Articles
- Page Path
- HOME > Restor Dent Endod > Volume 45(4); 2020 > Article
- Research Article A new phantom to evaluate the tissue dissolution ability of endodontic irrigants and activating devices
-
Kimia Khoshroo1
 , Brinda Shah1
, Brinda Shah1 , Alexander Johnson2
, Alexander Johnson2 , John Baeten2
, John Baeten2 , Katherine Barry2
, Katherine Barry2 , Mohammadreza Tahriri1
, Mohammadreza Tahriri1 , Mohamed S. Ibrahim1,3
, Mohamed S. Ibrahim1,3 , Lobat Tayebi1
, Lobat Tayebi1
-
Restor Dent Endod 2020;45(4):e45.
DOI: https://doi.org/10.5395/rde.2020.45.e45
Published online: August 24, 2020
1Marquette University School of Dentistry, Milwaukee, WI, USA.
2Inter Med-Vista Dental, Racine, WI, USA.
3Faculty of Dentistry, Mansoura University, Mansoura, Egypt.
- Correspondence to Lobat Tayebi, PhD. Associate Professor and Director of Research, Marquette University School of Dentistry, 1801 West Wisconsin Ave., Milwaukee, WI 53233, USA. lobat.tayebi@marquette.edu
• Received: November 16, 2019 • Revised: March 23, 2020 • Accepted: March 23, 2020
Copyright © 2020. The Korean Academy of Conservative Dentistry
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,863 Views
- 10 Download
- 1 Crossref
Abstract
-
Objective The aim of this study was to introduce a gelatin/bovine serum albumin (BSA) tissue standard, which provides dissolution properties identical to those of biological tissues. Further, the study evaluated whether the utilization of endodontic activating devices led to enhanced phantom dissolution rates.
-
Materials and Methods Bovine pulp tissue was obtained to determine a benchmark of tissue dissolution. The surface area and mass of samples were held constant while the ratio of gelatin and BSA were varied, ranging from 7.5% to 10% gelatin and 5% BSA. Each sample was placed in an individual test tube that was filled with an appropriate sodium hypochlorite solution for 1, 3, and 5 minutes, and then removed from the solution, blotted dry, and weighed again. The remaining tissue was calculated as the percent of initial tissue to determine the tissue dissolution rate. A radiopaque agent (sodium diatrizoate) and a fluorescent dye (methylene blue) were added to the phantom to allow easy quantification of phantom dissolution in a canal block model when activated using ultrasonic (EndoUltra) or sonic (EndoActivator) energy.
-
Results The 9% gelatin + 5% BSA phantom showed statistically equivalent dissolution to bovine pulp tissue at all time intervals. Furthermore, the EndoUltra yielded significantly more phantom dissolution in the canal block than the EndoActivator or syringe irrigation.
-
Conclusions Our phantom is comparable to biological tissue in terms of tissue dissolution and could be utilized for in vitro tests due to its injectability and detectability.
INTRODUCTION
Irrigation of the root canal system during endodontic therapy is an essential step in removing pulp tissue and debris and eradicating microorganisms from the complex root canal apparatus [1,2,3]. Currently, the irrigant of choice in endodontic therapy is sodium hypochlorite (NaOCl) solution due to its optimal tissue-dissolving capabilities and anti-microbial effectiveness [4,5]. Additionally, NaOCl serves as both a lubricant and a disinfectant, as it is capable of effectively dissolving necrotic pulp tissue and killing microbes in the canal system [6]. As an effective lubricant, NaOCl is able to penetrate the intricate structures of the canal and easily percolate through the root canal architecture due to its low viscosity, resulting in low friction between the instrument and root canal walls [7]. Furthermore, NaOCl is an effective disinfectant that is able to effectively rid the root canal system of organic pulp tissue, loose debris, and microbial biofilms [2,8].
Due to these desirable characteristics, numerous studies have measured and assessed the tissue-dissolving capabilities of NaOCl in various conditions [9,10,11,12,13]. While these studies independently hold significant merit, they used a wide range of different tissues, including bovine pulp tissue [14], rat abdominal tissue [15], porcine palatal mucosa [5,16], rabbit liver tissue [17,18], and bovine tendon collagen [19]. The variability in the results of these studies could be due to the varying experimental protocols, materials, and tissues used, making it difficult to draw clinically relevant conclusions [20]. The ideal biological tissue to investigate is dental pulp tissue because its histological characteristics are distinct from those of other anatomical tissues [21]. Dental pulp tissue is composed of loose, fibrous connective tissue with type I and type III collagen with a gelatinous consistency [22]. Bovine pulp tissue is more readily available and more easily preserved than other pulp tissues and is therefore a good benchmark for studies evaluating tissue dissolution [23].
