Abstract
-
Objectives
This study aimed to evaluate the capacity of 2% chlorhexidine gel associated with 8% papain gel in comparison with 5.25% sodium hypochlorite in bovine pulp tissue dissolution.
-
Materials and Methods
Ninety bovine pulps of standardized sizes were used and fragmented into 5-mm sizes. The fragments were removed from the root middle third region. They were divided into 6 experimental groups (n = 15), 1) 8% papain; 2) 2% chlorhexidine; 3) 2% chlorhexidine associated with 8% papain; 4) 0.9% saline solution; 5) 2.5% sodium hypochlorite; and 6) 5.25% sodium hypochlorite. The pulp fragments were weighed and put into immobile test tubes for dissolution for time intervals of 30, 60, 90, and 120 min.
-
Results
The 5.25% sodium hypochlorite had greater dissolution potential than the pure papain, and when associated with chlorhexidine, both promoted greater dissolution than did the saline solution and 2% chlorhexidine groups (p < 0.05). The 2.5% sodium hypochlorite promoted dissolution to a lesser extent than the groups with papain within a period of 30 min (p < 0.05), but, was comparable to the saline solution and chlorhexidine. After 120 min, the 2.5% and 5.25% sodium hypochlorite promoted dissolution of 100% of the pulp fragments, and papain, 61%, while chlorhexidine associated with papain and chlorhexidine alone dissolved only 55% and 3%, respectively.
-
Conclusions
The 8% papain in gel, both alone and in association with chlorhexidine, was able to dissolve bovine pulp tissue, but to a lesser extent than did 5.25% sodium hypochlorite.
-
Keywords: Chlorhexidine; Endodontics; Papain
Introduction
The efficacy of endodontic therapy is mainly associated with microbial control.
1 Thus, it is recommended that instruments that produce mechanical action be used with chemical substances that have antimicrobial activity, the capacity to dissolve organic matter, lubricant properties, and low cytotoxicity.
2 Sodium hypochlorite (NaOCl) is the most frequently used endodontic irrigant, and at present is the solution of choice. However, many studies have proposed other alternatives, considering that NaOCl is very cytotoxic, has allergenic potential, and compromises resin cement bonding. It also causes alterations in the collagen fibers of root dentin and alters the integrity of cancellous bone.
3-
5 In addition, there is the imminent risk that if injection of this solution occurs beyond the apical foramen, it may immediately cause intense pain, edema, and hyperemia, and also result in necrosis of the adjacent tissues, depending on the volume injected.
6,
7 The use of chlorhexidine gluconate as an irrigant has been justified by its antimicrobial action, and weaker odor and cytotoxicity than NaOCl.
8
The majority of researches related to endodontic irrigants have been restricted to the chemical and antimicrobial properties of the solutions, and the mechanical properties have been left aside. The use of viscous irrigants such as chlorhexidine gluconate in water soluble gel bases has maintained the antimicrobial efficacy and increased the lubricant capacity for endodontic files. Smear layer removal is another property that is possible due to the action of the gels. All dentin scrapings, organic remainders, and particles in suspension generated during instrumentation are eliminated together with the gel during irrigation and aspiration with saline solution.
9,
10
Chlorhexidine in gel has not shown the potential to dissolve organic matter.
11 The presence of infected pulp remainders, particularly in the regions in which mechanical instrumentation is not effective, is a major factor responsible for endodontic treatment failure.
12,
13 This suggests studies that seek to develop clinical or laboratory tests with the goal of arriving at an irrigant that best performs its function without harming the periapical tissues. The purpose of this study was to evaluate the capacity of 2% chlorhexidine associated with 8% papain to dissolve bovine pulp tissue, in comparison with 5.25% NaOCl.
