Articles
- Page Path
- HOME > Restor Dent Endod > Volume 46(3); 2021 > Article
- Research Article The effectiveness of the supplementary use of the XP-endo Finisher on bacteria content reduction: a systematic review and meta-analysis
-
Ludmila Smith de Jesus Oliveira1
 , Rafaella Mariana Fontes de Bragança2
, Rafaella Mariana Fontes de Bragança2 , Rafael Sarkis-Onofre3
, Rafael Sarkis-Onofre3 , André Luis Faria-e-Silva1,2
, André Luis Faria-e-Silva1,2
-
Restor Dent Endod 2021;46(3):e37.
DOI: https://doi.org/10.5395/rde.2021.46.e37
Published online: June 18, 2021
1Graduate Program in Health Sciences, Federal University of Sergipe, Aracaju, Brazil.
2Graduate Program in Dentistry, Federal University of Sergipe, Aracaju, Brazil.
3Graduate Program in Dentistry, IMED - Faculdade Meridional, Passo Fundo, Brazil.
- Correspondence to André Luis Faria-e-Silva, DDS, MSc, PhD. Associate Professor, Federal University of Sergipe, Rua Claudio Batista s/n, Aracaju, 49060-100, Brazil. fariaesilva.andre@gmail.com
Copyright © 2021. The Korean Academy of Conservative Dentistry
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Objectives This systematic review evaluated the efficacy of the supplementary use of the XP-endo Finisher on bacteria content reduction in the root canal system.
-
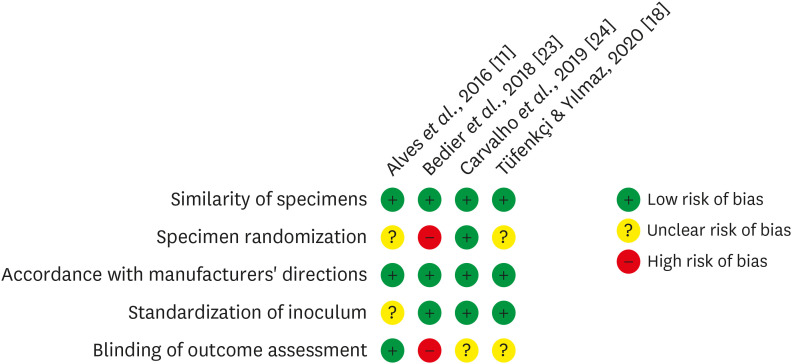
Materials and Methods In-vitro studies evaluating the use of the XP-endo Finisher on bacteria content were searched in four databases in July 2020. Two authors independently screened the studies for eligibility. Data were extracted, and risk of bias was assessed. Data were meta-analyzed by using random-effects model to compare the effect of the supplementary use (experimental) or not (control) of the XP-endo Finisher on bacteria counting reduction, and results from different endodontic protocols were combined. Four studies met the inclusion criteria while 1 study was excluded from the meta-analysis due to its high risk of bias and outlier data. The 3 studies that made it to the meta-analysis had an unclear risk of bias for at least one criterion.
-
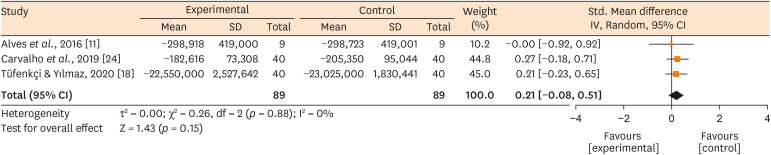
Results No heterogeneity was observed among the results of the studies included in the meta-analysis. The study excluded from the meta-analysis assessing the bacteria counting deep in the dentin demonstrated further bacteria reduction upon the use of the XP-endo Finisher.
-
Conclusions This systematic review found no evidence supporting the supplementary use of the XP-endo Finisher on further bacteria counting the reduction in the root canal.