The aim of this study was to introduce a gelatin/bovine serum albumin (BSA) tissue standard that can be used in lieu of pulp tissue to provide consistent results regarding the tissue dissolution properties of endodontic irrigants, including NaOCl. BSA and gelatin were used to mimic the dental pulp tissue in terms of its connective tissue composition and gelatinous consistency. Ultimately, this tissue-mimicking material could be potentially used in future research to standardize results when assessing different properties of endodontic medicaments. In this study, the tissue dissolution rate of this tissue phantom was evaluated in comparison to bovine pulp tissue by exposing various concentrations of the tissue phantom to NaOCl solution at 3-time intervals. In addition to its tissue-dissolution properties, the cleanliness of the developed tissue phantom material was examined with radiopaque fluorescent molecules.
MATERIALS AND METHODS
Bovine pulp tissue was obtained to determine a benchmark of tissue dissolution properties. Bovine pulp tissue was extracted from bovine teeth by splitting the bovine teeth in half and elevating the pulp from the teeth with anatomic forceps. The bovine pulp tissue was stored at −15°C. Next, the gelatin (G9391-500G, Type B, Sigma-Aldrich, St. Louis, MO, USA)/BSA (A2153-100 G, Sigma-Aldrich) tissue standard was created. This was also stored at −15°C. Of note, at room temperature, this tissue standard gelatinizes in order to mimic the consistency of biological pulp tissue.
Rectangular samples of bovine pulp tissue with similar size (10 × 4 mm) and mass (45 ± 3 mg) and different compositions of tissue-mimicking material, composed of 5% BSA and 7.5%, 8.5%, 9%, or 10% gelatin (n = 10) were prepared. Saline was used as a control group.
Then, 2 mL of 5.25% NaOCl solution (14.5%, Alfa Aesar Chemicals, Haverhill, MA, USA) as the root canal irrigant of choice was used at room temperature (25°C). Before immersing the samples into the NaOCl, each sample was weighed using a precision balance (Mettler Toledo Co., Columbus, OH, USA). Each sample was then placed in an individual test tube filled with 2 mL of 5.25% NaOCl solution. The samples with different compositions were inserted into the test tubes for 1, 3, and 5 minutes each. The samples were then removed from the solution, gently blotted dry, and weighed again. The remaining tissue weight was calculated as the percent of the initial tissue weight in order to determine the tissue dissolution rate when using 5.25% NaOCl for different concentrations of the pulp tissue-mimicking material under different exposure times to the irrigant. The amount of tissue dissolution was compared between groups using the t-test for statistical analysis.
Qualitatively, a radiopaque agent (sodium diatrizoate) and a fluorescent dye (methylene blue) were added to the phantom to enable easy quantification of the phantom. Methylene blue is strongly fluorescent, with an emission peak at 686 nm (λex, 665 nm) [24]. However, it was first necessary to test the effect of these chemicals on phantom dissolution to ensure that it showed similar dissolution rates to the bovine pulp. The 9% gelatin (G9391-500 G, Taye B, Sigma-Aldrich) + 5% BSA (A2153-100 G, Sigma-Aldrich) phantom was injected into an endodontic block (GuttaCore 4-Canal Practice Block, Dentsply Sirona, Charlotte, NC, USA) with 1 main canal and 2 lateral canals. During the synthesis of the tissue-mimicking material, methylene blue (0.01%) (code: 229801000, lot No. A0362510, Acros Organics, Pittsburgh, PA, USA), was added to the composition; therefore, after injecting the material into the endodontic block, it was detectable under the fluorescent microscope.