Materials and methods
A total of 90 bovine mandibular incisors were extracted and stored in physiological solution during transport to the laboratory of the State University of Southwest Bahia. The teeth were extracted from bovine jaws dissected after death in a refrigerated room where the animals were slaughtered for food. Given that the study did not interfere with or cause the death or slaughter of animals, there was no need for permission from the animal ethics committee. The pulps were removed by means of a cross-sectional fracture in the cervical region, separating the crown from the root. The root pulp was completely removed with the aid of hemostatic forceps using tractional movement. Pulp measurement and cutting was performed with a pachymeter and scalpel, at all times preserving the middle third portion, and discarding the cervical and apical portions. The pulp fragments were standardized to sizes of 5 mm. These fragments were washed for 30 seconds with physiological solution, enveloped in transparent plastic PVC film (Royal Pack, Paulista, Brazil), and stored at a temperature of -20℃ until they were used. All the formulations were prepared in the general Chemical Laboratory of the State University of Southwest Bahia. The sodium hypochlorite was formulated at a concentration of 5.25% and adjusted to a pH of 9.0 with the addition of boric acid. The 8% papain and 2% chlorhexidine were formulated in the same gel base and remainder under refrigeration until they were used. Six experimental groups were established with 15 experiments for each group (n = 15) as follows,
Group 1: 8% papain gel
Group 2: 2% chlorhexidine gel
Group 3: 2% chlorhexidine associated with 8% papain gel
Group 4: 0.9% saline solution
Group 5: 2.5% sodium hypochlorite
Group 6: 5.25% sodium hypochlorite
The pulp fragments in the process of dissolution were moved from -20℃ to room temperature, washed with saline solution for 30 seconds, dried with filter paper for another 30 seconds, and weighed on a precision analytic balance (AW-220, Shimadzu, Tokyo, Japan). They were placed in test tubes with 5 mL of the substance to be analyzed. At the end of each time interval (30, 60, 90, and 120 minutes) the substances were changed, and the pulp fragments were washed again, dried, and weighed according to the same pre-established criteria.
Statistical analyses were performed with the program SPSS 15.0 (SPSS Inc., Chicago, IL, USA). Descriptive statistical analysis including the mean and standard deviation were calculated for the groups evaluated. The mean pulp dissolution values were statistically analyzed using the Kruskal-Wallis and Mann-Whitney U-tests.
Results
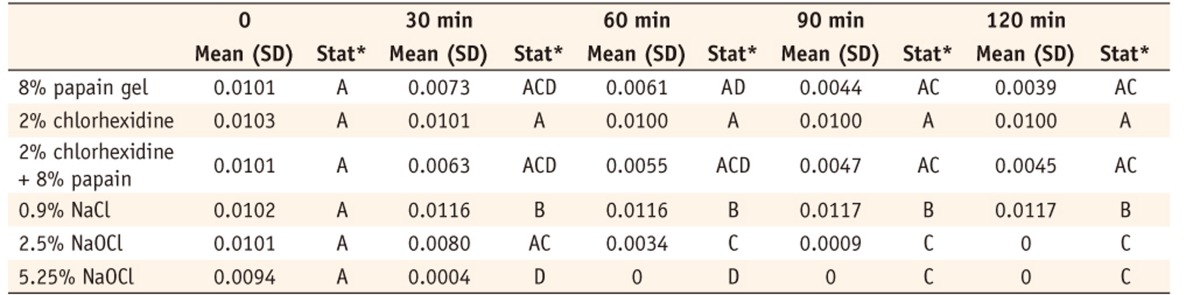
Table 1 shows the mean weight (grams) of the pulp fragments at each time interval during the dissolution process. Both 2.5% and 5.25% sodium hypochlorite promoted the dissolution of the fragments by 120 minutes. The 2.5% sodium hypochlorite dissolved less than did the papain and papain chlorhexidine by 30 minutes, but the differences were statistically significant as compared to the groups with 2% chlorhexidine and 0.9% saline solution. Both the 8% pure papain gel and that associated with 2% chlorhexidine exhibited significantly greater dissolution potential than the 0.9% saline solution and 2% chlorhexidine.
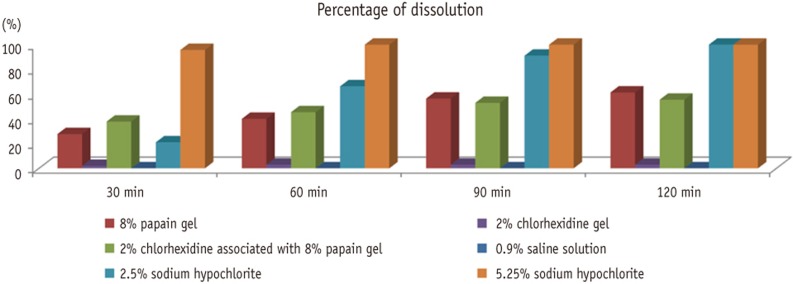
Figure 1 shows the percentage of pulp tissue dissolution that had occurred by the end of each time interval. Both 2.5% and 5.25% sodium hypochlorite achieved 100% dissolution at the end of the 120 minutes. The 8% papain gel presented 61% dissolution, and when it was associated with chlorhexidine, it presented 55%. The 2% chlorhexidine and 0.9% saline solution presented no statistically significant dissolution.