INTRODUCTION
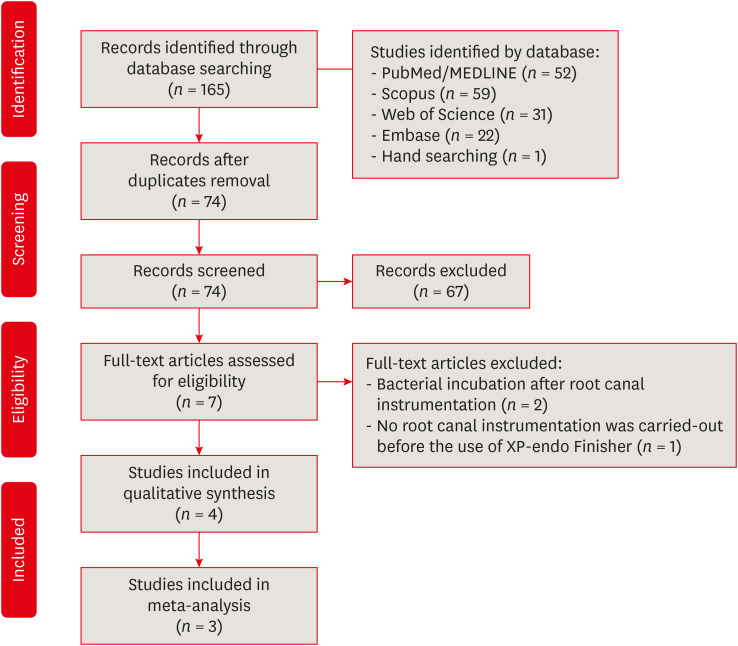
MATERIALS AND METHODS
Search strategy according to database
RESULTS
Characteristics of included studies
| Study | Alves et al., 2016 [11]* | Bedier et al., 2018 [23] | Carvalho et al., 2019 [24] | Tüfenkçi & Yılmaz, 2020 [18] |
|---|---|---|---|---|
| Teeth used | Mandibular molars | Mandibular molars | Mandibular incisors | Mandibular first molars |
| Contamination protocol | 1. Enlargement using BioRaCe BR2 (25/04) instrument. | 1. Enlargement up to a size 25 K-file | 1. Enlargement up to a size 25 K-file. | 1. Enlargement up to a file ISO 15. |
| 2. Smear layer removal with EDTA and 2.5% NaOCl. | 2. Sterilization in an autoclave | 2. Smear layer removal with EDTA. | 2. Smear layer removal with 5% NaOCl. | |
| 3. Specimens filled and immersed in TSB. | 3. Immersion of specimens in BHI. | 3. Immersion of specimens in BHI. | 3. Immersion of specimens in BHI. | |
| 4. Sterilization in an autoclave. | 4. Specimens contamination with E. faecalis (1 × 108 CFUs/mL). | 4. Sterilization in an autoclave. | 4. Sterilization in an autoclave. | |
| 5. Specimens contamination with E. faecalis. | 5. Incubation for 21 days at 37°C. | 5. Specimens contamination with E. faecalis (3 × 108 CFU/mL). | 5. Specimens contamination with E. faecalis (1 × 107 CFU/mL). | |
| 6. Incubation for 30 days at 37°C. | 6. Incubation for 10 days at 37°C. | 6. Incubation for 4 weeks at 37°C. | ||
| Bacteria counting method | 1. Rinsing with a sterile saline solution. | 1. Rinsing with a sterile saline solution. | 1. Rinsing with a sterile saline solution. | 1. Rinsing with a sterile saline solution. |
| 2. Bacteria recovered with sterile paper points. | 2. A sample measuring (2 × 2 × 4 mm in thickness) was removed from mild-third. | 2. Bacteria recovered with sterile stainless-steel a size 25 Hedstrom file and paper points. | 2. Bacteria recovered with sterile paper points. | |
| 3. Content transferred to tubes containing Tris-EDTA buffer. | 3. Staining procedure. | 3. Content transferred to tubes containing BHI. | 3. Content transferred to tubes containing phosphate-buffered solution. | |
| 4. DNA extraction and quantification of E. faecalis cells by using a 16S rRNA gene-targeted qPCR assay. | 4. Washing with PBS. | 4. Counting the CFU/mL on Agar-sheep blood plates after 48 hours of incubation at 37°C. | 4. Counting the CFU/mL on Agar-sheep blood plates after 24 hours of incubation at 37°C. | |
| 5. Percentage of dead bacteria at a depth of 50 μm was assessed using a CLSM. | ||||
| Systems used for root canal instrumentation | BT RaCe system (FKG Dentaire, La Chaux-de-Fonds, Switzerland) | XP-endo Shaper (FKG Dentaire) and iRaCe (FKG Dentaire) | XP-endo Shaper (FKG Dentaire) and Reciproc Blue (VDW, Munich, Germany) | ProTaper Next (Dentsply Maillefer, Ballaigues, Switzerland) and Reciproc (VDW) |
Forest plots showing the estimated effect of whether associating (Experimental) or not (Control) the use of XP-endo Finisher with root canal treatment on the bacteria counting reduction.
