Articles
- Page Path
- HOME > Restor Dent Endod > Volume 43(3); 2018 > Article
- Research Article Bacterial leakage and micro-computed tomography evaluation in round-shaped canals obturated with bioceramic cone and sealer using matched single cone technique
-
Kallaya Yanpiset1
 , Danuchit Banomyong1
, Danuchit Banomyong1 , Kanet Chotvorrarak1
, Kanet Chotvorrarak1 , Ratchapin Laovanitch Srisatjaluk2
, Ratchapin Laovanitch Srisatjaluk2
-
Restor Dent Endod 2018;43(3):e30.
DOI: https://doi.org/10.5395/rde.2018.43.e30
Published online: July 5, 2018
1Department of Operative Dentistry and Endodontics, Faculty of Dentistry, Mahidol University, Bangkok, Thailand.
2Department of Oral Microbiology, Faculty of Dentistry, Mahidol University, Bangkok, Thailand.
- Correspondence to Danuchit Banomyong, DDS, PhD. Assistant Professor, Department of Operative Dentistry and Endodontics, Faculty of Dentistry, Mahidol University, 6 Yothi Street, Ratchathewi, Bangkok 10400, Thailand. danuchit.ban@mahidol.ac.th
Copyright © 2018. The Korean Academy of Conservative Dentistry
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 2,317 Views
- 35 Download
- 32 Crossref
Abstract
-
Objectives To evaluate sealing ability of root canals obturated with bioceramic-impregnated gutta percha cone (BCC) or gutta percha (GP), with bioceramic sealer (BCS) or AH Plus (AH; Dentsply-Maillefer), in roundly-prepared canals using matched single-cone technique, based on bacterial leakage test, and to analyze obturation quality using micro-computed tomography (CT) analysis.
-
Materials and Methods Ninety-two distobuccal roots of maxillary molars were prepared using nickel-titanium files to apical size 40/0.06. The roots were divided into 4 groups (n = 20) that were obturated with a master cone and sealer: GP/AH, BCC/AH, GP/BCS, and BCC/BCS. Bacterial leakage model using Enterococcus faecalis was used to evaluate sealing ability for 60-day period. Obturated samples from each group (n = 4) were analyzed using micro-CT.
-
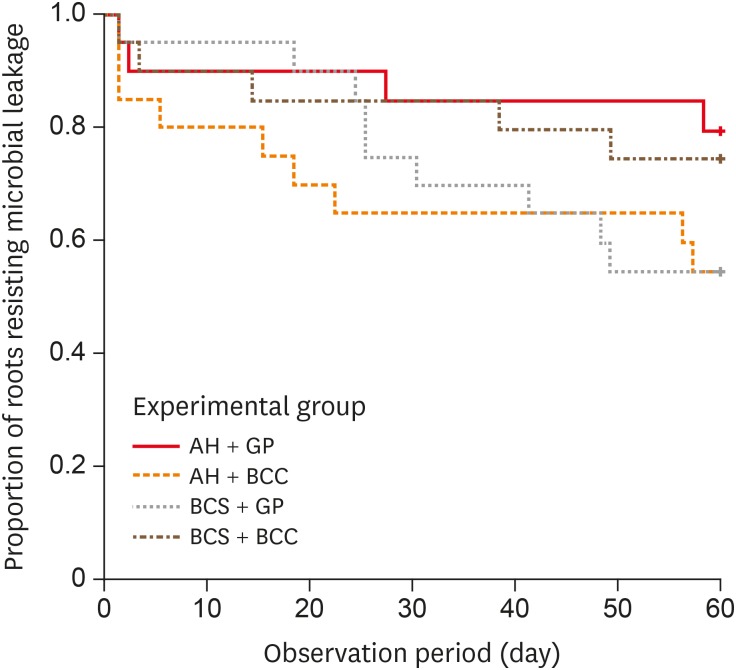
Results All groups showed bacterial leakage at 20%–45% of samples with mean leakage times of 42–52 days. There were no significant differences in bacterial leakage among the groups. Micro-CT showed minimal gaps and voids in all groups at less than 1%.
-
Conclusions In roundly-prepared canals, the single cone obturation with BCC/BCS was comparable to GP/AH for bacterial leakage at 60 days.
INTRODUCTION
MATERIALS AND METHODS
1. Sample preparation
2. Bacterial leakage model preparation
Bacterial leakage apparatus.
