari S, Kim D, Liu Y, Karabucak B, Koo H. Novel endodontic disinfection approach using catalytic nanoparticles. J Endod 2018;44:806-812.ArticlePubMedPMC
- 42. Rajeshkumar S, Bharath LV. Mechanism of plant-mediated synthesis of silver nanoparticles - A review on biomolecules involved, characterisation and antibacterial activity. Chem Biol Interact 2017;273:219-227.ArticlePubMed
- 43. Lee SH, Jun BH. Silver nanoparticles: synthesis and application for nanomedicine. Int J Mol Sci 2019;20:865.ArticlePubMedPMC
- 44. Zhang XF, Liu ZG, Shen W, Gurunathan S. Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. Int J Mol Sci 2016;17:1534.ArticlePubMedPMC
- 45. Singh R, Shedbalkar UU, Wadhwani SA, Chopade BA. Bacteriagenic silver nanoparticles: synthesis, mechanism, and applications. Appl Microbiol Biotechnol 2015;99:4579-4593.ArticlePubMedPDF
- 46. Mousavi SM, Hashemi SA, Ghasemi Y, Atapour A, Amani AM, Savar Dashtaki A, Babapoor A, Arjmand O. Green synthesis of silver nanoparticles toward bio and medical applications: review study. Artif Cells Nanomed Biotechnol 2018;46(sup3):S855-S872.ArticlePubMed
- 47. Patil MP, Kim GD. Eco-friendly approach for nanoparticles synthesis and mechanism behind antibacterial activity of silver and anticancer activity of gold nanoparticles. Appl Microbiol Biotechnol 2017;101:79-92.ArticlePubMedPDF
- 48. Hong X, Wen J, Xiong X, Hu Y. Shape effect on the antibacterial activity of silver nanoparticles synthesized via a microwave-assisted method. Environ Sci Pollut Res Int 2016;23:4489-4497.ArticlePubMedPDF
- 49. Mie R, Samsudin MW, Din LB, Ahmad A, Ibrahim N, Adnan SN. Synthesis of silver nanoparticles with antibacterial activity using the lichen Parmotrema praesorediosum . Int J Nanomedicine 2014;9:121-127.ArticlePubMed
- 50. Tang S, Zheng J. Antibacterial activity of silver nanoparticles: structural effects. Adv Healthc Mater 2018;7:e1701503.ArticlePubMedPDF
- 51. Raffi M, Hussain F, Bhatti TM, Akhter JI, Hameed A, Hasan MM. Antibacterial characterization of silver nanoparticles against E. coli ATCC-15224. J Mater Sci Technol 2008;24:192-196.
- 52. Bapat RA, Chaubal TV, Joshi CP, Bapat PR, Choudhury H, Pandey M, Gorain B, Kesharwani P. An overview of application of silver nanoparticles for biomaterials in dentistry. Mater Sci Eng C 2018;91:881-898.Article
- 53. Radzig MA, Nadtochenko VA, Koksharova OA, Kiwi J, Lipasova VA, Khmel IA. Antibacterial effects of silver nanoparticles on gram-negative bacteria: influence on the growth and biofilms formation, mechanisms of action. Colloids Surf B Biointerfaces 2013;102:300-306.ArticlePubMed
- 54. Shrivastava S, Bera T, Singh SK, Singh G, Ramachandrarao P, Dash D. Characterization of antiplatelet properties of silver nanoparticles. ACS Nano 2009;3:1357-1364.ArticlePubMed
- 55. Manikprabhu D, Lingappa K. Antibacterial activity of silver nanoparticles against methicillin-resistant Staphylococcus aureus synthesized using model Streptomyces sp. pigment by photo-irradiation method. J Pharm Res 2013;6:255-260.Article
- 56. Zawadzka K, Kądzioła K, Felczak A, Wrońska N, Piwoński I, Kisielewska A, Lisowska K. Surface area or diameter–which factor really determines the antibacterial activity of silver nanoparticles grown on TiO 2 coatings? New J Chem 2014;38:3275-3281.Article