Next, the endodontic block was instrumented using EndoSequence endodontic files up to size 40/04, with 2 mL of NaOCl irrigation after each file change. The canal was then irrigated using 3 different methods with 5 mL of NaOCl solution: 1) conventional syringe irrigation with NaOCl, 2) ultrasonic irrigation with EndoUltra, and 3) sonic irrigation with EndoActivator according to the manufacturer's instructions. Finally, the remaining tissue was observed under a fluorescent microscope. The obtained images were analyzed using the ImageJ program (LOCI, University of Wisconsin, Madison, WI, USA). The outcome variable determined was the amount of remaining tissue, which was calculated as a percentage of the initial tissue to determine the tissue dissolution rate. All measurements were conducted by 1 operator.
To compare the overall tissue dissolution at room temperature with the corresponding values obtained under various conditions, the sums of remaining area (%) in all main canals per model were averaged for each model (n = 10) and compared using the paired t-test. All tests were conducted with 95% confidence intervals (p < 0.05).
RESULTS
The results demonstrated that the 9% gelatin + 5% BSA phantom showed statistically equivalent dissolution to bovine pulp tissue at all time intervals (Figure 1). Furthermore, there was no significant difference in the 9% gelatin + 5% BSA phantom dissolution before and after adding reporter molecules (p > 0.05). Therefore, the 9% gelatin + 5% BSA phantom was chosen as the optimum composition for the cleanliness experiments.
Figure 1
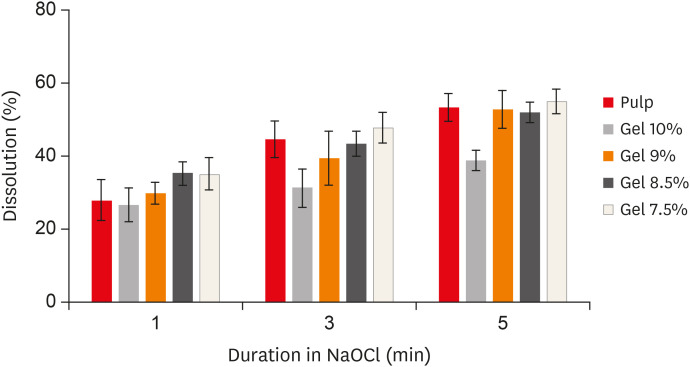
Dissolution (%) of the different phantoms at all time intervals.
NaOCl, sodium hypochlorite.

Figure 2A shows the results of fluorescence microscopy of endodontic blocks that were instrumented with endodontic files and then irrigated using 3 different methods. The results obtained for remaining tissue (%) showed that the endodontic blocks irrigated with EndoUltra (61.89% ± 11.57%) had a significantly higher dissolution rate than those irrigated using the other methods (syringe irrigation, 89.54% ± 6.71%; EndoActivator, 71.61% ± 6.18%) (Figure 2B).
Figure 2
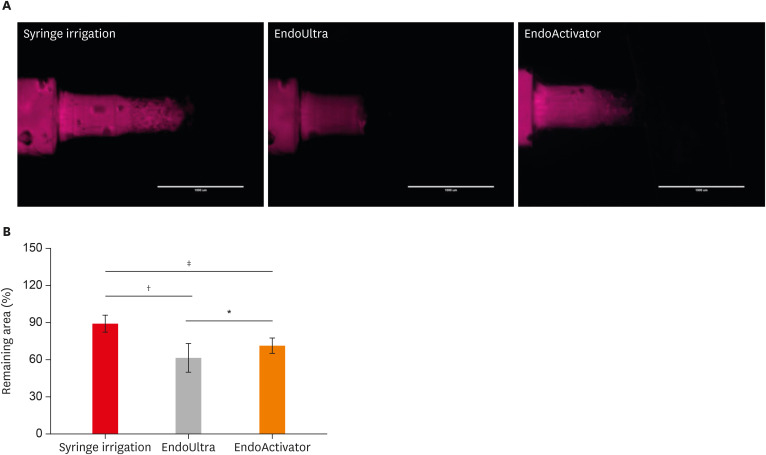
(A) Fluorescence microscopy of the endodontic blocks that were instrumented with endodontic files and then irrigated using 3 different methods. (B) Tissue remaining (%) in the endodontic blocks that were irrigated using 3 different methods.
*p < 0.05; †p < 0.001; ‡p < 0.0001.

