Articles
- Page Path
- HOME > Restor Dent Endod > Volume 38(4); 2013 > Article
-
Research Article
A comparative evaluation of cytotoxicity of root canal sealers: an
in vitro study - Gautam Pyarelal Badole1, Manjusha Madhukar Warhadpande2, Ganesh Kothiramji Meshram3, Rakesh Namdeoraoji Bahadure4, Shubha Gopal Tawani2, Gopal Tawani2, Shital Gautam Badole1
-
2013;38(4):-209.
DOI: https://doi.org/10.5395/rde.2013.38.4.204
Published online: November 12, 2013
1Department of Conservative Dentistry & Endodontics VSPM's Dental College & Research Center, Nagpur, India.
2Department of Conservative Dentistry & Endodontics Government Dental College & Hospital, Nagpur, India.
3Department of Conservative Dentistry & Endodontics, Peoples Dental College, Bhopal, India.
4Department of Pedodontics, Sharad Pawar Dental College, Sawangi, Wardha, India.
- Correspondence to Gautam Pyarelal Badole, MDS. Assistant Professor, Department of Conservative Dentistry & Endodontics VSPM's Dental College & Research Center, Digdoh hills, Hingna road, Nagpur, Maharashtra, India 440019. TEL, +91-9665410655; FAX, +91-7104306202; badole_g15@yahoo.co.in
©Copyights 2013. The Korean Academy of Conservative Dentistry.
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,708 Views
- 5 Download
- 14 Crossref
Abstract
-
Objectives The objective of this in vitro study was to evaluate and compare the cytotoxicity of four different root canal sealers i.e. Apexit Plus (Ivoclar Vivadent), Endomethasone N (Septodont), AH-26 (Dentsply) and Pulpdent Root Canal Sealer (Pulpdent), on a mouse fibroblast cell line (L929).
-
Materials and Methods Thirty two discs for each sealer (5 mm in diameter and 2 mm in height) were fabricated in Teflon mould. The sealer extraction was made in cell culture medium (Dulbecco's Modified Eagle's Medium, DMEM) using the ratio 1.25 cm2/mL between the surface of the sealer samples and the volume of medium in a shaker incubator. Extraction of each sealer was obtained at 24 hr, 7th day, 14th day, and one month of interval. These extracts were incubated with L929 cell line and 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide (MTT) assay was done. Two-way ANOVA for interaction effects between sealer and time and Post-hoc multiple comparison using Tukey's test across all the 16 different groups were used for statistical analysis.
-
Results Apexit Plus root canal sealer was significantly less toxic than other sealers (p < 0.05) and showed higher cellular growth than control. Endomethasone N showed mild cytotoxicity. AH-26 showed severe toxicity which became mild after one month while Pulpdent Root Canal Sealer showed severe to moderate toxicity.
-
Conclusions Apexit Plus was relatively biocompatible sealer as compared to other three sealers which were cytotoxic at their initial stages, however, they became biocompatible with time.
Introduction
Materials and Methods
1. Endodontic Sealers
1. Statistical analysis
Results
Discussion
Conclusions
Acknowledgements
- 1. Ingle JI, Backland KL. Chapter 30, Obturation of the radicular space. Endodontics. 6th ed. Hamilton: BC Decker Inc.; 2008. p. 1053-1087.
- 2. Grossman LI, Oliet S, Del Rio CE. Chapter 9, Obturation of radicular space. Endodontic practice. 12th ed. New Delhi: Walter Kluwer Pvt. Ltd.; 2010. p. 278-309.