DISCUSSION
CONCLUSIONS
-
Funding: This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001.
-
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
-
Author Contributions:
Conceptualization: Oliveira LSJ, Bragança RMF, Faria-e-Silva AL.
Data curation: Oliveira LSJ, Bragança RMF, Sarkis-Onofre R, Faria-e-Silva AL Formal analysis.
Investigation: Oliveira LSJ,Bragança RMF.
Methodology: Faria-e-Silva AL name.
Project administration: Faria-e-Silva AL.
Supervision: Faria-e-Silva AL.
Writing - original draft: Oliveira LSJ, Bragança RMF, Sarkis-Onofre R, Faria-e-Silva AL.
Writing - review & editing: Oliveira LSJ, Bragança RMF, Sarkis-Onofre R, Faria-e-Silva AL.
SUPPLEMENTARY MATERIAL
- 1. Gomes BP, Lilley JD, Drucker DB. Variations in the susceptibilities of components of the endodontic microflora to biomechanical procedures. Int Endod J 1996;29:235-241.ArticlePubMed
- 2. Siqueira JF Jr, Araújo MC, Garcia PF, Fraga RC, Dantas CJ. Histological evaluation of the effectiveness of five instrumentation techniques for cleaning the apical third of root canals. J Endod 1997;23:499-502.ArticlePubMed
- 3. Pourhajibagher M, Ghorbanzadeh R, Bahador A. Culture-dependent approaches to explore the prevalence of root canal pathogens from endodontic infections. Braz Oral Res 2017;31:e108.ArticlePubMed
- 4. Vieira GCS, Antunes HS, Pérez AR, Gonçalves LS, Antunes FE, Siqueira JF Jr, Rôças IN. Molecular analysis of the antibacterial effects of photodynamic therapy in endodontic surgery: a case series. J Endod 2018;44:1593-1597.ArticlePubMed
- 5. Plotino G, Cortese T, Grande NM, Leonardi DP, Di Giorgio G, Testarelli L, Gambarini G. New technologies to improve root canal disinfection. Braz Dent J 2016;27:3-8.ArticlePubMed
- 6. Estrela C, Holland R, Estrela CR, Alencar AH, Sousa-Neto MD, Pécora JD. Characterization of successful root canal treatment. Braz Dent J 2014;25:3-11.ArticlePubMed
- 7. Peters OA, Schönenberger K, Laib A. Effects of four Ni-Ti preparation techniques on root canal geometry assessed by micro computed tomography. Int Endod J 2001;34:221-230.ArticlePubMedPDF
- 8. Siqueira Junior JF, Rôças IDN, Marceliano-Alves MF, Pérez AR, Ricucci D. Unprepared root canal surface areas: causes, clinical implications, and therapeutic strategies. Braz Oral Res 2018;32(Supplement 1):e65.PubMed
- 9. Pacheco-Yanes J, Provenzano JC, Marceliano-Alves MF, Gazzaneo I, Pérez AR, Gonçalves LS, Siqueira JF Jr. Distribution of sodium hypochlorite throughout the mesial root canal system of mandibular molars after adjunctive irrigant activation procedures: a micro-computed tomographic study. Clin Oral Investig 2020;24:907-914.ArticlePubMedPDF
- 10. Uygun AD, Kol E, Topcu MK, Seckin F, Ersoy I, Tanriver M. Variations in cyclic fatigue resistance among ProTaper Gold, ProTaper Next and ProTaper Universal instruments at different levels. Int Endod J 2016;49:494-499.ArticlePubMed