DISCUSSION
In the present study, a method of preparing a gelatin/BSA phantom to simulate the dissolution properties of dental pulp tissue was described. Phantoms that simulate various characteristics of tissues are commonly used in biomedical applications to mimic living tissue properties for reliable reference measurements, to provide quantitative information, and to calibrate systems such as ultrasound imaging, spectroscopy, and dosimetry [25,26,27]. Acoustic and optical properties are among the most studied properties using phantoms in the literature [25,27]. In particular, magnesium silicate-based materials, agarose-based materials, condensed milk-based materials, polyvinyl alcohol-based materials and gelatin-based materials are widely used as tissue substitutes [25].
The implementation of soft tissue substitutes in dental research is a novel topic. In the current endodontics literature, tissue dissolution properties have been studied in a vast variety of vital and necrotic tissue samples from different tissue sources such as human pulp tissue, porcine oral mucosa, and bovine muscle samples [28]. Due to the wide range of variation, the need for standardized models with known and controllable dissolution properties is of paramount importance in order to obtain repeatable and comparable results.
The loose connective tissue of the pulp is roughly composed of 75% water and 25% organic material (mainly collagen) [29]. In the present study, aqueous solution of gelatin/BSA was proposed as a substitute for pulp tissue. Gelatin is derived from collagen by destruction of cross-linkage of polypeptide chains and has a long history of application as a tissue-mimicking material for ultrasound phantoms [30].
Extensive research has evaluated the tissue-dissolving ability of NaOCl. It has been demonstrated that the solvent capability of NaOCl depends on its concentration, volume, pH, temperature, time, agitation, and the type, amount, and surface area of the tissue [12,13,31,32]. As such, the considerable variation in these factors across studies makes it difficult to draw comparative conclusions based on the existing research and to assess the relative significance of each factor [33].
The present study examined the influence of agitation on the capability of NaOCl to dissolve organic materials in a standardized setting and demonstrated that activation improved tissue dissolution. Previous research has ascertained that the tissue-dissolving ability of NaOCl solution decreases if it is diluted [31,34]. It is also worth mentioning that the results obtained from the present study showed that 5% NaOCl was effective. The significance of agitation on the tissue-dissolving ability of NaOCl has been reported in the literature, but relatively few studies have specifically analyzed the effects of agitation [35].
An aqueous solution of NaOCl is a dynamic balance of NaOH and HClO. When NaOCl is in contact with organic material, NaOH reacts with fatty acids to form soap and glycerol, in a reaction known as saponification. It also reacts with amino acids to form salt and water (neutralization). Additionally, HClO reacts with amino acids to form NH2Cl (chloramine) and H2O. These reactions, which generally occur at the surface, cause the liquefaction of organic tissue [36].
In the meantime, the molecules of NaOCl that take part in these reactions are consumed, bringing about a decrease of nearby activity. Thus, it is critical to supply active hypochlorite to the region and to expel the remnants of dissolved tissue. In this study, the effects of different agitation techniques on the tissue-dissolving ability of NaOCl were evaluated, and it was found that agitation of the solution enhanced its dissolving ability to a statistically significant extent.
It was noteworthy that after injecting the tissue-mimicking material into the simulated canals, we let the material set at room temperature for 15 minutes, after which the process of instrumentation and irrigation and data acquisition should be performed as soon as possible. The reason for this is that the tissue-mimicking material dries out over time; this also occurs with natural pulp tissue, since water is a major component of both materials. Therefore, handling ought to be performed cautiously and immediately to preserve the similarity with in vivo conditions.
The utilization of ultrasound energy in root canal fabrication and irrigation has long been a disputed issue. Although some researchers reported the successful use of ultrasonic tools in root canal cleaning [37,38,39,40], others did not find ultrasound to be advantageous compared with root canal irrigation utilizing a syringe [41,42].
The mechanism of passive ultrasonic action has been proposed to involve acoustic streaming (microstreaming) and cavitation. Sonic energy has a comparable mechanism to that of ultrasonic energy, although the pattern of the oscillating file is different [43]. However, as indicated by Estrela et al. [36], cavitation is constrained to a distance of under 100 µm. It is important to point out that acoustic streaming results in a stirring action and causes rapid movement of the liquid away from the energy source. This is most likely the mechanism through which ultrasonic energy influences the cleaning of the peripheral sections of the root canal in vivo. Within the limitations of the present study, the method of agitation enhanced tissue dissolution.