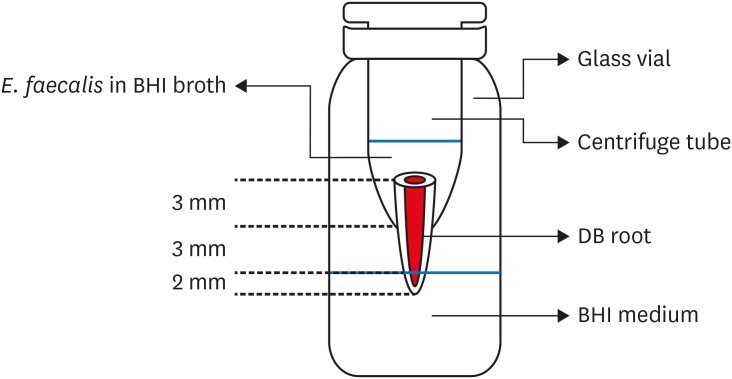
3. Bacterial leakage test with E. faecalis
RESULTS
Numbers of teeth and percentages of bacterial leakage of obturated root canals in each experimental group (n = 20 each) after exposure to Enterococcus faecalis for 60 days
Kaplan-Meier survival curves of the 4 experimental groups.
Total gaps/voids of leaked and non-leaked samples (percentage, in range), evaluated by micro-computed tomographic analysis
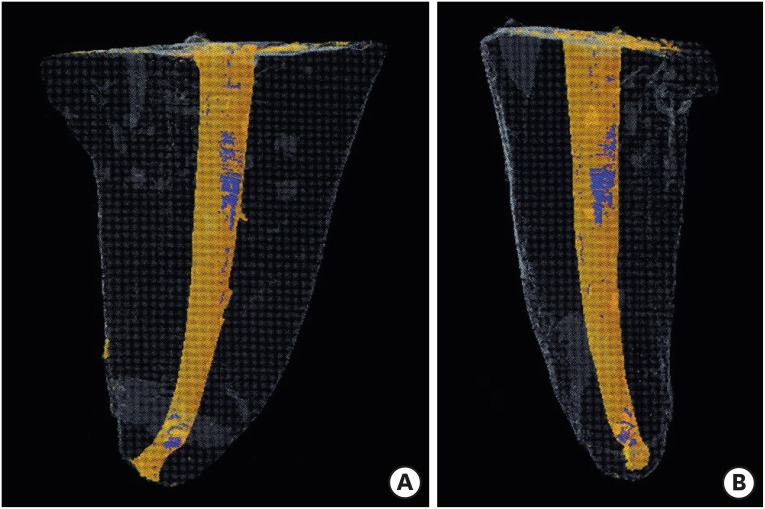
Micro-computed tomographic images in 3 dimensions of the obturated roots. Obturation cones and sealers are in the orange colour. The blue color within the obturation materials indicates gaps/voids at coronal and apical third levels. (A) Mesiodistal view; (B) Buccolingual view.
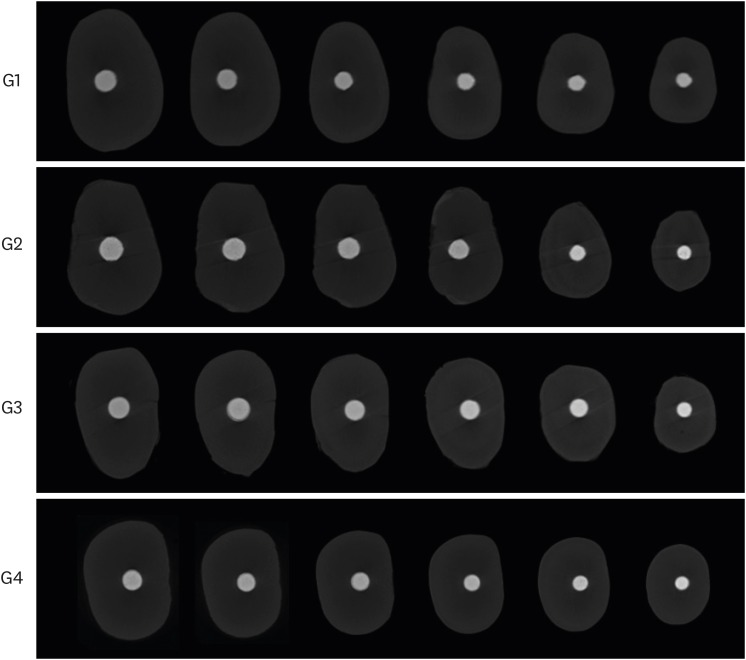
Two-dimensional slices from micro-computed tomographic scanning of axial cross-sections of obturated root canals from coronal third to apical third level (left to right) in each experimental group. Group 1 (G1), GP/AH; Group 2 (G2), BCC/AH; Group 3 (G3), GP/BCS; Group 4 (G4), BCC/BCS.
DISCUSSION
CONCLUSIONS
ACKNOWLEDGEMENT
-
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
-
Author Contributions:
Conceptualization: Yanpiset K, Banomyong D.