- 3. De Deus QD. Frequency, location, and direction of lateral, secondary, and accessory canals. J Endod 1975;1:361-366.PubMed
- 4. Dongari A, Lambrianidis T. Periodontally derived pulpal lesions. Endod Dent Traumatol 1988;4:49-54.ArticlePubMed
- 5. Dahl JE, Frangou-Polyzois MJ, Polyzois GL. In vitro biocompatibility of denture relining materials. Gerodontology 2006;23:17-22.ArticlePubMed
- 6. Miletić I, Devcić N, Anić I, Borcić J, Karlović Z, Osmak M. The cytotoxicity of Roeko Seal and AH plus compared during different setting periods. J Endod 2005;31:307-309.ArticlePubMed
- 7. Al-Nazhan S, Spangberg L. Morphological cell changes due to chemical toxicity of a dental material: an electron microscopic study on human periodontal ligament fibroblasts and L929 cells. J Endod 1990;16:129-134.ArticlePubMed
- 8. ISO-Standards ISO 10993. Biological compatibility of medical devices. Test for cytotoxicity: in vitro methods. part 5. International Organization for Standardization; 2009. cited 2013 July 9]. https://law.resource.org/pub/ie/ibr/is.en.iso.10993.5.2009.html.
- 9. Leonardo RT, Consolaro A, Carlos IZ, Leonardo MR. Evaluation of cell culture cytotoxicity of five root canal sealers. J Endod 2000;26:328-330.ArticlePubMed
- 10. Huang FM, Hsieh YS, Tai KW, Chou MY, Chang YC. Induction of c-fos and c-jun protooncogenes expression by formaldehyde-releasing and epoxy resin-based root-canal sealers in human osteoblastic cells. J Biomed Mater Res 2002;59:460-465.ArticlePubMed
- 11. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983;65:55-63.ArticlePubMed
- 12. Oztan MD, Yilmaz S, Kalayci A, Zaimoğlu A. A comparison of the in vitro cytotoxicity of two root canal sealers. J Oral Rehabil 2003;30:426-429.ArticlePubMedPDF
- 13. Koulaouzidou EA, Papazisis KT, Beltes P, Geromichalos GD, Kortsaris AH. Cytotoxicity of three resin-based root canal sealers: an in vitro evaluation. Endod Dent Traumatol 1998;14:182-185.ArticlePubMed
- 14. Pinna L, Brackett MG, Lockwood PE, Huffman BP, Mai S, Cotti E, Dettori C, Pashley DH, Tay FR. In vitro cytotoxicity evaluation of a self-adhesive, methacrylate resin-based root canal sealer. J Endod 2008;34:1085-1088.ArticlePubMed
- 15. Osorio RM, Hefti A, Vertucci FJ, Shawley AL. Cytotoxicity of endodontic materials. J Endod 1998;24:91-96.ArticlePubMed
- 16. Azar NG, Heidari M, Bahrami ZS, Shokri F. In vitro cytotoxicity of a new epoxy resin root canal sealer. J Endod 2000;26:462-465.ArticlePubMed
- 17. Miletić I, Anić I, Karlović Z, Marsan T, Pezelj-Ribarić S, Osmak M. Cytotoxic effect of four root filling materials. Endod Dent Traumatol 2000;16:287-290.ArticlePubMed
- 18. Geurtsen W, Leinenbach F, Krage T, Leyhausen G. Cytotoxicity of four root canal sealers in permanent 3T3 cells and primary human periodontal ligament fibroblast cultures. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1998;85:592-597.ArticlePubMed
- 19. Nakamura H, Sakakibara F, Matsumoto Y, Hirano S, Hayakawa H, Sakai K, Yip M. Study on cytotoxicity of root canal filling materials. J Endod 1986;12:156-160.PubMed
- 20. Guigand M, Pellen-Mussi P, Le Goff A, Vulcain JM, Bonnaure-Mallet M. Evaluation of the cytotocompatibility of three endodontic materials. J Endod 1999;25:419-423.PubMed