- 57. Qing Y, Cheng L, Li R, Liu G, Zhang Y, Tang X, Wang J, Liu H, Qin Y. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int J Nanomedicine 2018;13:3311-3327.ArticlePubMedPMCPDF
- 58. Markowska K, Grudniak AM, Wolska KI. Silver nanoparticles as an alternative strategy against bacterial biofilms. Acta Biochim Pol 2013;60:523-530.ArticlePubMedPDF
- 59. Fissan H, Ristig S, Kaminski H, Asbach C, Epple M. Comparison of different characterization methods for nanoparticle dispersions before and after aerosolization. Anal Methods 2014;6:7324-7334.ArticlePubMed
- 60. Blanchard PY, Sun T, Yu Y, Wei Z, Matsui H, Mirkin MV. Scanning electrochemical microscopy study of permeability of a thiolated aryl multilayer and imaging of single nanocubes anchored to it. Langmuir 2016;32:2500-2508.ArticlePubMedPMC
- 61. Eaton P, Batziou K. Artifacts and practical issues in atomic force microscopy. Methods Mol Biol 2019;1886:3-28.ArticlePubMed
- 62. Leung AB, Suh KI, Ansari RR. Particle-size and velocity measurements in flowing conditions using dynamic light scattering. Appl Opt 2006;45:2186-2190.ArticlePubMed
- 63. Das R, Nath S, Chakdar D, Gope G, Bhattacharjee R. Preparation of silver nanoparticles and their characterization. J Nanotechnol 2009;5:1-6.
- 64. Paddock SW, Eliceiri KW. Laser scanning confocal microscopy: history, applications, and related optical sectioning techniques. Methods Mol Biol 2014;1075:9-47.ArticlePubMed
- 65. Lotfi M, Vosoughhosseini S, Ranjkesh B, Khani S, Saghiri M, Zand V. Antimicrobial efficacy of nanosilver, sodium hypochlorite and chlorhexidine gluconate against Enterococcus faecalis . Afr J Biotechnol 2011;10:6799-6803.
- 66. Hiraishi N, Yiu CK, King NM, Tagami J, Tay FR. Antimicrobial efficacy of 3.8% silver diamine fluoride and its effect on root dentin. J Endod 2010;36:1026-1029.ArticlePubMed
- 67. Rodrigues CT, de Andrade FB, de Vasconcelos LR, Midena RZ, Pereira TC, Kuga MC, Duarte MA, Bernardineli N. Antibacterial properties of silver nanoparticles as a root canal irrigant against Enterococcus faecalis biofilm and infected dentinal tubules. Int Endod J 2018;51:901-911.ArticlePubMedPDF
- 68. Wu D, Fan W, Kishen A, Gutmann JL, Fan B. Evaluation of the antibacterial efficacy of silver nanoparticles against Enterococcus faecalis biofilm. J Endod 2014;40:285-290.ArticlePubMed
- 69. Charannya S, Duraivel D, Padminee K, Poorni S, Nishanthine C, Srinivasan MR. Comparative evaluation of antimicrobial efficacy of silver nanoparticles and 2% chlorhexidine gluconate when used alone and in combination assessed using agar diffusion method: an in vitro study. Contemp Clin Dent 2018;9(Supplement 2):S204-S209.ArticlePubMedPMC
- 70. Yousefshahi H, Aminsobhani M, Shokri M, Shahbazi R. Anti-bacterial properties of calcium hydroxide in combination with silver, copper, zinc oxide or magnesium oxide. Eur J Transl Myol 2018;28:7545.ArticlePubMedPMCPDF
- 71. Shantiaee Y, Dianat O, Mohammadkhani H, Akbarzadeh BA. Cytotoxicity comparison of nanosilver coated gutta-percha with Guttaflow and normal gutta-percha on L929 fibroblast with MTT assay. Shahid Beheshti Univ Dent J 2011;29:62-68.