Data curation: Yanpiset K, Banomyong D, Chotvorrarak K.
Formal analysis: Yanpiset K, Banomyong D, Chotvorrarak K.
Funding acquisition: Yanpiset K, Srisatjaluk RL.
Investigation: Chotvorrarak K.
Methodology: Yanpiset K, Banomyong D, Chotvorrarak K, Srisatjaluk RL.
Project administration: Chotvorrarak K.
Resources: Yanpiset K, Srisatjaluk RL.
Software: Banomyong D, Chotvorrarak K.
Supervision: Yanpiset K, Banomyong, Srisatjaluk RL.
Validation: Yanpiset K, Banomyong D.
Visualization: Yanpiset K, Banomyong D.
Writing - original draft: Yanpiset K, Banomyong D, Chotvorrarak K, Srisatjaluk RL.
Writing - review & editing: Yanpiset K, Banomyong D, Chotvorrarak K, Srisatjaluk RL.
- 1. Sundqvist G, Figdor D, Persson S, Sjögren U. Microbiologic analysis of teeth with failed endodontic treatment and the outcome of conservative re-treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1998;85:86-93.ArticlePubMed
- 2. Zhang W, Li Z, Peng B. Assessment of a new root canal sealer's apical sealing ability. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009;107:e79-e82.ArticlePubMed
- 3. Zhang W, Li Z, Peng B. Ex vivo cytotoxicity of a new calcium silicate-based canal filling material. Int Endod J 2010;43:769-774.ArticlePubMed
- 4. Zoufan K, Jiang J, Komabayashi T, Wang YH, Safavi KE, Zhu Q. Cytotoxicity evaluation of Gutta Flow and Endo Sequence BC sealers. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2011;112:657-661.ArticlePubMed
- 5. Willershausen I, Callaway A, Briseño B, Willershausen B. In vitro analysis of the cytotoxicity and the antimicrobial effect of four endodontic sealers. Head Face Med 2011;7:15.ArticlePubMedPMCPDF
- 6. Zhou HM, Shen Y, Zheng W, Li L, Zheng YF, Haapasalo M. Physical properties of 5 root canal sealers. J Endod 2013;39:1281-1286.ArticlePubMed
- 7. Zhang H, Shen Y, Ruse ND, Haapasalo M. Antibacterial activity of endodontic sealers by modified direct contact test against Enterococcus faecalis. J Endod 2009;35:1051-1055.ArticlePubMed
- 8. Chotvorrarak K, Yanpiset K, Banomyong D, Srisatjaluk R. In vitro antibacterial activity of oligomer-based and calcium silicate-based root canal sealers. Mahidol Dent J 2017;37:145-154.
- 9. Adib V, Spratt D, Ng YL, Gulabivala K. Cultivable microbial flora associated with persistent periapical disease and coronal leakage after root canal treatment: a preliminary study. Int Endod J 2004;37:542-551.ArticlePubMed
- 10. Timpawat S, Amornchat C, Trisuwan WR. Bacterial coronal leakage after obturation with three root canal sealers. J Endod 2001;27:36-39.ArticlePubMed
- 11. Shipper G, Trope M. In vitro microbial leakage of endodontically treated teeth using new and standard obturation techniques. J Endod 2004;30:154-158.ArticlePubMed
- 12. Pinheiro CR, Guinesi AS, de Camargo EJ, Pizzolitto AC, Filho IB. Bacterial leakage evaluation of root canals filled with different endodontic sealers. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009;108:e56-e60.Article
- 13. Jafari F, Jafari S. Importance and methodologies of endodontic microleakage studies: a systematic review. J Clin Exp Dent 2017;9:e812-e819.ArticlePubMedPMC
- 14. Veríssimo DM, do Vale MS. Methodologies for assessment of apical and coronal leakage of endodontic filling materials: a critical review. J Oral Sci 2006;48:93-98.ArticlePubMed
- 15. Wu MK, Wesselink PR. Endodontic leakage studies reconsidered. Part I. Methodology, application and relevance. Int Endod J 1993;26:37-43.ArticlePubMed
- 16. Celikten B, Uzuntas CF, Orhan AI, Tufenkci P, Misirli M, Demiralp KO, Orhan K. Micro-CT assessment of the sealing ability of three root canal filling techniques. J Oral Sci 2015;57:361-366.ArticlePubMed
- 17. Celikten B, Uzuntas CF, Orhan AI, Orhan K, Tufenkci P, Kursun S, Demiralp KO. Evaluation of root canal sealer filling quality using a single-cone technique in oval shaped canals: an in vitro Micro-CT study. Scanning 2016;38:133-140.ArticlePubMedPDF