CONCLUSIONS
In summary, our developed phantom in this study is comparable to biological tissue in terms of tissue dissolution and could be utilized for in vitro tests due to its injectability and detectability. Furthermore, the data obtained from the cleanliness experiments demonstrated that the percentage of remaining material in the endodontic blocks irrigated with EndoUltra was significantly lower than in the blocks irrigated using the other methods.
-
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
-
Author Contributions:
- 1. Nair PN, Henry S, Cano V, Vera J. Microbial status of apical root canal system of human mandibular first molars with primary apical periodontitis after “one-visit” endodontic treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2005;99:231-252.ArticlePubMed
- 2. Zehnder M. Root canal irrigants. J Endod 2006;32:389-398.ArticlePubMed
- 3. Peters OA, Peters CI, Basrani B. Cleaning and shaping of the root canal system. In: Cohen S, Hargreaves KM, editors. Cohen's pathways of the pulp. St. Louis, MO: Elsevier; 2006. p. 209-357.
- 4. Clarkson RM, Podlich HM, Savage NW, Moule AJ. A survey of sodium hypochlorite use by general dental practitioners and endodontists in Australia. Aust Dent J 2003;48:20-26.ArticlePubMed
- 5. Naenni N, Thoma K, Zehnder M. Soft tissue dissolution capacity of currently used and potential endodontic irrigants. J Endod 2004;30:785-787.ArticlePubMed
- 6. Baumgartner JC, Cuenin PR. Efficacy of several concentrations of sodium hypochlorite for root canal irrigation. J Endod 1992;18:605-612.ArticlePubMed
- 7. Poggio C, Ceci M, Beltrami R, Colombo M, Dagna A. Viscosity of endodontic irrigants: influence of temperature. Dent Res J (Isfahan) 2015;12:425-430.ArticlePubMedPMC
- 8. Cheung GS, Stock CJ. In vitro cleaning ability of root canal irrigants with and without endosonics. Int Endod J 1993;26:334-343.ArticlePubMed
- 9. Yang SF, Rivera EM, Baumgardner KR, Walton RE, Stanford C. Anaerobic tissue-dissolving abilities of calcium hydroxide and sodium hypochlorite. J Endod 1995;21:613-616.ArticlePubMed
- 10. Cunningham WT, Balekjian AY. Effect of temperature on collagen-dissolving ability of sodium hypochlorite endodontic irrigant. Oral Surg Oral Med Oral Pathol 1980;49:175-177.ArticlePubMed
- 11. Zehnder M, Kosicki D, Luder H, Sener B, Waltimo T. Tissue-dissolving capacity and antibacterial effect of buffered and unbuffered hypochlorite solutions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2002;94:756-762.ArticlePubMed
- 12. Christensen CE, McNeal SF, Eleazer P. Effect of lowering the pH of sodium hypochlorite on dissolving tissue in vitro . J Endod 2008;34:449-452.ArticlePubMed
- 13. Stojicic S, Zivkovic S, Qian W, Zhang H, Haapasalo M. Tissue dissolution by sodium hypochlorite: effect of concentration, temperature, agitation, and surfactant. J Endod 2010;36:1558-1562.ArticlePubMed
- 14. Koskinen KP, Stenvall H, Uitto VJ. Dissolution of bovine pulp tissue by endodontic solutions. Eur J Oral Sci 1980;88:406-411.Article