- 72. Bahador A, Pourakbari B, Bolhari B, Hashemi FB. In vitro evaluation of the antimicrobial activity of nanosilver-mineral trioxide aggregate against frequent anaerobic oral pathogens by a membrane-enclosed immersion test. Biomed J 2015;38:77-83.ArticlePubMed
- 73. Baras BH, Melo MA, Sun J, Oates TW, Weir MD, Xie X, Bai Y, Xu HH. Novel endodontic sealer with dual strategies of dimethylaminohexadecyl methacrylate and nanoparticles of silver to inhibit root canal biofilms. Dent Mater 2019;35:1117-1129.ArticlePubMed
- 74. de Almeida J, Cechella BC, Bernardi AV, de Lima Pimenta A, Felippe WT. Effectiveness of nanoparticles solutions and conventional endodontic irrigants against Enterococcus faecalis biofilm. Indian J Dent Res 2018;29:347-351.ArticlePubMed
- 75. Halkai KR, Mudda JA, Shivanna V, Rathod V, Halkai R. Evaluation of antibacterial efficacy of fungal-derived silver nanoparticles against Enterococcus faecalis . Contemp Clin Dent 2018;9:45-48.ArticlePubMedPMC
- 76. Athanassiadis B, Abbott PV, Walsh LJ. The use of calcium hydroxide, antibiotics and biocides as antimicrobial medicaments in endodontics. Aust Dent J 2007;52(Supplement):S64-S82.ArticlePubMed
- 77. Suzuki TY, Gallego J, Assunção WG, Briso AL, Dos Santos PH. Influence of silver nanoparticle solution on the mechanical properties of resin cements and intrarradicular dentin. PLoS One 2019;14:e0217750.ArticlePubMedPMC
- 78. Afkhami F, Pourhashemi SJ, Sadegh M, Salehi Y, Fard MJ. Antibiofilm efficacy of silver nanoparticles as a vehicle for calcium hydroxide medicament against Enterococcus faecalis . J Dent 2015;43:1573-1579.ArticlePubMed
- 79. Fan W, Wu Y, Ma T, Li Y, Fan B. Substantivity of Ag-Ca-Si mesoporous nanoparticles on dentin and its ability to inhibit Enterococcus faecalis . J Mater Sci Mater Med 2016;27:16.ArticlePubMedPDF
- 80. Marin S, Vlasceanu GM, Tiplea RE, Bucur IR, Lemnaru M, Marin MM, Grumezescu AM. Applications and toxicity of silver nanoparticles: a recent review. Curr Top Med Chem 2015;15:1596-1604.ArticlePubMed
- 81. Mathur P, Jha S, Ramteke S, Jain NK. Pharmaceutical aspects of silver nanoparticles. Artif Cells Nanomed Biotechnol 2018;46(sup1):115-126.ArticlePubMed
- 82. Reidy B, Haase A, Luch A, Dawson KA, Lynch I. Mechanisms of silver nanoparticle release, transformation and toxicity: a critical review of current knowledge and recommendations for future studies and applications. Materials (Basel) 2013;6:2295-2350.ArticlePubMedPMC
- 83. Palacios-Hernandez T, Diaz-Diestra DM, Nguyen AK, Skoog SA, Vijaya Chikkaveeraiah B, Tang X, Wu Y, Petrochenko PE, Sussman EM, Goering PL. Cytotoxicity, cellular uptake and apoptotic responses in human coronary artery endothelial cells exposed to ultrasmall superparamagnetic iron oxide nanoparticles. J Appl Toxicol 2020;40:918-930.ArticlePubMedPDF
- 84. Panáček A, Smékalová M, Večeřová R, Bogdanová K, Röderová M, Kolář M, Kilianová M, Hradilová Š, Froning JP, Havrdová M, Prucek R, Zbořil R, Kvítek L. Silver nanoparticles strongly enhance and restore bactericidal activity of inactive antibiotics against multiresistant Enterobacteriaceae . Colloids Surf B Biointerfaces 2016;142:392-399.ArticlePubMed
- 85. Chowdhury NR, MacGregor-Ramiasa M, Zilm P, Majewski P, Vasilev K. ‘Chocolate’ silver nanoparticles: Synthesis, antibacterial activity and cytotoxicity. J Colloid Interface Sci 2016;482:151-158.ArticlePubMed
REFERENCES
Tables & Figures
REFERENCES
Citations

- Endodontic Intracanal Medicaments and Agents
Anu Priya Guruswamy Pandian, Depti Bellani, Ritya Mary Jibu, Varsha Agnihotri
Dental Clinics of North America.2026; 70(1): 45. CrossRef - Time-dependent Tooth Color Changes Following Conventional, Silver-based, and Photodynamic Root Canal Irrigants: An In Vitro Study
Laila Mohamed Mohamed Kenawi, Mohamed Fattouh, Khaled Abid Althaqafi, Abla Arafa
The Open Dentistry Journal.2026;[Epub] CrossRef - Advanced nanoparticle-based antibacterial delivery for endodontic disinfection: A systematic review and meta-analysis
Kanwalpreet Kaur, Seerat Kaura, Ravinder S Saini, Maurya Manjunath, Shashit Shetty Bavabeedu, Mario Alberto Alarcón-Sánchez, Javier Flores-Fraile, Artak Heboyan
Journal of Dentistry.2026; 166: 106347. CrossRef - Scoping review on the genotoxicity of silver nanoparticles in endodontics: therapeutic saviors or genetic saboteurs?