- 18. Ersahan S, Aydin C. Solubility and apical sealing characteristics of a new calcium silicate-based root canal sealer in comparison to calcium hydroxide-, methacrylate resin- and epoxy resin-based sealers. Acta Odontol Scand 2013;71:857-862.ArticlePubMed
- 19. Pawar SS, Pujar MA, Makandar SD. Evaluation of the apical sealing ability of bioceramic sealer, AH plus & epiphany: an in vitro study. J Conserv Dent 2014;17:579-582.ArticlePubMedPMC
- 20. Vertucci FJ. Root canal anatomy of the human permanent teeth. Oral Surg Oral Med Oral Pathol 1984;58:589-599.ArticlePubMed
- 21. Torabinejad M, Ung B, Kettering JD. In vitro bacterial penetration of coronally unsealed endodontically treated teeth. J Endod 1990;16:566-569.ArticlePubMed
- 22. Chailertvanitkul P, Saunders WP, MacKenzie D, Weetman DA. An in vitro study of the coronal leakage of two root canal sealers using an obligate anaerobe microbial marker. Int Endod J 1996;29:249-255.ArticlePubMed
- 23. Tay FR, Loushine RJ, Lambrechts P, Weller RN, Pashley DH. Geometric factors affecting dentin bonding in root canals: a theoretical modeling approach. J Endod 2005;31:584-589.ArticlePubMed
- 24. Kontakiotis EG, Wu MK, Wesselink PR. Effect of sealer thickness on long-term sealing ability: a 2-year follow-up study. Int Endod J 1997;30:307-312.ArticlePubMed
- 25. Candeiro GT, Correia FC, Duarte MA, Ribeiro-Siqueira DC, Gavini G. Evaluation of radiopacity, pH, release of calcium ions, and flow of a bioceramic root canal sealer. J Endod 2012;38:842-845.ArticlePubMed
- 26. Yücel AC, Güler E, Güler AU, Ertaş E. Bacterial penetration after obturation with four different root canal sealers. J Endod 2006;32:890-893.ArticlePubMed
- 27. Zmener O, Spielberg C, Lamberghini F, Rucci M. Sealing properties of a new epoxy resin-based root-canal sealer. Int Endod J 1997;30:332-334.ArticlePubMed
- 28. Eldeniz AU, Ørstavik D. A laboratory assessment of coronal bacterial leakage in root canals filled with new and conventional sealers. Int Endod J 2009;42:303-312.ArticlePubMed
- 29. Loushine BA, Bryan TE, Looney SW, Gillen BM, Loushine RJ, Weller RN, Pashley DH, Tay FR. Setting properties and cytotoxicity evaluation of a premixed bioceramic root canal sealer. J Endod 2011;37:673-677.ArticlePubMed
- 30. De-Deus G. Research that matters - root canal filling and leakage studies. Int Endod J 2012;45:1063-1064.ArticlePubMed
- 31. Brosco VH, Bernardineli N, Torres SA, Consolaro A, Bramante CM, de Moraes IG, Ordinola-Zapata R, Garcia RB. Bacterial leakage in obturated root canals-part 2: a comparative histologic and microbiologic analyses. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010;109:788-794.ArticlePubMed
- 32. Hammad M, Qualtrough A, Silikas N. Evaluation of root canal obturation: a three-dimensional in vitro study. J Endod 2009;35:541-544.ArticlePubMed
- 33. Epley SR, Fleischman J, Hartwell G, Cicalese C. Completeness of root canal obturations: epiphany techniques versus gutta-percha techniques. J Endod 2006;32:541-544.ArticlePubMed
- 34. James BL, Brown CE, Legan JJ, Moore BK, Vail MM. An in vitro evaluation of the contents of root canals obturated with gutta percha and AH-26 sealer or Resilon and Epiphany sealer. J Endod 2007;33:1359-1363.ArticlePubMed
- 35. Sevimay S, Dalat D. Evaluation of penetration and adaptation of three different sealers: a SEM study. J Oral Rehabil 2003;30:951-955.ArticlePubMedPDF
- 36. Silva RV, Silveira FF, Horta MC, Duarte MA, Cavenago BC, Morais IG, Nunes E. Filling effectiveness and dentinal penetration of endodontic sealers: a stereo and confocal laser scanning microscopy study. Braz Dent J 2015;26:541-546.ArticlePubMed
- 37. Zhejun W. Bioceramic materials in endodontics. Endod Topics 2015;32:86-96.