- 15. Thé SD. The solvent action of sodium hypochlorite on fixed and unfixed necrotic tissue. Oral Surg Oral Med Oral Pathol 1979;47:558-561.ArticlePubMed
- 16. Clarkson RM, Moule AJ, Podlich H, Kellaway R, Macfarlane R, Lewis D, Rowell J. Dissolution of porcine incisor pulps in sodium hypochlorite solutions of varying compositions and concentrations. Aust Dent J 2006;51:245-251.ArticlePubMedPDF
- 17. Macedo RG, Wesselink PR, Zaccheo F, Fanali D, Van Der Sluis LW. Reaction rate of NaOCl in contact with bovine dentine: effect of activation, exposure time, concentration and pH. Int Endod J 2010;43:1108-1115.ArticlePubMed
- 18. Cameron JA. The use of 4 per cent sodium hypochlorite, with or without ultrasound, in cleansing of uninstrumented immature root canals; SEM study. Aust Dent J 1987;32:204-213.ArticlePubMed
- 19. Pascon FM, Puppin-Rontani RM. The influence of cleansers on the permeability index of primary tooth root dentin. J Clin Pediatr Dent 2006;31:93-97.ArticlePubMedPDF
- 20. Gu LS, Kim JR, Ling J, Choi KK, Pashley DH, Tay FR. Review of contemporary irrigant agitation techniques and devices. J Endod 2009;35:791-804.ArticlePubMed
- 21. Christo JE, Zilm PS, Sullivan T, Cathro PR. Efficacy of low concentrations of sodium hypochlorite and low-powered Er,Cr:YSGG laser activated irrigation against an Enterococcus faecalis biofilm. Int Endod J 2016;49:279-286.PubMed
- 22. Linde A. The extracellular matrix of the dental pulp and dentin. J Dent Res 1985;64 Spec No:523-529.PubMed
- 23. de Almeida LH, Leonardo NG, Gomes AP, Giardino L, Souza EM, Pappen FG. Pulp tissue dissolution capacity of sodium hypochlorite combined with cetrimide and polypropylene glycol. Braz Dent J 2013;24:477-481.ArticlePubMed
- 24. Selvam S, Sarkar I. Bile salt induced solubilization of methylene blue: study on methylene blue fluorescence properties and molecular mechanics calculation. J Pharm Anal 2017;7:71-75.ArticlePubMed
- 25. Culjat MO, Goldenberg D, Tewari P, Singh RS. A review of tissue substitutes for ultrasound imaging. Ultrasound Med Biol 2010;36:861-873.ArticlePubMed
- 26. Moffitt T, Chen YC, Prahl SA. Preparation and characterization of polyurethane optical phantoms. J Biomed Opt 2006;11:041103.ArticlePubMed
- 27. Pogue BW, Patterson MS. Review of tissue simulating phantoms for optical spectroscopy, imaging and dosimetry. J Biomed Opt 2006;11:041102.PubMed
- 28. Kutty SK, Lekshmi MS, Mohan A, Isaac L. Pulp tissue dissolution in endodontics- A review. Int J Appl Dent Sci 2017;3:193-196.
- 29. Frank RM, Nalbandian J. Structure and ultrastructure of the dental pulp. In: Berkovitz BK, Boyde A, Frank RM, Höhling HJ, Moxham BJ, Nalbandian J, Tonge CH, editors. Teeth. Berlin: Springer; 1989. p. 249-307.
- 30. Madsen EL, Zagzebski JA, Banjavie RA, Jutila RE. Tissue mimicking materials for ultrasound phantoms. Med Phys 1978;5:391-394.ArticlePubMedPDF
- 31. Hand RE, Smith ML, Harrison JW. Analysis of the effect of dilution on the necrotic tissue dissolution property of sodium hypochlorite. J Endod 1978;4:60-64.ArticlePubMed