Discussion
Papain is a proteolytic enzyme extracted from the latex of
Carica papaya, Caricaceae family, better known as papaya fruit. In 1526, this papaya tree was first described by the Spaniard Oviedo in Panama and Colombia.
Carica papaya latex produces an enzymatic mixture basically formed of papain and chymopapain. Since 1968, many researchers have studied the digestive action of papaya latex and attributed various nomenclatures to the active ingredients. First 'Caricina', then 'papaiotin' and finally 'papain'.
14
In the pharmaceutical industry, papain has been used in diverse formulations of medications, mainly due to its proteolytic action. In medicine, its main clinical application is in debriding wounds and burns that exhibit necrotic areas, in order to accelerate the cicatrization process.
15,
16 In dentistry, papain in gel form associated with chloramine is used in chemomechanical caries removal, with the object of removing the infected dentin without causing any damage whatsoever to the healthy oral structure.
14,
15,
17-
19 Based on evaluating the apical sealing of endodontically treated teeth, Duarte et al. argued that 0.8% papain gel can be used as an irrigant solution.
16
When we evaluated the antibacterial activity of the extract papain gel against
Enterococcus faecalis, an efficacy of 67.3% was observed, while 2% chlorhexidine gel showed 100%.
20 Further studies are needed to evaluate the antibacterial action of papain associated with chlorhexidine. Moreover, molecular studies on the association of papain with the stability of chlorhexidine must be carefully made, mainly in order to evaluate the useful life of the product and the activity of chlorhexidine. The proteolytic activity of papain was observed in this work, and was maintained when associated with chlorhexidine, indicating that no denaturation of the enzyme occurred, since no statistical difference existed between the dissolution with pure papain and that associated with chlorhexidine.
For this study, the option was to use a methodology that is better suited to the type of experiment presented. A previous study reported that three factors influence the dissolution of tissues: the rate of agitation, the amount of organic matter in relation to the quantity of irrigating solution, and the area of tissue contact with the solution.
12 In comparing substances of different viscosities, such as a solution and gel, the medium of agitation may influence the dissolution potential, mainly because of the volume of solution that would be coming into contact with the fragment. For this reason, a method with immobile test tubes was used, in order to reduce the variables that could confound the results. Moreover, it was observed that the sodium hypochlorite had an advantage in the dissolution due to the differences in viscosity of the solutions, as the pulp fragment was able to come into contact with the entire solution, whereas with the gel, this did not happen. This study prioritized the comparison of papain and chlorhexidine with the substance that is most used and considered the gold standard for dissolution in endodontics, which is 5.25% sodium hypochlorite.
This increased viscosity of the gel could be a disadvantage for the performance of the papain because it is important that an irrigator penetrate the dentinal tubules to promote greater disinfection. However, studies comparing the antibacterial and mechanical ability of chlorhexidine gel and sodium hypochlorite showed that chlorhexidine gel promotes better cleaning of the dentin surface, removing the greatest quantity of the smear layer. When we analyzed the ability to inhibit
E. faecalis at different depths of the dentinal tubules (200 to 400 µm), we found that the chlorhexidine gel promoted 100% inhibition. Thus the mechanical properties of the gel seem to be the main factor for this enhanced cleaning ability, as chlorhexidine, when used in its liquid form, showed a lower efficiency.
9,
20
The pulp fragments were standardized to an approximate size and weight. The exposure times of the fragments were standardized to 30, 60, 90, and 120 minutes, following other studies.
21,
22 Long periods of time were tested to verify the hypothesis that papain would actually be able to promote dissolution of tissues, since this work is an exploratory study. Clinically, these times would fit in endodontic treatments with more than one clinical section of 30 minutes each.