- 38. Ricucci D, Gröndahl K, Bergenholtz G. Periapical status of root-filled teeth exposed to the oral environment by loss of restoration or caries. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2000;90:354-359.ArticlePubMed
REFERENCES
Tables & Figures
REFERENCES
Citations

- Effect of Root Dentin Moisture on the Apical Sealing Ability of Root Canal Sealers: In vitro Study
Zahraa Khalil Alani, Manal Hussain Abd-alla
Al-Rafidain Journal of Medical Sciences ( ISSN 2789-3219 ).2025; 8(2): 122. CrossRef - Synthesis, physical properties, and root canal sealing of experimental MTA- and salicylate-based root canal sealers
Rafael Pino Vitti, Kusai Baroudi, Tarun Walia, Raghavandra M. Shetty, Flávia Goulart da Rosa Cardoso, Flávia de Moura Pereira, Evandro Piva, Cesar Henrique Zanchi, Gabriel Flores Abuna, Carolina Oliveira de Lima, Emmanuel João Nogueira Leal Silva, Flávio
PLOS One.2025; 20(7): e0329476. CrossRef - Impact of cone system compatibility on single cone bioceramic obturation in canals prepared with variable taper NiTi rotary files
Reem M. Barakat, Rahaf A. Almohareb, Njoom Aleid, Hoor Almowais, Aljawhara Alharbi, Meshal Al-Sharafa, Ali Alrahlah
Scientific Reports.2025;[Epub] CrossRef - Estudio de la obturación con selladores biocerámicos de conductos radiculares de premolares inferiores
Alicia Beatriz Bonafé, Cecilia Inés Rourera, Carla Pedraza, Yamila Victoria Zanoni, Soledad Salduna, Cecilia Noemi De Caso, Gabriela Martín
Methodo Investigación Aplicada a las Ciencias Biológicas.2025; 10(3): 31. CrossRef - Sealing ability of mineral trioxide aggregate: A scoping review of laboratory assessment methods
Kenta Tsuchiya, Salvatore Sauro, Jukka P. Matinlinna, Hidehiko Sano, Monica Yamauti, Deepak Mehta, Kyung‐San Min, Atsushi Tomokiyo
European Journal of Oral Sciences.2025;[Epub] CrossRef - Bacterial Leakage Testing in Dentistry: A Comprehensive Review on Methods, Models, and Clinical Relevance
Niher Tabassum Snigdha, Mohmed Isaqali Karobari, Sukhamoy Gorai
Scientifica.2025;[Epub] CrossRef - In vitro comparative evaluation of apical leakage using a bioceramic sealer with three different obturating techniques: A glucose leakage model
Tanvi S Agrawal, Shishir Singh, Rajesh S Podar, Gaurav Kulkarni, Anuprita Gadkari, Navin Agarwal
Journal of Conservative Dentistry and Endodontics.2024; 27(1): 76. CrossRef - In Vitro Microscopical and Microbiological Assessment of the Sealing Ability of Calcium Silicate-Based Root Canal Sealers
Karin Christine Huth, Sabina Noreen Wuersching, Leander Benz, Stefan Kist, Maximilian Kollmuss
Journal of Functional Biomaterials.2024; 15(11): 341. CrossRef - Comparison between AH plus sealer and total fill bioceramic sealer performance in previously untreated and retreatment cases of maxillary incisors with large-sized periapical lesion: a randomized controlled trial
Eisa Wahbi, Hassan Achour, Yasser Alsayed Tolibah
BDJ Open.2024;[Epub] CrossRef - Bacterial sealing ability of calcium silicate-based sealer for endodontic surgery: an in-vitro study
Mai M. Mansour, Sybel M. Moussa, Marwa A. Meheissen, Mahmoud R. Aboelseoud
BMC Oral Health.2024;[Epub] CrossRef - Assessment the bioactivity of zinc oxid eugenol sealer after the addition of different concentrations of nano hydroxyapatite-tyrosine amino acid
Rasha M. Al-Shamaa, Raghad A. Al-Askary
Brazilian Journal of Oral Sciences.2024; 23: e243733. CrossRef - Assessment of Bacterial Sealing Ability of Two Different Bio-Ceramic Sealers in Single-Rooted Teeth Using Single Cone Obturation Technique: An In Vitro Study
Doaa M. AlEraky, Ahmed M. Rahoma, Hatem M. Abuohashish, Abdullh AlQasser, Abbas AlHamali, Hussain M. AlHussain, Hussain M. AlShoalah, Zakrya AlSaghah, Abdulrahman Khattar, Shimaa Rifaat
Applied Sciences.2023; 13(5): 2906. CrossRef - How do imaging protocols affect the assessment of root-end fillings?