- 11. Alves FR, Marceliano-Alves MF, Sousa JC, Silveira SB, Provenzano JC, Siqueira JF Jr. Removal of root canal fillings in curved canals using either reciprocating single- or rotary multi-instrument systems and a supplementary step with the XP-endo Finisher. J Endod 2016;42:1114-1119.ArticlePubMed
- 12. Leoni GB, Versiani MA, Silva-Sousa YT, Bruniera JF, Pécora JD, Sousa-Neto MD. Ex vivo evaluation of four final irrigation protocols on the removal of hard-tissue debris from the mesial root canal system of mandibular first molars. Int Endod J 2017;50:398-406.ArticlePubMedPDF
- 13. Bao P, Shen Y, Lin J, Haapasalo M. In vitro efficacy of XP-endo Finisher with 2 different protocols on biofilm removal from apical root canals. J Endod 2017;43:321-325.ArticlePubMed
- 14. Souza LC, Brito PR, de Oliveira JC, Alves FR, Moreira EJ, Sampaio-Filho HR, Rôças IN, Siqueira JF Jr. Photodynamic therapy with two different photosensitizers as a supplement to instrumentation/irrigation procedures in promoting intracanal reduction of Enterococcus faecalis . J Endod 2010;36:292-296.ArticlePubMed
- 15. Sasanakul P, Ampornaramveth RS, Chivatxaranukul P. Influence of adjuncts to irrigation in the disinfection of large root canals. J Endod 2019;45:332-337.ArticlePubMed
- 16. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097.ArticlePubMedPMC
- 17. In: Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions 5.1.0 [Internet]. London: The Cochrane Collaboration; 2011. updated 2011 Mar]. cited 2020 Jun 22]. Available from: https://handbook-5-1.cochrane.org/.
- 18. Tüfenkçi P, Yılmaz K. The effects of different endodontic access cavity design and using XP-endo Finisher on the reduction of Enterococcus faecalis in the root canal system. J Endod 2020;46:419-424.ArticlePubMed
- 19. Plotino G, Nagendrababu V, Bukiet F, Grande NM, Veettil SK, De-Deus G, Aly Ahmed HM. Influence of negotiation, glide path, and preflaring procedures on root canal shaping-terminology, basic concepts, and a systematic review. J Endod 2020;46:707-729.ArticlePubMed
- 20. Valle AD, Dotto L, Morgental RD, Pereira-Cenci T, Pereira GKDR, Sarkis-Onofre R. Influence of root canal preparation on formation of dentinal microcracks: a systematic review. Braz Dent J 2020;31:201-220.ArticlePubMed
- 21. Lima VP, Soares K, Caldeira VS, Faria-e-Silva AL, Loomans B, Moraes RR. Airborne-particle abrasion and dentin bonding: systematic review and meta-analysis. Oper Dent 2021;46:E21-E33.ArticlePubMedPDF
- 22. Alves FR, Andrade-Junior CV, Marceliano-Alves MF, Pérez AR, Rôças IN, Versiani MA, Sousa-Neto MD, Provenzano JC, Siqueira JF Jr. Adjunctive steps for disinfection of the mandibular molar root canal system: a correlative bacteriologic, micro-computed tomography, and cryopulverization approach. J Endod 2016;42:1667-1672.ArticlePubMed