Another important fact to consider is that the pulp fragments, when exposed to groups containing papain, developed a fairly soft consistency, whereas when exposed to chlorhexidine or NaOCl, the pulps became hardened and dried. When exposed to 0.9% saline solution, they maintained their morphology and consistency. Indeed, this important clinically because it can facilitate the mechanical action of instruments while cleaning the root canals.
Sodium hypochlorite was the substance that presented the greatest pulp tissue dissolution potential, being statistically significant in comparison with the other groups evaluated. At the end of 30 minutes, the 5.25% sodium hypochlorite had already degraded almost the entire fragment, 96%, demonstrating great effectiveness in the dissolution of organic matter, as has been described in the literature.
20,
21,
23,
24 The sodium hypochlorite, when diluted to 2.5%, did not show the same effectiveness, promoting only approximately 20% dissolution. The corroborates other authors' findings that this dilution promotes a decrease in the irrigant's ability to dissolve.
21 Both pure papain and papain in association with chlorhexidine only achieved approximately 60% pulp dissolution by 120 minutes. Nevertheless, the papain promoted statistically significant dissolution of the fragments in comparison with 0.9% saline solution and 2% chlorhexidine gel. It was also observed that the fragments exposed to saline solution underwent hydration and gained weight in the first 30 minutes, thus demonstrating that it did not cause dissolution. The chlorhexidine group underwent a small weight loss during the process, but this was not statistically significant in comparison with the saline solution, corroborating other studies.
11,
22-
24
Conclusions
The 8% papain in gel, both alone and in association with chlorhexidine, performed bovine pulp tissue dissolution, but in a lower proportion than did the 5.25% sodium hypochlorite.
-
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1. Kakehashi S, Stanley HR, Fitzgerald RJ. The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral Surg Oral Med Oral Pathol 1965;20:340-349.ArticlePubMed
- 2. Safavi KE, Spangberg LS, Langeland K. Root canal dentinal tubule disinfection. J Endod 1990;16:207-210.ArticlePubMed
- 3. Moreira DM, Almeida JF, Ferraz CC, Gomes BP, Line SR, Zaia AA. Structural analysis of bovine root dentin after use of different endodontics auxiliary chemical substances. J Endod 2009;35:1023-1027.ArticlePubMed
- 4. Morris MD, Lee KW, Agee KA, Bouillaguet S, Pashley DH. Effects of sodium hypochlorite and RC-prep on bond strengths of resin cement to endodontic surfaces. J Endod 2001;27:753-757.ArticlePubMed
- 5. Kerbl FM, DeVilliers P, Litaker M, Eleazer PD. Physical effects of sodium hypochlorite on bone: an ex vivo study. J Endod 2012;38:357-359.ArticlePubMed
- 6. Paschoalino Mde A, Hanan AA, Marques AA, Garcia Lda F, Garrido AB, Sponchiado EC Jr. Injection of sodium hypochlorite beyond the apical foramen-a case report. Gen Dent 2012;60:16-19.PubMed
- 7. Behrents KT, Speer ML, Noujeim M. Sodium hypochlorite accident with evaluation by cone beam computed tomography. Int Endod J 2012;45:492-498.ArticlePubMed
- 8. Dametto FR, Ferraz CC, Gomes BP, Zaia AA, Teixeira FB, de Souza-Filho FJ. In vitro assessment of the immediate and prolonged antimicrobial action of chlorhexidine gel as an endodontic irrigant against Enterococcus faecalis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2005;99:768-772.ArticlePubMed
- 9. Ferraz CC, Gomes BP, Zaia AA, Teixeira FB, Souza-Filho FJ. In vitro assessment of the antimicrobial action and the mechanical ability of chlorhexidine gel as an endodontic irrigant. J Endod 2001;27:452-455.ArticlePubMed
- 10. Ferraz CC, Gomes BP, Zaia AA, Teixeira FB, Souza-Filho FJ. Comparative study of the antimicrobial efficacy of chlorhexidine gel, chlorhexidine solution and sodium hypochlorite as endodontic irrigants. Braz Dent J 2007;18:294-298.ArticlePubMed
- 11. Okino LA, Siqueira EL, Santos M, Bombana AC, Figueiredo JA. Dissolution of pulp tissue by aqueous solution of chlorhexidine digluconate and chlorhexidine digluconate gel. Int Endod J 2004;37:38-41.ArticlePubMed
- 12. Moorer WR, Wesselink PR. Factors promoting the tissue dissolving capability of sodium hypochlorite. Int Endod J 1982;15:187-196.ArticlePubMed
- 13. Callahan JR. Sulfuric acid for opening root canals. Dent Cosmos 1984;36:957-959.
