Articles
- Page Path
- HOME > Restor Dent Endod > Volume 45(4); 2020 > Article
- Research Article Effect of phytic acid as an endodontic chelator on resin adhesion to sodium hypochlorite-treated dentin
-
Mohannad Nassar1
 , Noriko Hiraishi2
, Noriko Hiraishi2 , Md. Sofiqul Islam3
, Md. Sofiqul Islam3 , Maria JRH. Romero4
, Maria JRH. Romero4 , Masayuki Otsuki2
, Masayuki Otsuki2 , Junji Tagami2
, Junji Tagami2
-
Restor Dent Endod 2020;45(4):e44.
DOI: https://doi.org/10.5395/rde.2020.45.e44
Published online: August 24, 2020
1Preventive and Restorative Dentistry, College of Dental Medicine, University of Sharjah, Sharjah, UAE.
2Cariology and Operative Dentistry, Department of Oral Health Sciences, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University, Tokyo, Japan.
3RAK College of Dental Sciences (RAKCODS), RAK Medical and Health Sciences University (RAKMHSU), Ras Al Khaimah, UAE.
4Department of Cariology, Operative Dentistry and Dental Public Health, Indiana University School of Dentistry, Indianapolis, IN, USA.
- Correspondence to Mohannad Nassar, BDS, MSc, PhD. Assistant Professor, Preventive and Restorative Dentistry, College of Dental Medicine, University of Sharjah, University City Rd, Sharjah 27272, UAE. minassar@sharjah.ac.ae
Copyright © 2020. The Korean Academy of Conservative Dentistry
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 2,157 Views
- 17 Download
- 17 Crossref
Abstract
-
Objectives Phytic acid (IP6), a naturally occurring agent, has been previously reported as a potential alternative to ethylenediaminetetraacetic acid (EDTA). However, its effect on adhesion to sodium hypochlorite (NaOCl)-treated dentin and its interactions with NaOCl have not been previously reported. Thus, in this study, the effects of IP6 on resin adhesion to NaOCl-treated dentin and the failure mode were investigated and the interactions between the used agents were analyzed.
-
Materials and Methods Micro-tensile bond strength (µTBS) testing was performed until failure on dentin treated with either distilled water (control), 5% NaOCl, or 5% NaOCl followed with chelators: 17% EDTA for 1 minute or 1% IP6 for 30 seconds or 1 minute. The failed specimens were assessed under a scanning electron microscope. The reaction of NaOCl with EDTA or IP6 was analyzed in terms of temperature, pH, effervescence, and chlorine odor, and the effects of the resulting mixtures on the color of a stained paper were recorded.
-
Results The µTBS values of the control and NaOCl with chelator groups were not significantly different, but were all significantly higher than that of the group treated with NaOCl only. In the failure analysis, a distinctive feature was the presence of resin tags in samples conditioned with IP6 after treatment with NaOCl. The reaction of 1% IP6 with 5% NaOCl was less aggressive than the reaction of the latter with 17% EDTA.
-
Conclusions IP6 reversed the adverse effects of NaOCl on resin-dentin adhesion without the chlorine-depleting effect of EDTA.
INTRODUCTION
MATERIALS AND METHODS
RESULTS
Micro-tensile bond strength values of each tested group
| Group | Values |
|---|---|
| Control | 71.6 ± 19.9a |
| Group I (NaOCl) | 51.3 ± 16.4b |
| Group II (NaOCl/EDTA 1 minute) | 68.5 ± 22.5a |
| Group III (NaOCl/IP6 1 minute) | 65.5 ± 19.7a |
| Group IV (NaOCl/IP6 30 seconds) | 64.2 ± 12.5a |
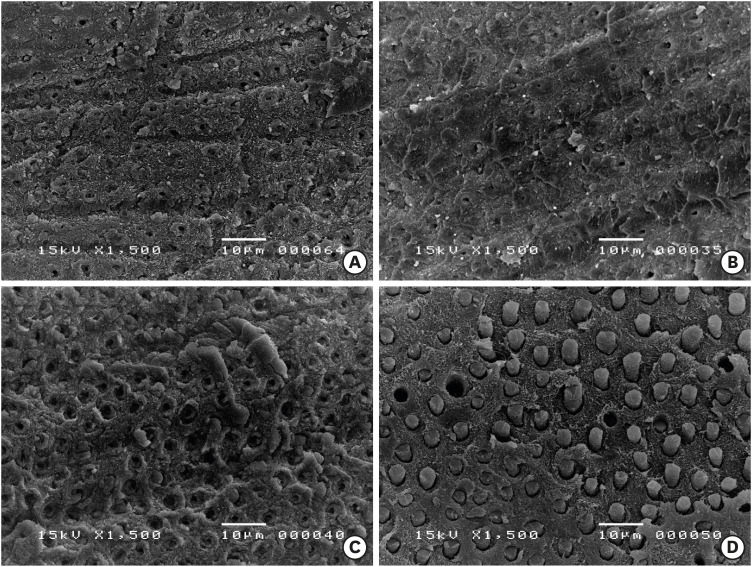
Scanning electron microscopy images of the dentin side of the fractured specimens. (A) Control group, (B) 5% NaOCl treatment for 5 minutes, (C) 1 minute of 17% EDTA treatment of the NaOCl-treated dentin surfaces, and (D) 1 minute of 1% IP6 treatment of the NaOCl-treated dentin surfaces. Resin tags were formed along the surface treated with IP6.