- 23. Bedier MM, Hashem AAR, Hassan YM. Improved dentin disinfection by combining different-geometry rotary nickel-titanium files in preparing root canals. Restor Dent Endod 2018;43:e46.ArticlePubMedPMCPDF
- 24. Carvalho MC, Zuolo ML, Arruda-Vasconcelos R, Marinho ACS, Louzada LM, Francisco PA, Pecorari VGA, Gomes BPFA. Effectiveness of XP-endo Finisher in the reduction of bacterial load in oval-shaped root canals. Braz Oral Res 2019;33:e021.ArticlePubMed
- 25. Ran S, Wang J, Jiang W, Zhu C, Liang J. Assessment of dentinal tubule invasion capacity of Enterococcus faecalis under stress conditions ex vivo . Int Endod J 2015;48:362-372.ArticlePubMed
- 26. Brignardello-Petersen R, Carrasco-Labra A, Shah P, Azarpazhooh A. A practitioner's guide to developing critical appraisal skills: what is the difference between clinical and statistical significance? J Am Dent Assoc 2013;144:780-786.PubMed
- 27. Faggion CM Jr. Guidelines for reporting pre-clinical in vitro studies on dental materials. J Evid Based Dent Pract 2012;12:182-189.Article
- 28. Krithikadatta J, Gopikrishna V, Datta M. CRIS guidelines (Checklist for Reporting In-vitro Studies): a concept note on the need for standardized guidelines for improving quality and transparency in reporting in-vitro studies in experimental dental research. J Conserv Dent 2014;17:301-304.ArticlePubMedPMC
- 29. Siddique R, Nivedhitha MS. Effectiveness of rotary and reciprocating systems on microbial reduction: a systematic review. J Conserv Dent 2019;22:114-122.ArticlePubMedPMC
- 30. Paqué F, Rechenberg DK, Zehnder M. Reduction of hard-tissue debris accumulation during rotary root canal instrumentation by etidronic acid in a sodium hypochlorite irrigant. J Endod 2012;38:692-695.ArticlePubMed
- 31. Tawakoli PN, Ragnarsson KT, Rechenberg DK, Mohn D, Zehnder M. Effect of endodontic irrigants on biofilm matrix polysaccharides. Int Endod J 2017;50:153-160.ArticlePubMedPDF
REFERENCES
Tables & Figures
REFERENCES
Citations

- Mapping risk of bias criteria in systematic reviews of in vitro endodontic studies: an umbrella review
Rafaella Rodrigues da Gama, Lucas Peixoto de Araújo, Evandro Piva, Leandro Perello Duro, Adriana Fernandes da Silva, Wellington Luiz de Oliveira da Rosa
Evidence-Based Dentistry.2025; 26(4): 179. CrossRef - Characteristics and Effectiveness of XP‐Endo Files and Systems: A Narrative Review
Sarah M. Alkahtany, Rana Alfadhel, Aseel AlOmair, Sarah Bin Durayhim, Kee Y. Kum
International Journal of Dentistry.2024;[Epub] CrossRef - Impact XP-endo finisher on the 1-year follow-up success of posterior root canal treatments: a randomized clinical trial
Ludmila Smith de Jesus Oliveira, Fabricio Eneas Diniz de Figueiredo, Janaina Araújo Dantas, Maria Amália Gonzaga Ribeiro, Carlos Estrela, Manoel Damião Sousa-Neto, André Luis Faria-e-Silva