- 32. Türkün M, Cengiz T. The effects of sodium hypochlorite and calcium hydroxide on tissue dissolution and root canal cleanliness. Int Endod J 1997;30:335-342.ArticlePubMed
- 33. Beltz RE, Torabinejad M, Pouresmail M. Quantitative analysis of the solubilizing action of MTAD, sodium hypochlorite, and EDTA on bovine pulp and dentin. J Endod 2003;29:334-337.ArticlePubMed
- 34. Abou-Rass M, Oglesby SW. The effects of temperature, concentration, and tissue type on the solvent ability of sodium hypochlorite. J Endod 1981;7:376-377.ArticlePubMed
- 35. Moorer WR, Wesselink PR. Factors promoting the tissue dissolving capability of sodium hypochlorite. Int Endod J 1982;15:187-196.ArticlePubMed
- 36. Estrela C, Estrela CR, Barbin EL, Spanó JC, Marchesan MA, Pécora JD. Mechanism of action of sodium hypochlorite. Braz Dent J 2002;13:113-117.ArticlePubMed
- 37. Ahmad M, Pitt Ford TR, Crum LA. Ultrasonic debridement of root canals: an insight into the mechanisms involved. J Endod 1987;13:93-101.ArticlePubMed
- 38. Cameron JA. The synergistic relationship between ultrasound and sodium hypochlorite: a scanning electron microscope evaluation. J Endod 1987;13:541-545.ArticlePubMed
- 39. Weller RN, Brady JM, Bernier WE. Efficacy of ultrasonic cleaning. J Endod 1980;6:740-743.ArticlePubMed
- 40. Sabins RA, Johnson JD, Hellstein JW. A comparison of the cleaning efficacy of short-term sonic and ultrasonic passive irrigation after hand instrumentation in molar root canals. J Endod 2003;29:674-678.ArticlePubMed
- 41. Mayer BE, Peters OA, Barbakow F. Effects of rotary instruments and ultrasonic irrigation on debris and smear layer scores: a scanning electron microscopic study. Int Endod J 2002;35:582-589.ArticlePubMed
- 42. Cymerman JJ, Jerome LA, Moodnik RM. A scanning electron microscope study comparing the efficacy of hand instrumentation with ultrasonic instrumentation of the root canal. J Endod 1983;9:327-331.ArticlePubMed
- 43. van der Sluis LW, Versluis M, Wu MK, Wesselink PR. Passive ultrasonic irrigation of the root canal: a review of the literature. Int Endod J 2007;40:415-426.ArticlePubMed
REFERENCES
Tables & Figures
REFERENCES
Citations
Citations to this article as recorded by 

- Evaluation of pulp tissue dissolving efficiency of sodium and calcium hypochlorite solutions activated by ultrasonics and laser: an in vitro study
Oznur Ozturk, Ozgur Genc Sen
BMC Oral Health.2024;[Epub] CrossRef
A new phantom to evaluate the tissue dissolution ability of endodontic irrigants and activating devices
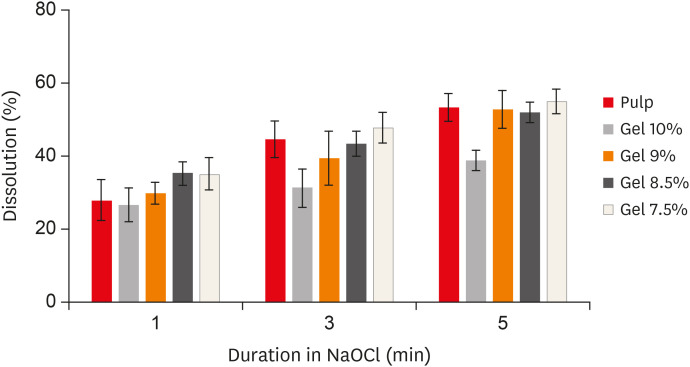
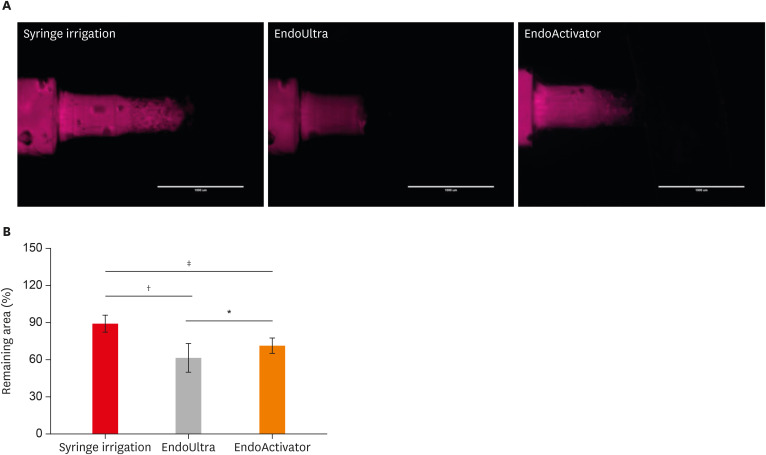
Figure 1 Dissolution (%) of the different phantoms at all time intervals.NaOCl, sodium hypochlorite.
Figure 2 (A) Fluorescence microscopy of the endodontic blocks that were instrumented with endodontic files and then irrigated using 3 different methods. (B) Tissue remaining (%) in the endodontic blocks that were irrigated using 3 different methods.*p < 0.05; †p < 0.001; ‡p < 0.0001.
Figure 1
Figure 2
A new phantom to evaluate the tissue dissolution ability of endodontic irrigants and activating devices

 KACD
KACD
 ePub Link
ePub Link Cite
Cite