Galvin Sim Siang Lin, Widya Lestari, Mohd Haikal Muhamad Halil, Mohd Syafiq Abd Aziz
Odontology.2025; 113(2): 457. CrossRef - Bioceramics in Endodontics: Limitations and Future Innovations—A Review
Peramune Arachchilage Amila Saman Prasad Kumara, Paul Roy Cooper, Peter Cathro, Maree Gould, George Dias, Jithendra Ratnayake
Dentistry Journal.2025; 13(4): 157. CrossRef - Recent advances in antibacterial nanoformulations for endodontic applications
Tiago Dionísio, Pedro Brandão, Vanessa Machado, João Botelho, José João Mendes, Pedro Fonte
Expert Opinion on Drug Delivery.2025; 22(8): 1117. CrossRef - Systematic review of silver and vanadium-based antibiofilm agents: mechanisms and efficacy in oral biofilms
João Marcos Carvalho-Silva, Andréa Cândido dos Reis
Future Microbiology.2025; 20(10): 639. CrossRef - Nanomaterial-Enhanced Dentistry: A Clinical Perspective
Selvam Manoj, Radhakrishnan Sreena, Rajkumar Divya, Starlin Ebinesh, Shenbagaraman Akshaya, Srikumar Sugantha Angel, Arputharaj Joseph Nathanael
ACS Biomaterials Science & Engineering.2025; 11(8): 4671. CrossRef - Antimicrobial Effects of Formulations of Various Nanoparticles and Calcium Hydroxide as Intra-canal Medications Against Enterococcus faecalis: A Systematic Review
Seema H Bukhari, Dax Abraham, Shakila Mahesh
Cureus.2024;[Epub] CrossRef - The Push-Out Bond Strength, Surface Roughness, and Antimicrobial Properties of Endodontic Bioceramic Sealers Supplemented with Silver Nanoparticles
Karla Navarrete-Olvera, Nereyda Niño-Martínez, Idania De Alba-Montero, Nuria Patiño-Marín, Facundo Ruiz, Horacio Bach, Gabriel-Alejandro Martínez-Castañón
Molecules.2024; 29(18): 4422. CrossRef - Synergistic bactericidal activity of chlorhexidine loaded on positively charged ionic liquid-protected silver nanoparticles as a root canal disinfectant against Enterococcus faecalis: An ex vivo study
Abbas Abbaszadegan, Elham Tayebikhorami, Ahmad Gholami, Nazanin Bonyanpour, Bahar Asheghi, Sara Nikmanesh
Journal of Ionic Liquids.2024; 4(2): 100117. CrossRef - Improving the Antimicrobial Potency of Berberine for Endodontic Canal Irrigation Using Polymeric Nanoparticles
Célia Marques, Liliana Grenho, Maria Helena Fernandes, Sofia A. Costa Lima
Pharmaceutics.2024; 16(6): 786. CrossRef - A narrative review on application of metal and metal oxide nanoparticles in endodontics
Roohollah Sharifi, Ahmad Vatani, Amir Sabzi, Mohsen Safaei
Heliyon.2024; 10(15): e34673. CrossRef - The Effectiveness of Silver Nanoparticles Mixed with Calcium Hydroxide against Candida albicans: An Ex Vivo Analysis
Maha Alghofaily, Jood Alfraih, Aljohara Alsaud, Norah Almazrua, Terrence S. Sumague, Sayed H. Auda, Fahd Alsalleeh
Microorganisms.2024; 12(2): 289. CrossRef - Evaluation of the efficacy of a novel disinfecting material on the surface topography of gutta-percha: An in vitro study
KHanisha Reddy, Lekshmi Chandran, TMurali Mohan, K Sudha, DL Malini, Bonney Dominic
Journal of Conservative Dentistry.2023; 26(1): 94. CrossRef - Silver Nanoparticles and Their Therapeutic Applications in Endodontics: A Narrative Review