Clinical Oral Investigations.2023; 27(12): 7595. CrossRef - Comparative analysis of the effectiveness of modern irrigants activation techniques in the process of mechanical root canal system treatment (Literature review)
Anatoliy Potapchuk, Vasyl Almashi, Arsenii Horzov, Victor Buleza
InterConf.2023; (34(159)): 200. CrossRef - Comparative analysis of the effectiveness of modern irrigants activation techniques in the protocol of chemomechanical root canal system treatment (literature review)
A. Potapchuk, V. Almashi, Y. Rak, Y. Melnyk, V. Buleza, A. Horzov
SUCHASNA STOMATOLOHIYA.2023; 114(3): 4. CrossRef - Methodological quality assessment criteria for the evaluation of laboratory‐based studies included in systematic reviews within the specialty of Endodontology: A development protocol
Venkateshbabu Nagendrababu, Paul V. Abbott, Christos Boutsioukis, Henry F. Duncan, Clovis M. Faggion, Anil Kishen, Peter E. Murray, Shaju Jacob Pulikkotil, Paul M. H. Dummer
International Endodontic Journal.2022; 55(4): 326. CrossRef
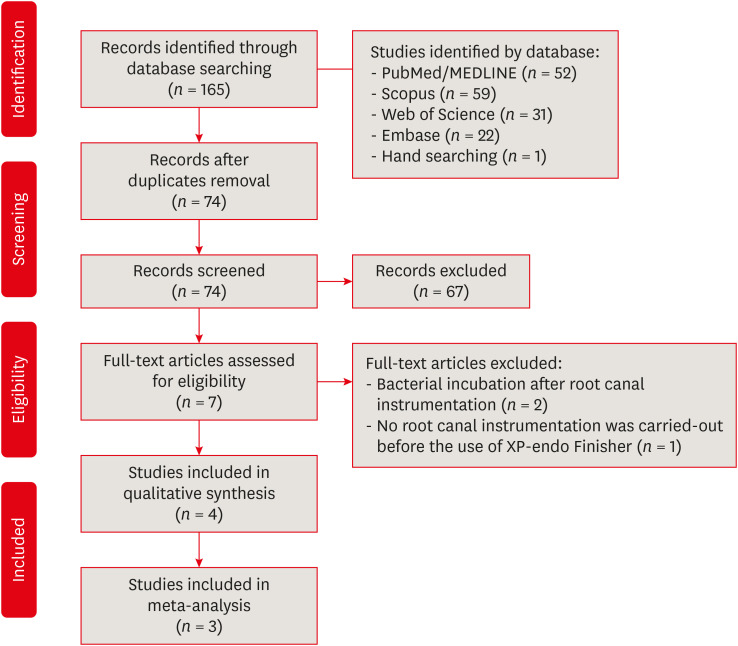
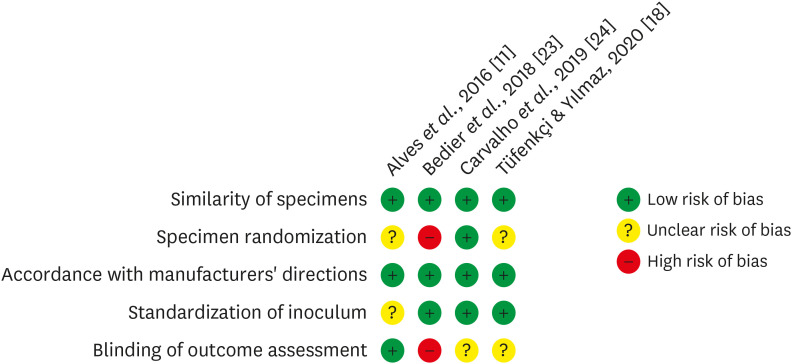
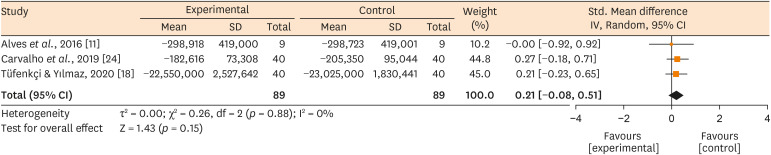
Figure 1
Figure 2
Figure 3
Search strategy according to database
| Database | Search strategy |
|---|---|
| PubMed/MEDLINE | (“Root Canal Preparation” [Mesh] OR “Root Canal Therapy” [Mesh] OR “Canal Preparation, Root” [Title/Abstract] OR “Canal Preparations, Root” [Title/Abstract] OR “Preparation, Root Canal” [Title/Abstract] OR “Root Canal Preparations” [Title/Abstract] OR “Canal Therapy, Root” [Title/Abstract] OR “Root Canal Therapies” [Title/Abstract] OR “Therapy, Root Canal” [Title/Abstract]) AND (“XP endo” [Title/Abstract] OR “XP-endo” [Title/Abstract]) |
| Scopus | (TITLE-ABS-KEY (“Endodontic treatment” OR “Root Canal Preparation” OR “Canal Preparation, Root” OR “Canal Preparations, Root” OR “Preparation, Root Canal” OR “Preparations, Root Canal” OR “Root Canal Preparations”)) AND (TITLE-ABS-KEY (“XP endo finisher” OR “XP-endo”)) |
| Web of Science | (TS = (Root Canal Preparation OR Root Canal Preparations)) AND (TS = (XP endo OR XP-endo)) |
| Embase | (‘endodontic treatment’:ti,ab,kw OR ‘root canal preparation’:ti,ab,kw OR ‘canal preparation, root’:ti,ab,kw OR ‘canal preparations, root’:ti,ab,kw OR ‘preparation, root canal’:ti,ab,kw OR ‘preparations, root canal’:ti,ab,kw OR ‘root canal preparations’:ti,ab,kw) AND (‘xp endo’:ti,ab,kw OR ‘XP-endo’:ti,ab,kw) |
Characteristics of included studies
| Study | Alves et al., 2016 [ | Bedier et al., 2018 [ | Carvalho et al., 2019 [ | Tüfenkçi & Yılmaz, 2020 [ |
|---|---|---|---|---|
| Teeth used | Mandibular molars | Mandibular molars | Mandibular incisors | Mandibular first molars |
| Contamination protocol | 1. Enlargement using BioRaCe BR2 (25/04) instrument. | 1. Enlargement up to a size 25 K-file | 1. Enlargement up to a size 25 K-file. | 1. Enlargement up to a file ISO 15. |