The formation of chlorine gas bubbles in the mixed solutions. (A) 17% EDTA and 5% NaOCl, (B) 1% IP6 and 5% NaOCl.

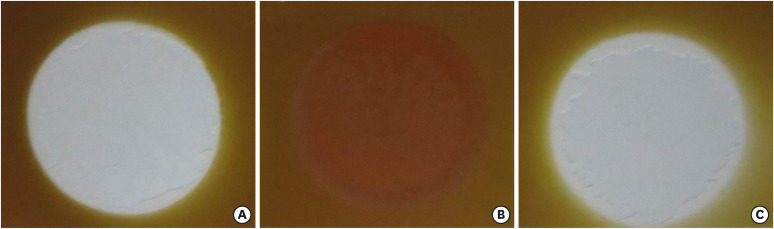
The effect of different solutions on the color of a stained paper. (A) NaOCl, (B) EDTA/NaOCl mixture, and (C) IP6/NaOCl mixture.
DISCUSSION
CONCLUSIONS
-
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
-
Author Contributions:
Conceptualization: Nassar M.
Data curation: Hiraishi N.
Formal analysis: Romero MJRH.
Funding acquisition: Hiraishi N.
Investigation: Nassar M.
Methodology: Nassar M, Otsuki M.
Project administration: Tagami J.
Resources: Otsuki M.
Software: Islam MS.
Supervision: Tagami J.
Validation: Hiraishi N.
Writing - original draft: Nassar M.
Writing - review & editing: Romero MJRH.
- 1. Zehnder M. Root canal irrigants. J Endod 2006;32:389-398.ArticlePubMed
- 2. Taşman F, Cehreli ZC, Oğan C, Etikan I. Surface tension of root canal irrigants. J Endod 2000;26:586-587.ArticlePubMed
- 3. Giardino L, Ambu E, Becce C, Rimondini L, Morra M. Surface tension comparison of four common root canal irrigants and two new irrigants containing antibiotic. J Endod 2006;32:1091-1093.ArticlePubMed
- 4. Crane AB. A predictable root canal technique. Philadelphia, PA: Lea & Febiger; 1920.
- 5. Haapasalo M, Endal U, Zandi H, Coil JM. Eradication of endodontic infection by instrumentation and irrigation solutions. Endod Topics 2005;10:77-102.Article
- 6. Byström A, Sundqvist G. Bacteriologic evaluation of the effect of 0.5 percent sodium hypochlorite in endodontic therapy. Oral Surg Oral Med Oral Pathol 1983;55:307-312.ArticlePubMed
- 7. Estrela C, Estrela CR, Barbin EL, Spanó JC, Marchesan MA, Pécora JD. Mechanism of action of sodium hypochlorite. Braz Dent J 2002;13:113-117.ArticlePubMed
- 8. Schwartz RS, Fransman R. Adhesive dentistry and endodontics: materials, clinical strategies and procedures for restoration of access cavities: a review. J Endod 2005;31:151-165.ArticlePubMed
- 9. Rueggeberg FA, Margeson DH. The effect of oxygen inhibition on an unfilled/filled composite system. J Dent Res 1990;69:1652-1658.ArticlePubMedPDF
- 10. Nikaido T, Nakabayashi N. Relationship between polymerization and adhesion to teeth. Adhes Dent 1988;6:229-234.