Fernanda Ferrari Esteves Torres, Reinhilde Jacobs, Mostafa EzEldeen, Karla de Faria-Vasconcelos, Juliane Maria Guerreiro-Tanomaru, Bernardo Camargo dos Santos, Mário Tanomaru-Filho
Restorative Dentistry & Endodontics.2022;[Epub] CrossRef - The impact of Morse taper implant design on microleakage at implant-healing abutment interface
Soyeon KIM, Joo Won LEE, Jae-Heon KIM, Van Mai TRUONG, Young-Seok PARK
Dental Materials Journal.2022; 41(5): 767. CrossRef - A critical analysis of research methods and experimental models to study root canal fillings
Gustavo De‐Deus, Erick Miranda Souza, Emmanuel João Nogueira Leal Silva, Felipe Gonçalves Belladonna, Marco Simões‐Carvalho, Daniele Moreira Cavalcante, Marco Aurélio Versiani
International Endodontic Journal.2022; 55(S2): 384. CrossRef - Micro‐CT assessment of gap‐containing areas along the gutta‐percha‐sealer interface in oval‐shaped canals
Gustavo De‐Deus, Gustavo O. Santos, Iara Zamboni Monteiro, Daniele M. Cavalcante, Marco Simões‐Carvalho, Felipe G. Belladonna, Emmanuel J. N. L. Silva, Erick M. Souza, Raphael Licha, Carla Zogheib, Marco A. Versiani
International Endodontic Journal.2022; 55(7): 795. CrossRef - Comparison of Sealing Ability of Bioceramic Sealer, AH Plus, and GuttaFlow in Conservatively Prepared Curved Root Canals Obturated with Single-Cone Technique: An In vitro Study
Shalan Kaul, Ajay Kumar, Bhumika Kamal Badiyani, Laxmi Sukhtankar, M. Madhumitha, Amit Kumar
Journal of Pharmacy and Bioallied Sciences.2021; 13(Suppl 1): S857. CrossRef - Micro-CT Evaluation of Four Root Canal Obturation Techniques
Mahmood Reza Kalantar Motamedi, Amin Mortaheb, Maryam Zare Jahromi, Brett E. Gilbert, Marilena Vivona
Scanning.2021; 2021: 1. CrossRef - Effects of Both Fiber Post/Core Resin Construction System and Root Canal Sealer on the Material Interface in Deep Areas of Root Canal
Hiroki Miura, Shinji Yoshii, Masataka Fujimoto, Ayako Washio, Takahiko Morotomi, Hiroshi Ikeda, Chiaki Kitamura
Materials.2021; 14(4): 982. CrossRef - Sealing ability and microbial leakage of root-end filling materials: MTA versus epoxy resin: A systematic review and meta-analysis
Mario Dioguardi, Mario Alovisi, Diego Sovereto, Giuseppe Troiano, Giancarlo Malagnino, Michele Di Cosola, Angela Pia Cazzolla, Luigi Laino, Lorenzo Lo Muzio
Heliyon.2021; 7(7): e07494. CrossRef - Development of A Nano-Apatite Based Composite Sealer for Endodontic Root Canal Filling
Angelica Bertacci, Daniele Moro, Gianfranco Ulian, Giovanni Valdrè