| 2. Smear layer removal with EDTA and 2.5% NaOCl. | 2. Sterilization in an autoclave | 2. Smear layer removal with EDTA. | 2. Smear layer removal with 5% NaOCl. | |
| 3. Specimens filled and immersed in TSB. | 3. Immersion of specimens in BHI. | 3. Immersion of specimens in BHI. | 3. Immersion of specimens in BHI. | |
| 4. Sterilization in an autoclave. | 4. Specimens contamination with E. faecalis (1 × 108 CFUs/mL). | 4. Sterilization in an autoclave. | 4. Sterilization in an autoclave. | |
| 5. Specimens contamination with E. faecalis. | 5. Incubation for 21 days at 37°C. | 5. Specimens contamination with E. faecalis (3 × 108 CFU/mL). | 5. Specimens contamination with E. faecalis (1 × 107 CFU/mL). | |
| 6. Incubation for 30 days at 37°C. | 6. Incubation for 10 days at 37°C. | 6. Incubation for 4 weeks at 37°C. | ||
| Bacteria counting method | 1. Rinsing with a sterile saline solution. | 1. Rinsing with a sterile saline solution. | 1. Rinsing with a sterile saline solution. | 1. Rinsing with a sterile saline solution. |
| 2. Bacteria recovered with sterile paper points. | 2. A sample measuring (2 × 2 × 4 mm in thickness) was removed from mild-third. | 2. Bacteria recovered with sterile stainless-steel a size 25 Hedstrom file and paper points. | 2. Bacteria recovered with sterile paper points. | |
| 3. Content transferred to tubes containing Tris-EDTA buffer. | 3. Staining procedure. | 3. Content transferred to tubes containing BHI. | 3. Content transferred to tubes containing phosphate-buffered solution. | |
| 4. DNA extraction and quantification of E. faecalis cells by using a 16S rRNA gene-targeted qPCR assay. | 4. Washing with PBS. | 4. Counting the CFU/mL on Agar-sheep blood plates after 48 hours of incubation at 37°C. | 4. Counting the CFU/mL on Agar-sheep blood plates after 24 hours of incubation at 37°C. | |
| 5. Percentage of dead bacteria at a depth of 50 μm was assessed using a CLSM. | ||||
| Systems used for root canal instrumentation | BT RaCe system (FKG Dentaire, La Chaux-de-Fonds, Switzerland) | XP-endo Shaper (FKG Dentaire) and iRaCe (FKG Dentaire) | XP-endo Shaper (FKG Dentaire) and Reciproc Blue (VDW, Munich, Germany) | ProTaper Next (Dentsply Maillefer, Ballaigues, Switzerland) and Reciproc (VDW) |
BHI, brain heart infusion; CFU/mL: colony-forming unit per milliliter; CLSM, confocal laser scanning microscopy; EDTA, ethylenediaminetetraacetic acid; NaOCl, sodium hypochlorite; PBS, phosphate buffer saline; qPCR, quantitative polymerase chain reaction; TSB, trypticase soy broth.
*Only the phase 1 of the study was included.
BHI, brain heart infusion; CFU/mL: colony-forming unit per milliliter; CLSM, confocal laser scanning microscopy; EDTA, ethylenediaminetetraacetic acid; NaOCl, sodium hypochlorite; PBS, phosphate buffer saline; qPCR, quantitative polymerase chain reaction; TSB, trypticase soy broth.
*Only the phase 1 of the study was included.

 KACD
KACD
 ePub Link
ePub Link Cite
Cite