- 11. Pimentel Corrêa AC, Cecchin D, de Almeida JF, Gomes BP, Zaia AA, Ferraz CC. Sodium thiosulfate for recovery of bond strength to dentin treated with sodium hypochlorite. J Endod 2016;42:284-288.ArticlePubMed
- 12. Cecchin D, Farina AP, Bedran-Russo AK. Efficacy of natural collagen crosslinkers on the compromised adhesive bond strength to NaOCl-treated pulp chamber dentin. J Adhes Dent 2018;20:365-369.PubMed
- 13. Zhang K, Kim YK, Cadenaro M, Bryan TE, Sidow SJ, Loushine RJ, Ling JQ, Pashley DH, Tay FR. Effects of different exposure times and concentrations of sodium hypochlorite/ethylenediaminetetraacetic acid on the structural integrity of mineralized dentin. J Endod 2010;36:105-109.ArticlePubMed
- 14. Stevens CD. Immediate shear bond strength of resin cements to sodium hypochlorite-treated dentin. J Endod 2014;40:1459-1462.ArticlePubMed
- 15. Begotka BA, Hartwell GR. The importance of the coronal seal following root canal treatment. Va Dent J 1996;73:8-10.
- 16. Siqueira JF Jr, Rôças IN, Favieri A, Abad EC, Castro AJ, Gahyva SM. Bacterial leakage in coronally unsealed root canals obturated with 3 different techniques. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2000;90:647-650.ArticlePubMed
- 17. Pommel L, Camps J. In vitro apical leakage of system B compared with other filling techniques. J Endod 2001;27:449-451.ArticlePubMed
- 18. Weston CH, Ito S, Wadgaonkar B, Pashley DH. Effects of time and concentration of sodium ascorbate on reversal of NaOCl-induced reduction in bond strengths. J Endod 2007;33:879-881.ArticlePubMed
- 19. Nassar M, Awawdeh L, Jamleh A, Sadr A, Tagami J. Adhesion of Epiphany self-etch sealer to dentin treated with intracanal irrigating solutions. J Endod 2011;37:228-230.ArticlePubMed
- 20. Baumgartner JC, Mader CL. A scanning electron microscopic evaluation of four root canal irrigation regimens. J Endod 1987;13:147-157.ArticlePubMed
- 21. Torabinejad M, Khademi AA, Babagoli J, Cho Y, Johnson WB, Bozhilov K, Kim J, Shabahang S. A new solution for the removal of the smear layer. J Endod 2003;29:170-175.ArticlePubMed
- 22. Segura JJ, Calvo JR, Guerrero JM, Sampedro C, Jimenez A, Llamas R. The disodium salt of EDTA inhibits the binding of vasoactive intestinal peptide to macrophage membranes: endodontic implications. J Endod 1996;22:337-340.ArticlePubMed
- 23. Sillanpää M. Environmental fate of EDTA and DTPA. Rev Environ Contam Toxicol 1997;152:85-111.PubMed
- 24. Grawehr M, Sener B, Waltimo T, Zehnder M. Interactions of ethylenediamine tetraacetic acid with sodium hypochlorite in aqueous solutions. Int Endod J 2003;36:411-417.ArticlePubMedPDF
- 25. Grande NM, Plotino G, Falanga A, Pomponi M, Somma F. Interaction between EDTA and sodium hypochlorite: a nuclear magnetic resonance analysis. J Endod 2006;32:460-464.ArticlePubMed
- 26. Amaral KF, Rogero MM, Fock RA, Borelli P, Gavini G. Cytotoxicity analysis of EDTA and citric acid applied on murine resident macrophages culture. Int Endod J 2007;40:338-343.ArticlePubMed