Journal of Composites Science.2021; 5(1): 30. CrossRef - BIOCERAMIC-BASED ROOT CANAL SEALERS
L Somolová, Z Zapletalová, M Rosa, B Novotná, I Voborná, Y Morozova
Česká stomatologie a praktické zubní lékařství.2021; 121(4): 116. CrossRef - Calcium Silicate-Based Root Canal Sealers: A Narrative Review and Clinical Perspectives
Germain Sfeir, Carla Zogheib, Shanon Patel, Thomas Giraud, Venkateshbabu Nagendrababu, Frédéric Bukiet
Materials.2021; 14(14): 3965. CrossRef - Physico-Chemical Properties of Calcium-Silicate vs. Resin Based Sealers—A Systematic Review and Meta-Analysis of Laboratory-Based Studies
Viresh Chopra, Graham Davis, Aylin Baysan
Materials.2021; 15(1): 229. CrossRef - Comparison of apical sealing ability of bioceramic sealer and epoxy resin-based sealer using the fluid filtration technique and scanning electron microscopy
Widcha Asawaworarit, Thitapa Pinyosopon, Kanittha Kijsamanmith
Journal of Dental Sciences.2020; 15(2): 186. CrossRef - Micro-computed tomographic evaluation of a new system for root canal filling using calcium silicate-based root canal sealers
Mario Tanomaru-Filho, Fernanda Ferrari Esteves Torres, Jader Camilo Pinto, Airton Oliveira Santos-Junior, Karina Ines Medina Carita Tavares, Juliane Maria Guerreiro-Tanomaru
Restorative Dentistry & Endodontics.2020;[Epub] CrossRef - A micro-computed tomographic evaluation of root canal filling with a single gutta-percha cone and calcium silicate sealer
Jong Cheon Kim, Maung Maung Kyaw Moe, Sung Kyo Kim
Restorative Dentistry & Endodontics.2020;[Epub] CrossRef - Comparative evaluation of sealing ability of gutta percha and resilon as root canal filling materials- a systematic review
Pragya Pandey, Himanshi Aggarwal, A.P. Tikku, Arpit Singh, Rhythm Bains, Shambhavi Mishra
Journal of Oral Biology and Craniofacial Research.2020; 10(2): 220. CrossRef - Micro-computed tomographic evaluation of the flow and filling ability of endodontic materials using different test models
Fernanda Ferrari Esteves Torres, Juliane Maria Guerreiro-Tanomaru, Gisselle Moraima Chavez-Andrade, Jader Camilo Pinto, Fábio Luiz Camargo Villela Berbert, Mario Tanomaru-Filho
Restorative Dentistry & Endodontics.2020;[Epub] CrossRef - Root fillings with a matched-taper single cone and two calcium silicate–based sealers: an analysis of voids using micro-computed tomography
Eugenio Pedullà, Roula S. Abiad, Gianluca Conte, Giusy R. M. La Rosa, Ernesto Rapisarda, Prasanna Neelakantan