- 21. Gerosa R, Menegazzi G, Borin M, Cavalleri G. Cytotoxicity evaluation of six root canal sealers. J Endod 1995;21:446-448.ArticlePubMed
- 22. Schwarze T, Fiedler I, Leyhausen G, Geurtsen W. The cellular compatibility of five endodontic sealers during the setting period. J Endod 2002;28:784-786.ArticlePubMed
- 23. Torneck CD, Moe H, Howley TP. The effect of calcium hydroxide on porcine pulp fibroblasts in vitro. J Endod 1983;9:131-136.ArticlePubMed
- 24. Swierenga SH, MacManus JP, Whitfield JF. Regulation by calcium of the proliferation of heart cells from young adult rats. In Vitro 1976;12:31-36.ArticlePubMedPDF
- 25. Whitfield JF, MacManus JP, Rixon RH, Boynton AL, Yondale T, Swierenga S. The positive control of cell proliferation by the interplay on calcium ions and cyclic nucleotides. A review. In Vitro 1976;12:1-18.PubMed
- 26. Kim CK, Ryu HW, Chang HS, Lee BD, Min KS, Hong CU. Evaluation of the radiopacity and cytotoxicity of resinous root canal sealers. J Korean Acad Conserv Dent 2007;32:419-425.Article
- 27. Schwarze T, Leyhausen G, Geursten W. Long-term cytocompatibility of various endodontic sealers using a new root canal model. J Endod 2002;28:749-753.ArticlePubMed
- 28. Kim HJ, Baek SH, Bae KS. Cytotoxicity and genotoxicity of newly developed calcium phosphate-based root canal sealers. J Korean Acad Conserv Dent 2006;31:36-49.Article
- 29. Park SY, Lee WC, Lim SS. Cytotoxicity and antibacterial property of new resin-based sealers. J Korean Acad Conserv Dent 2003;28:162-168.Article
- 30. Cohen BI, Pagnillo MK, Musikant BL, Deutsch AS. An in vitro study of the cytotoxicity of two root canal sealers. J Endod 2000;26:228-229.ArticlePubMed
- 31. Vajrabhaya L, Sithisarn P. Multilayer and monolayer cell cultures in a cytotoxicity assay of root canal sealers. Int Endod J 1997;30:141-144.ArticlePubMed
- 32. Spångberg LS, Barbosa SV, Lavigne GD. AH-26 releases formaldehyde. J Endod 1993;19:596-598.ArticlePubMed
- 33. Kaplan AE, Goldberg F, Artaza LP, de Silvio A, Macchi RL. Disintegration of endodontic cements in water. J Endod 1997;23:439-441.ArticlePubMed
- 34. Araki K, Suda H, Barbosa SV, Spångberg LS. Reduced cytotoxicity of a root canal sealer through eugenol substitution. J Endod 1993;19:554-557.ArticlePubMed
- 35. Key JE, Rahemtulla FG, Eleazer PD. Cytotoxicity of a new root canal filling material on human gingival fibroblasts. J Endod 2006;32:756-758.ArticlePubMed
- 36. Watts A, Paterson RC. Cellular responses in the dental pulp: a review. Int Endod J 1981;14:10-19.ArticlePubMed
- 37. Wataha JC, Hanks CT, Strawn SE, Fat JC. Cytotoxicity of components of resins and other dental restorative materials. J Oral Rehabil 1994;21:453-462.ArticlePubMed
- 38. Wiegand C, Hipler UC. Methods for the measurement of cell and tissue compatibility including tissue regeneration processes. GMS Krankenhhyg Interdiszip 2008;3:Doc12.PubMedPMC
REFERENCES
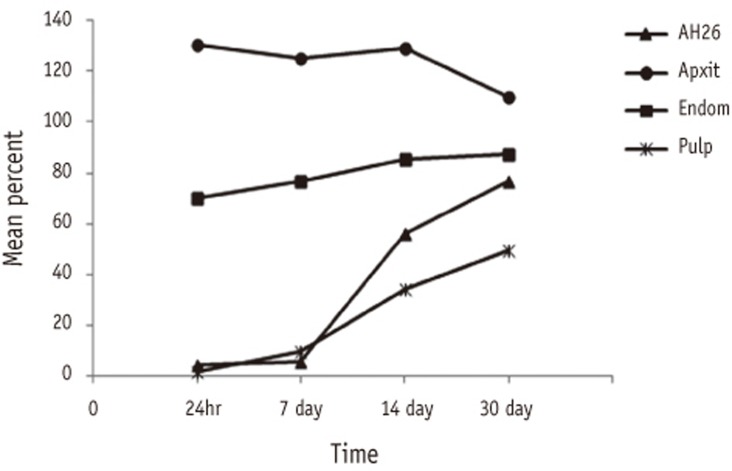
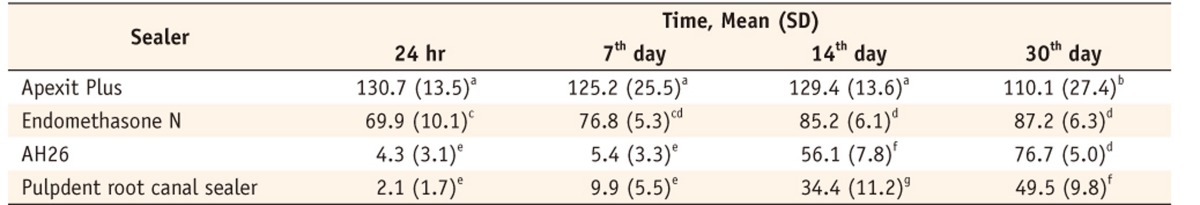
The main effects and the interaction of Sealer and Time were significant (p < 0.001) therefore multiple comparision using Tukey's test was applied in sixteen different groups.