Farzaneh Afkhami, Parisa Forghan, James L. Gutmann, Anil Kishen
Pharmaceutics.2023; 15(3): 715. CrossRef - Nanopartículas antimicrobianas en endodoncia: Revisión narrativa
Gustavo Adolfo Tovar Rangel , Fanny Mildred González Sáenz , Ingrid Ximena Zamora Córdoba , Lina María García Zapata
Revista Estomatología.2023;[Epub] CrossRef - Functionalized Nanoparticles: A Paradigm Shift in Regenerative Endodontic Procedures
Vinoo Subramaniam Ramachandran, Mensudar Radhakrishnan, Malathi Balaraman Ravindrran, Venkatesh Alagarsamy, Gowri Shankar Palanisamy
Cureus.2022;[Epub] CrossRef
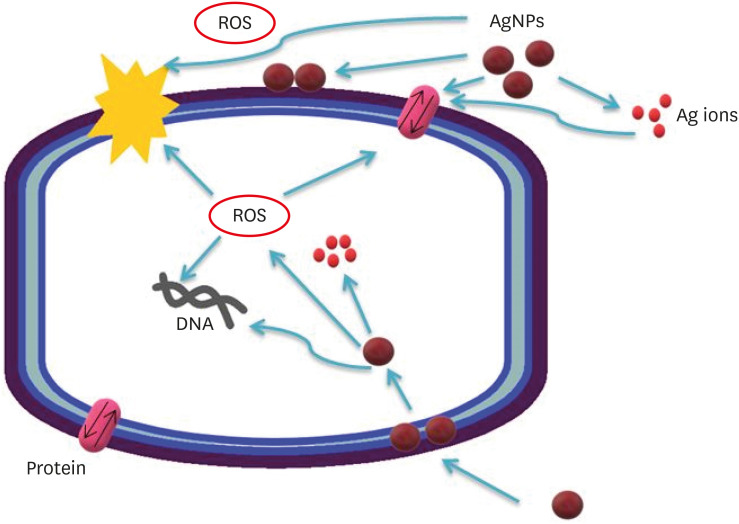
Figure 1
Figure 2
Figure 3
Figure 4
Current analysis methods used for silver nanoparticle characterization
| Methods | Abbreviation | Uniqueness of the method | Reference |
|---|---|---|---|
| Scanning electron microscopy | SEM | SEM can be used to completely differentiate particle sizes, size distributions, nanomaterial shapes, and surface morphologies of synthesized particles in microscale and nanoscale. Additionally, a histogram can be derived from the images by manually measuring and counting particles or using specific software. | Fissan et al., 2014 [ |
| Transmission electron microscopy | TEM | While TEM has advantages that include good spatial resolution and additional analytical measurements, sample preparation is time-consuming. | Zhang et al., 2016 [ |
| Scanning electrochemical microscopy | SECM | SECM is a noninvasive method developed to measure load/mass transport rates across surface film using electrodes. | Blanchard et al., 2016 [ |
| Atomic force microscopy | AFM | AFM can also be used to characterize the real-time interaction of nanomaterials with supported lipid layers. However, a major disadvantage is that the lateral dimensions of the samples are overestimated. | Zhang et al., 2016 [ |
| Dynamic light scattering | DLS | DLS, a method that depends on the interaction of light with particles, is used to characterize the particle size and dimension distribution in aqueous or physiological solutions. | Leung et al., 2006 [ |
| Ultraviolet-visible spectroscopy | UVS | UV-Vis spectroscopy is quick, simple, precise, and selective for nanoparticles. Additionally, it requires only a short time for measurement, and calibration is not required for particle characterization of colloidal suspensions. | Zhang et al., 2016 [ |
| Confocal laser scanning microscopy | CLSM | Rapid visualization of dynamic processes in fixed and live cells enables the detailed morphological analysis of tissues and automatic collection of 3-dimensional data. | Paddock and Eliceiri, 2014 [ |
Recent applications of silver nanoparticles in endodontics
| Related area | Application procedure | Main results | Reference |
|---|---|---|---|
| Irrigation solution | The antimicrobial effects of 6 solutions were compared: 0.85% saline (control), 2% CHX, 5% NaOCl, 1% NaOCl, 1% AgNP, and 26% ZnONP. | The 1% AgNP and 26% ZnONP solutions were similarly effective against E. faecalis biofilm relative to conventional endodontic irrigants. | De Almeida et al., 2018 [ |