- 27. Nassar M, Hiraishi N, Tamura Y, Otsuki M, Aoki K, Tagami J. Phytic acid: an alternative root canal chelating agent. J Endod 2015;41:242-247.ArticlePubMed
- 28. Raboy V. myo-Inositol-1,2,3,4,5,6-hexakisphosphate. Phytochemistry 2003;64:1033-1043.ArticlePubMed
- 29. Luttrell BM. The biological relevance of the binding of calcium ions by inositol phosphates. J Biol Chem 1993;268:1521-1524.ArticlePubMed
- 30. Torres J, Domínguez S, Cerdá MF, Obal G, Mederos A, Irvine RF, Díaz A, Kremer C. Solution behaviour of myo-inositol hexakisphosphate in the presence of multivalent cations. Prediction of a neutral pentamagnesium species under cytosolic/nuclear conditions. J Inorg Biochem 2005;99:828-840.ArticlePubMed
- 31. Nassar M, Hiraishi N, Islam MS, Aizawa M, Tamura Y, Otsuki M, Kasugai S, Ohya K, Tagami J. Effect of phytic acid used as etchant on bond strength, smear layer, and pulpal cells. Eur J Oral Sci 2013;121:482-487.ArticlePubMed
- 32. Kong K, Islam MS, Nassar M, Hiraishi N, Otsuki M, Yiu CK, Tagami J. Effect of phytic acid etchant on the structural stability of demineralized dentine and dentine bonding. J Mech Behav Biomed Mater 2015;48:145-152.ArticlePubMed
- 33. Kong K, Hiraishi N, Nassar M, Otsuki M, Yiu CK, Tagami J. Effect of phytic acid etchant on resin-dentin bonding: monomer penetration and stability of dentin collagen. J Prosthodont Res 2017;61:251-258.ArticlePubMed
- 34. Ray HA, Trope M. Periapical status of endodontically treated teeth in relation to the technical quality of the root filling and the coronal restoration. Int Endod J 1995;28:12-18.ArticlePubMed
- 35. Trope M, Chow E, Nissan R. In vitro endotoxin penetration of coronally unsealed endodontically treated teeth. Endod Dent Traumatol 1995;11:90-94.ArticlePubMed
- 36. Fathi B, Bahcall J, Maki JS. An in vitro comparison of bacterial leakage of three common restorative materials used as an intracoronal barrier. J Endod 2007;33:872-874.ArticlePubMed
- 37. Ozturk B, Ozer F. Effect of NaOCl on bond strengths of bonding agents to pulp chamber lateral walls. J Endod 2004;30:362-365.ArticlePubMed
- 38. Belli S, Zhang Y, Pereira PN, Ozer F, Pashley DH. Regional bond strengths of adhesive resins to pulp chamber dentin. J Endod 2001;27:527-532.ArticlePubMed
- 39. Santos JN, Carrilho MR, De Goes MF, Zaia AA, Gomes BP, Souza-Filho FJ, Ferraz CC. Effect of chemical irrigants on the bond strength of a self-etching adhesive to pulp chamber dentin. J Endod 2006;32:1088-1090.ArticlePubMed
- 40. Prado M, Santos Júnior HM, Rezende CM, Pinto AC, Faria RB, Simão RA, Gomes BP. Interactions between irrigants commonly used in endodontic practice: a chemical analysis. J Endod 2013;39:505-510.ArticlePubMed
- 41. Bui TB, Baumgartner JC, Mitchell JC. Evaluation of the interaction between sodium hypochlorite and chlorhexidine gluconate and its effect on root dentin. J Endod 2008;34:181-185.ArticlePubMed
- 42. Nikaido T, Takano Y, Sasafuchi Y, Burrow MF, Tagami J. Bond strengths to endodontically-treated teeth. Am J Dent 1999;12:177-180.PubMed