Clinical Oral Investigations.2020; 24(12): 4487. CrossRef - Influence of different disinfection protocols on gutta-percha cones surface roughness assessed by two different methods
A.M. Nunes, J.P. Gouvea, L. da Silva
Journal of Materials Research and Technology.2019; 8(6): 5464. CrossRef - Endodontic sealers based on calcium silicates: a systematic review
David Donnermeyer, Sebastian Bürklein, Till Dammaschke, Edgar Schäfer
Odontology.2019; 107(4): 421. CrossRef
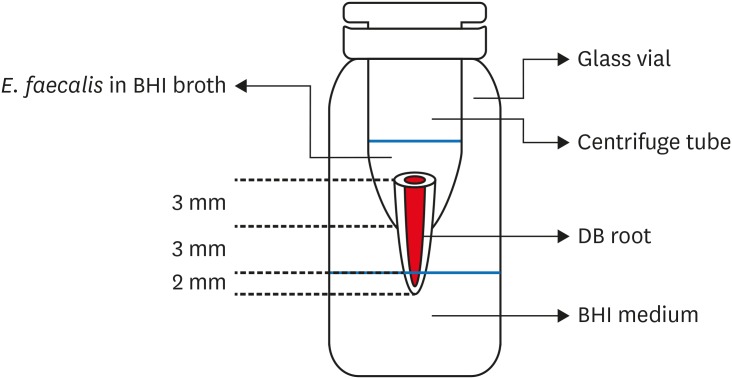
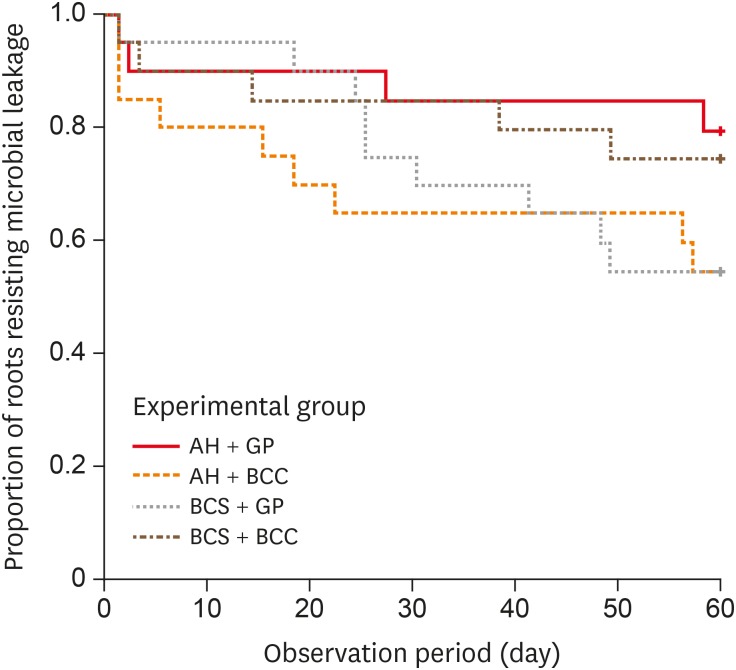
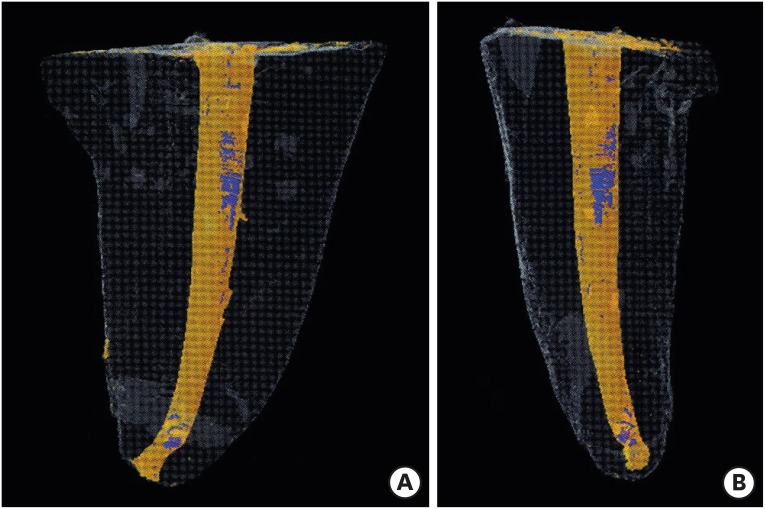
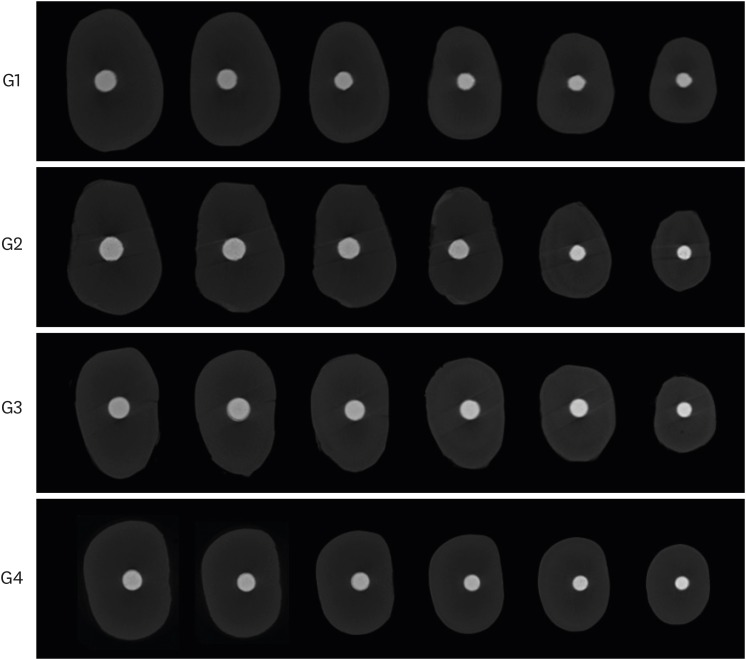
Figure 1
Figure 2
Figure 3
Figure 4
Numbers of teeth and percentages of bacterial leakage of obturated root canals in each experimental group (n = 20 each) after exposure to Enterococcus faecalis for 60 days
| Group | No. | Without leakage | With leakage | Time to leakage | p value* |
|---|---|---|---|---|---|
| 1. GP/AH | 20 | 16 (80%) | 4 (20%) | 52.40 ± 4.12 | 0.216 |
| 2. BCC/AH | 20 | 11 (55%) | 9 (45%) | 41.80 ± 5.50 | |
| 3. GP/BCS | 20 | 11 (55%) | 9 (45%) | 46.05 ± 4.07 | |
| 4. BCC/BCS | 20 | 15 (75%) | 5 (25%) | 50.25 ± 4.34 |
The values in the column of ‘Time to leakage’ are shown as means ± standard deviations in days.
GP, gutta percha; AH, AH Plus; BCC, bioceramic-impregnated gutta percha cone; BCS, bioceramic sealer.
*Using the log-rank test (α = 0.05), no significant difference of bacterial leakage was found among the 4 groups.
Total gaps/voids of leaked and non-leaked samples (percentage, in range), evaluated by micro-computed tomographic analysis
| Group | Sample (n = 4 of each) | Percentage of total gaps/voids (%) |
|---|---|---|
| 1. GP/AH | Leaked | 0.127–0.139 |
| Non-leaked | 0.048–0.125 | |
| 2. BCC/AH | Leaked | 0.038–0.819 |
| Non-leaked | 0.482–0.063 | |
| 3. GP/BCS | Leaked | 0.002–0.004 |
| Non-leaked | 0.001–0.002 | |
| 4. BCC/BCS | Leaked | < 0.001–0.077 |
| Non-leaked | < 0.001 |
GP, gutta percha; AH, AH Plus; BCC, bioceramic-impregnated gutta percha cone; BCS, bioceramic sealer.
The values in the column of ‘Time to leakage’ are shown as means ± standard deviations in days.
GP, gutta percha; AH, AH Plus; BCC, bioceramic-impregnated gutta percha cone; BCS, bioceramic sealer.
*Using the log-rank test (
GP, gutta percha; AH, AH Plus; BCC, bioceramic-impregnated gutta percha cone; BCS, bioceramic sealer.

 KACD
KACD
 ePub Link
ePub Link Cite
Cite