R-squared = 0.932 (Adjusted R-squared = 0.930).
Percentage values with the same superscripts in different groups imply statistical insignificance by Tukey's test.
Tables & Figures
REFERENCES
Citations

- The Evolution of In Vitro Toxicity Assessment Methods for Oral Cavity Tissues—From 2D Cell Cultures to Organ-on-a-Chip
Alexandra Jităreanu, Luminița Agoroaei, Ioana-Cezara Caba, Florina-Daniela Cojocaru, Liliana Vereștiuc, Mădălina Vieriu, Ioana Mârțu
Toxics.2025; 13(3): 195. CrossRef - Comparative analysis of the inflammatory response of human gingival fibroblasts to NeoSEALER Flo and CeraSeal bioceramic sealers: an in vitro study
Sarah Salah Gaafar, Abdel Rahman O. El Mekkawi, Rehab Ali Farag, Mohamed H. A. Gadelmawla, Ahmad Mostafa Hussein Mohamad Hussein, Mohamed Sayed, Mohammad Rayyan, Doaa Gamal AbdelMouez Basta
BMC Oral Health.2025;[Epub] CrossRef - Surface characteristics of nanostructured titanium surface modified via UV irradiation during SLA
S. Khorasani, G. Faraji
Applied Physics A.2025;[Epub] CrossRef - Cytotoxicity and Cell Viability of Two Bioactive Root Canal Sealers, Mineral Trioxide Aggregate, and BioRoot Root Canal Sealer: An In Vitro Study
Emmanuel Samson, Lata B Gangurde, Jaiprakash R Rathod, Pradnya S Jadhav, Sangeeta Ambhore, Pranav S Jadhav
CODS - Journal of Dentistry.2023; 14(2): 57. CrossRef - Human Gingival Fibroblasts Response to Different Endodontic Sealers: An In Vitro Study
Rita Noites, Inês Tavares, Miguel Cardoso, Isabel M. Carreira, Maria Bartolomeu, Ana S. Duarte, Ilda P. Ribeiro
Applied Sciences.2023; 13(19): 10976. CrossRef - Antibacterial and Cytotoxicity of Root Canal Sealer with the Addition of Chitosan Nanoparticle at Various Concentrations
Diatri Nari Ratih, Ema Mulyawati, Rika Kurnia Santi, Yulita Kristanti
European Journal of Dentistry.2023; 17(02): 398. CrossRef - Transforaminal and systemic diffusion of an active agent from a zinc oxide eugenol-based endodontic sealer containing hydrocortisone—in an in vivo model
Davy Aubeux, Anne Valot-Salengro, Gaelle Gautier, Arnaud Malet, Fabienne Pérez
Clinical Oral Investigations.2020; 24(12): 4395. CrossRef - PEGylated curcumin-loaded nanofibrous mats with controlled burst release through bead knot-on-spring design
Mahdi Saeed, Hamid Mirzadeh, Mojgan Zandi, Jalal Barzin
Progress in Biomaterials.2020; 9(4): 175. CrossRef - A New Calcium Silicate-Based Root Canal Dressing: Physical and Chemical Properties, Cytotoxicity and Dentinal Tubule Penetration