- 43. Lohbauer U, Nikolaenko SA, Petschelt A, Frankenberger R. Resin tags do not contribute to dentin adhesion in self-etching adhesives. J Adhes Dent 2008;10:97-103.PubMed
REFERENCES
Tables & Figures
REFERENCES
Citations

- The Effect of Chemical Surface Modification on the Repair Bond Strength of Resin Composite: An In Vitro Study
Md Sofiqul Islam, Shadi El Bahra, Smriti Aryal A C, Vivek Padmanabhan, Abdulaziz Al Tawil, Ihab Saleh, Muhammed Mustahsen Rahman, Upoma Guha
Polymers.2025; 17(4): 513. CrossRef - Advancing Adhesive Strategies for Endodontically Treated Teeth—Part I: Impact of Endodontic Irrigation Protocols on the Chemical Composition and Structural Integrity of Coronal Dentin
Joana A. Marques, Rui I. Falacho, Sara Fateixa, Francisco Caramelo, João Miguel Santos, João Rocha, Markus B. Blatz, João Carlos Ramos, Paulo J. Palma
Journal of Esthetic and Restorative Dentistry.2025; 37(7): 1848. CrossRef - Effect of collagen crosslinkers on sodium hypochlorite treated dentin bond strength: a systematic review and meta-analysis
Weiqing Zhou, Shuting Feng, Xiaojun Chu, Shuaimei Xu, Xiongqun Zeng
Frontiers in Bioengineering and Biotechnology.2025;[Epub] CrossRef - Advancing Adhesive Strategies for Endodontically Treated Teeth—Part II: Dentin Sealing Before Irrigation Increases Long‐Term Microtensile Bond Strength to Coronal Dentin
Joana A. Marques, Rui I. Falacho, Gabriela Almeida, Francisco Caramelo, João Miguel Santos, João Rocha, Markus B. Blatz, João Carlos Ramos, Paulo J. Palma
Journal of Esthetic and Restorative Dentistry.2025; 37(7): 1865. CrossRef - Effects of phytic acid and etidronic acid using continuous and sequential chelation on the removal of smear layer, dentin microhardness, and push-out bond strength of calcium silicate-based cement
Ecehan Hazar, Ahmet Hazar
BMC Oral Health.2025;[Epub] CrossRef - Comparative evaluation of free available chlorine in sodium hypochlorite solutions admixed with novel chelating agents
Somya Tyagi, Sonali Taneja, Kandasamy Nagarajan, Divya Chowdhary
Endodontology.2025; 37(2): 188. CrossRef - Effect of different chelating agents, with and without activation, including XP-endo Finisher, on root dentin microhardness: An in vitro study
Mahmoud Mohamed A. Sherif, Mai Hamdy Ragab, Marwa ElSayed Sharaan
Saudi Endodontic Journal.2025; 15(3): 282. CrossRef - Oracle of phytic acid in dental panacea – Insight into properties, therapeutic effect, regeneration, materials interaction and oral physiology
Ummey Salma, C. Pushpalatha, SV. Sowmya, Dominic Augustine, Ahmed Alamoudi, Bassam Zidane, Nassreen Hassan Mohammad Albar, Shilpa Bhandi
The Saudi Dental Journal.2024; 36(8): 1093. CrossRef - In Vitro Bond Strength of Dentin Treated with Sodium Hypochlorite: Effects of Antioxidant Solutions
Guillermo Grazioli, Elisa de León Cáceres, Romina Tessore, Rafael Lund, Ana Monjarás-Ávila, Monika Lukomska-Szymanska, Louis Hardan, Rim Bourgi, Carlos Cuevas-Suárez
Antioxidants.2024; 13(9): 1116. CrossRef - Is a mix – A fix? “A microscopic analysis of depth of penetration of three combinations of irrigants”