| Irrigation solution | Equal amounts of 2% CHX and 15 µg/mL AgNPs were mixed homogeneously and compared with the solutions used individually. | The CHX-AgNP combined solution exhibited higher efficacy than the individual solutions. | Charannya et al., 2018 [ |
| Root canal medicaments solution and gel form | The antibacterial efficacy of silver nanoparticles as an irrigant (0.1% AgNP) or medicament (0.02% and 0.01% AgNP) against E. faecalis biofilms was evaluated. | As a medication, 0.02% AgNP gel significantly impaired the structural integrity of the biofilm and resulted in the fewest viable E. faecalis cells remaining after treatment. | Wu et al., 2014 [ |
| Development of bioactive material | The antibacterial activities of NanoAg and NanoAg-MTA against 4 types of anaerobic pathogens were tested in vitro. Each gram of MTA powder was mixed with 350 µL of 25 ppm, 12.5 ppm, and 6.25 ppm preparations of NanoAg solution on sterile glass slabs using a sterile spatula. | AgNPs can effectively enhance the antibacterial activity of MTA against anaerobic periodontal/endodontic pathogens. | Bahador et al., 2015 [ |
| Root canal sealer | Methacrylate-resin dual-cured root canal sealer contained 5% dimethylaminohexadecyl methacrylate (DMAHDM), 0.15% AgNP, and nanoparticles of amorphous calcium phosphate (NACP) at 10%, 20%, and 30% mass fractions. Antibacterial properties against E. faecalis were measured. | The novel therapeutic root canal sealer with triple bioactive agents of DMAHDM, AgNP, and NACP neutralized acid and raised the pH, regenerated dentin minerals, and increased root dentin hardness. | Baras et al., 2019 [ |
| Regenerative endodontic procedures | In regenerative endodontics, the antibacterial effectiveness of double antibiotic paste (1 mg/mL DAP), silver nanoparticle (0.02% AgNP) gel, and tailored amorphous multiporous bioactive glass (100 mg/mL TAMP-BG) against 3 weeks of E. faecalis biofilms were evaluated. | These medicaments can function as potent intracanal drugs for regenerative endodontic procedures. However, complete elimination of E. faecalis biofilms occurred only at recommended concentrations and was made possible with AgNPs. | Athanassiads et al., 2007 [ |
| Fiber post cementation | The effect of AgNP solution on the mechanical properties of resin cements used for fiber post bonding was investigated. | The results indicate that the AgNP solution can be used as an irrigation protocol before glass fiber post cementation. | Suzuki et al., 2019 [ |
CHX, chlorhexidine; NaOCl, sodium hypochlorite; AgNP, silver nanoparticle; ZnONP, zinc oxide nanoparticle; MTA, mineral trioxide aggregate; E. faecalis, Enterococcus faecalis.
CHX, chlorhexidine; NaOCl, sodium hypochlorite; AgNP, silver nanoparticle; ZnONP, zinc oxide nanoparticle; MTA, mineral trioxide aggregate;

 KACD
KACD
 ePub Link
ePub Link Cite
Cite