- 14. Carrillo CM, Tanaka MH, Cesar MF, Camargo MA, Juliano Y, Novo NF. Use of papain gel in disabled patients. J Dent Child (Chic) 2008;75:222-228.PubMed
- 15. Ferreira CM, Bonifácio KC, Fröner IC, Ito IY. Evaluation of the antimicrobial activity of three irrigating solutions in teeth with pulpal necrosis. Braz Dent J 1999;10:15-21.PubMed
- 16. Duarte MAH, Yamashita JC, Lanza P, de Campos Fraga S, Kuga MC. Influência da irrigação endodôntica com gel de papaína no selamento apical. Salusvita 2001;20:27-33.
- 17. Gianini RJ, do Amaral FL, Flório FM, Basting RT. Microtensile bond strength of etch-and-rinse and self-etch adhesive systems to demineralized dentin after the use of a papain-based chemomechanical method. Am J Dent 2010;23:23-28.PubMed
- 18. Piva E, Ogliari FA, Moraes RR, Corá F, Henn S, Correr-Sobrinho L. Papain-based gel for biochemical caries removal: influence on microtensile bond strength to dentin. Braz Oral Res 2008;22:364-370.ArticlePubMed
- 19. Botelho Amaral FL, Martão Florio F, Bovi Ambrosano GM, Basting RT. Morphology and microtensile bond strength of adhesive systems to in situ-formed caries-affected dentin after the use of a papain-based chemomechanical gel method. Am J Dent 2011;24:13-19.PubMed
- 20. Irala LE, Grazziotin-Soares R, Salles AA, Munari AZ, Pereira JS. Dissolution of bovine pulp tissue in solutions consisting of varying NaOCl concentrations and combined with EDTA. Braz Oral Res 2010;24:271-276.ArticlePubMed
- 21. Stojicic S, Zivkovic S, Qian W, Zhang H, Haapasalo M. Tissue dissolution by sodium hypochlorite: effect of concentration, temperature, agitation, and surfactant. J Endod 2010;36:1558-1562.ArticlePubMed
- 22. Christensen CE, McNeal SF, Eleazer P. Effect of lowering the pH of sodium hypochlorite on dissolving tissue in vitro. J Endod 2008;34:449-452.ArticlePubMed
- 23. Marending M, Luder HU, Brunner TJ, Knecht S, Stark WJ, Zehnder M. Effect of sodium hypochlorite on human root dentine-mechanical, chemical and structural evaluation. Int Endod J 2007;40:786-793.ArticlePubMed
- 24. Zehnder M, Grawehr M, Hasselgren G, Waltimo T. Tissue-dissolution capacity and dentin-disinfecting potential of calcium hydroxide mixed with irrigating solutions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2003;96:608-613.ArticlePubMed
Figure 1Percentage of pulp tissue dissolution in different time intervals.
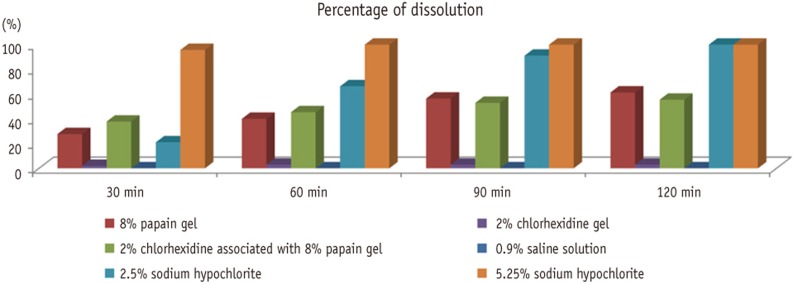
Table 1Mean weights (gr) of pulp fragments in different time intervals



 KACD
KACD


 ePub Link
ePub Link Cite
Cite