Natália Villa, Vanessa Valgas Dos Santos, Ubirajara Maciel da Costa, Aline Teixeira Mendes, Pedro Henrique Marks Duarte, Ricardo Abreu da Rosa, Jefferson Ricardo Pereira, Marcus Vinícius Reis Só
Brazilian Dental Journal.2020; 31(6): 598. CrossRef - Evaluation of the Antimicrobial Efficacy of Herbal Extracts Added to Root Canal Sealers of Different Bases: An In Vitro Study
Abhay M Tripathi, Minarani T Devi, Sonali K Kalra, Ujjala Ghoshal
International Journal of Clinical Pediatric Dentistry.2019; 12(5): 398. CrossRef - Endodontic-related inferior alveolar nerve injuries: A review and a therapeutic flow chart
R. Castro, M. Guivarc'h, J.M. Foletti, J.H. Catherine, C. Chossegros, L. Guyot
Journal of Stomatology, Oral and Maxillofacial Surgery.2018; 119(5): 412. CrossRef - Comparison between direct contact and extract exposure methods for PFO cytotoxicity evaluation
Girish K. Srivastava, Maria L. Alonso-Alonso, Ivan Fernandez-Bueno, Maria T. Garcia-Gutierrez, Fernando Rull, Jesús Medina, Rosa M. Coco, J. Carlos Pastor
Scientific Reports.2018;[Epub] CrossRef - Novel endodontic sealers induce cell cytotoxicity and apoptosis in a dose-dependent behavior and favorable response in mice subcutaneous tissue
L. A. B. Silva, L. U. Azevedo, A. Consolaro, F. Barnett, Y. Xu, R. A. Battaglino, P. S. Cañadas, Katharina Morant Holanda de Oliveira, R. A. B. Silva
Clinical Oral Investigations.2017; 21(9): 2851. CrossRef - Designing and fabrication of curcumin loaded PCL/PVA multi-layer nanofibrous electrospun structures as active wound dressing
Seyed Mahdi Saeed, Hamid Mirzadeh, Mojgan Zandi, Jalal Barzin
Progress in Biomaterials.2017; 6(1-2): 39. CrossRef
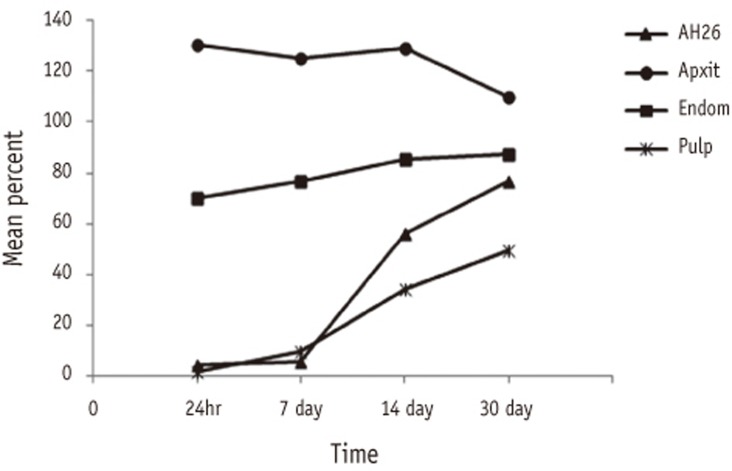
Figure 1
Comparison of percentage cell viability (mean ± SD, n = 32) for all sealers at different time periods

The main effects and the interaction of Sealer and Time were significant (p < 0.001) therefore multiple comparision using Tukey's test was applied in sixteen different groups.
R-squared = 0.932 (Adjusted R-squared = 0.930).
Percentage values with the same superscripts in different groups imply statistical insignificance by Tukey's test.
The main effects and the interaction of Sealer and Time were significant ( Percentage values with the same superscripts in different groups imply statistical insignificance by

 KACD
KACD
 ePub Link
ePub Link Cite
Cite