Yantrapragada Lakshmi Sunanda, Krishna Prasad Parvathaneni, T. B. V. G. Raju, Abitha Seshadri, Nadimpalli Mahendra Varma, Gowtam Dev Dondapati
Journal of Conservative Dentistry and Endodontics.2024; 27(2): 186. CrossRef - Effect of phytic acid on dentinal collagen solubilization and its binding and debinding potentials to dentin
Diletta Forgione, Mohannad Nassar, Roda Seseogullari-Dirihan, Ahmed Jamleh, Arzu Tezvergil-Mutluay
Journal of Dentistry.2023; 128: 104361. CrossRef - Application of Inositol Hexaphosphate and Inositol in Dental Medicine: An Overview
Ana Druzijanic, Mare Kovic, Marija Roguljic, Livia Cigic, Martina Majstorovic, Ivana Vucenik
Biomolecules.2023; 13(6): 913. CrossRef - Ex-vivo study about antimicrobial effectiveness of phytic acid against Enterococcus faecalis into root canals
Giulia BOSCHI, Giorgio PICCINELLI, Carlo BONFANTI, Stefano A. SALGARELLO
Minerva Dental and Oral Science.2023;[Epub] CrossRef - Effect of phytic acid on bond strength and interfacial integrity of universal adhesive to deep dentin
Ahmed Mostafa Attia, Ahmed Fawzy Abo-Elezz, Rehab Khalil Safy
Brazilian Dental Journal.2022; 33(5): 116. CrossRef - Resin-Based Cement Applied to Enamel and Dentin Pre-Treated with Phytic Acid: An In Vitro Study
Mohannad Nassar, Md. Sofiqul Islam, Smriti Aryal A C, Hatem Mostafa El-Damanhoury, Salvatore Sauro, Noriko Hiraishi
Applied Sciences.2021; 11(24): 11976. CrossRef - Postspace pretreatment with 17% ethylenediamine tetraacetic acid, 7% maleic acid, and 1% phytic acid on bond strength of fiber posts luted with a self-adhesive resin cement
PriyaC Yadav, Ramya Raghu, Ashish Shetty, Subhashini Rajasekhara
Journal of Conservative Dentistry.2021; 24(6): 558. CrossRef - Phytic Acid: Properties and Potential Applications in Dentistry
Mohannad Nassar, Rania Nassar, Husain Maki, Abdullah Al-Yagoob, Mahmood Hachim, Abiola Senok, David Williams, Noriko Hiraishi
Frontiers in Materials.2021;[Epub] CrossRef
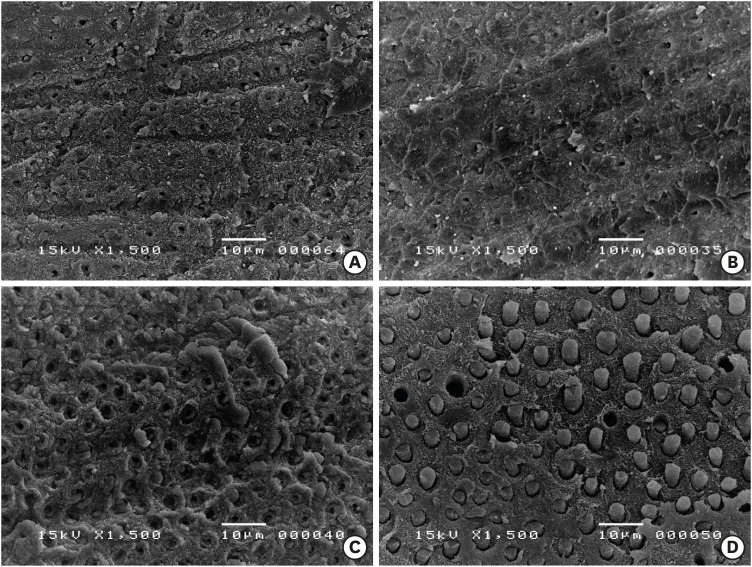

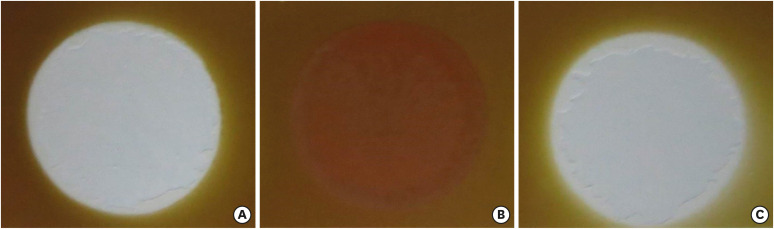
Figure 1
Figure 2
Figure 3
Micro-tensile bond strength values of each tested group
| Group | Values |
|---|---|
| Control | 71.6 ± 19.9a |
| Group I (NaOCl) | 51.3 ± 16.4b |
| Group II (NaOCl/EDTA 1 minute) | 68.5 ± 22.5a |
| Group III (NaOCl/IP6 1 minute) | 65.5 ± 19.7a |
| Group IV (NaOCl/IP6 30 seconds) | 64.2 ± 12.5a |
Values are presented as mean (MPa) ± standard deviation. Groups identified by different superscript letters indicate statistically significant differences (1-way analysis of variance with the Tukey post hoc test, p < 0.05).
NaOCl, sodium hypochlorite; EDTA, ethylenediaminetetraacetic acid; IP6, phytic acid.
Values are presented as mean (MPa) ± standard deviation. Groups identified by different superscript letters indicate statistically significant differences (1-way analysis of variance with the Tukey
NaOCl, sodium hypochlorite; EDTA, ethylenediaminetetraacetic acid; IP6, phytic acid.

 KACD
KACD
 ePub Link
ePub Link Cite
Cite

