Articles
- Page Path
- HOME > Restor Dent Endod > Volume 34(6); 2009 > Article
- Original Article Changes in µ-TBS to pulp chamber dentin after the application of NaOCl & reversal effect by using sodium ascorbate
- Su-Mi Kwon, Tae-Gun Kim, Mi-Kyung Yu, Kwang-Won Lee
-
2009;34(6):-525.
DOI: https://doi.org/10.5395/JKACD.2009.34.6.515
Published online: November 30, 2009
Department of Conservative Dentistry, School of Dentistry & Institute of Oral Bioscience, Chonbuk National University, Korea.
- Corresponding Author: Kwang-Won Lee. Department of Conservative Dentistry, School of Dentistry, Chonbuk National University, 664-14, Duckjin-Dong, Duckjin-Gu, Jeonju 561-756, Korea. Tel: 82-63-250-2016, lkw@chonbuk.ac.kr
• Received: October 9, 2009 • Revised: October 21, 2009 • Accepted: October 22, 2009
Copyright © 2009 The Korean Academy of Conservative Dentistry
- 1,082 Views
- 4 Download
- 1 Crossref
Abstract
-
Clinical suggestion for the limitation of application time of NaOCl solution is needed to avoid large reductions in resin-dentin bond strength. The aim of this study was to measure the change of µ-tensile bond strength after the various application time of 5.25% NaOCl solution to pulp chamber dentin in endodontic access cavity, and to evaluate the effect of 10% sodium ascorbate application for 10 min on bond strength after the treatment of 5.25% NaOCl solution. In this experiment, there were no statistical differences(p>0.05) in bond strengths between upper chamber dentin and lower chamber dentin. NaOCl-treated group for 20 min did not show any significant decrease(p>0.05) in bond strength than non-treated control group. In contrast to that, bond strengths of NaOCl-treated groups for 40 & 80 min were significantly lower(p<0.05) than that of non-treated control group.10% sodium ascorbate retreated group for 10 min after 5.25% NaOCl application for 40 min to chamber dentin showed the recovery of bond strength significantly. However, the bond strength of sodium ascorbate retreated group after 5.25% NaOCl application for 80 min was still significantly lower(p<0.05) compared to the non-treated control group, which means the reductions in resin-dentin bond strength were not fully reversed. On the contrary, sodium ascorbate retreated group after 5.25% NaOCl application for 5 min showed significantly higher(p<0.05) bond strength compared to the control group, which demonstrates its superior recovery effect. In SEM exminations of specimens retreated with 10% sodium ascorbate after NaOCl application for 40 & 80 min showed that resin tags were formed clearly and densely, but weakly in density and homogeneity of individual resin tag compared to the control specimen.
- 1. Ray HA, Trope M. Periapical status of endodontically treated teeth in relation to the technical quality of the root filling and the coronal restoration. Int Endod J. 1995;28: 12-18.ArticlePubMed
- 2. Davidson CL, De Gee AJ, Feilzer AJ. The competition between the composite-dentin bond strength and the polymerization contraction stress. J Dent Res. 1984;63: 1396-1399.ArticlePubMedPDF
- 3. Feilzer AJ, De Gee AJ, Davidson CL. Quantitative determination of stress reduction by flow in composite restorations. Dent Mater. 1990;6: 167-171.ArticlePubMed
- 4. Carvalho RM, Pereira JC, Yoshiyama M, Pashley DH. A review of polymerization contraction: the influence of stress development versus stress relief. Oper Dent. 1996;21: 17-24.PubMed
- 5. Ausiello P, De Gee AJ, Rengo S, Davidson CL. Fracture resistance of endodontically-treated premolars adhesively restored. Am J Dent. 1995;10: 237-241.
- 6. Hernandez R, Bader S, Boston D, Trope M. Resistance to fracture of endodontically treated premolars restored with new generation dentine bonding systems. Int Endod J. 1995;27: 281-284.Article
- 7. Belli S, Zhang Y, Pereira PNR, Pashley DH. Adhesive sealing of the pulp chamber. J Endod. 2001;27: 521-526.PubMed
- 8. Haak R, Wicht MJ, Noack MJ. Does chemomechanical caries removal affect dentin adhesion? Eur J Oral Sci. 2000;108: 449-455.PubMed
- 9. Cox CF, Hafez AA, Akimoto N, Otsuki M, Suzuki S, Tarim B. Biocompatibility of primer, adhesive and resin composite systems on non-exposed and exposed pulps of non-human primate teeth. Am J Dent. 1998;11(Spec No):S55-S63.PubMed
- 10. Nikaido T, Takano Y, Sasafuchi Y, Burrow MF, Tagami J. Bond strength to endodontically-treated teeth. Am J Dent. 1999;12: 177-180.PubMed
- 11. Morris MD, Lee KW, Agee KA, Bouillaguet S, Pashley DH. Effects of Sodium Hypochlorite and RC-Prep on Bond Strengths of Resin Cement to Endodontic Surfaces. J Endod. 2001;27(12):753-757.PubMed
- 12. Frankenberger R, Kramer N, Oberschachtsiek H, Petschelt A. Dentin bond strength and marginal adaption after NaOCl pre-treatment. Oper Dent. 2000;25: 40-45.PubMed
- 13. Lai SCN, Mak UF, Cheung GSP, Osorio R, Toledano M, Carvalho RM, Tay FR, Pashley DH. Reversal of Compromised Bonding to Oxidized Etched Dentin. J Dent Res. 2001;80(10):1919-1924.ArticlePubMedPDF
- 14. Rose RC, Bode AM. Biology of free radical scavengers : an evaluation of ascorbate. FASEB J. 1993;7: 1135-1142.ArticlePubMedPDF
- 15. Jeon SY, Lee KW, Yoo MK. Influence of Sodium Ascorbate on Microtensile Bond Strengths to Pulp Chamber Dentin Treated with NaOCL. J Korean Acad Conserv Dent. 2008;33(6):545-552.Article
- 16. Torneck CD, Titley KC, Smith DC, Adibfar A. Adhesion of light-cured composite resin to bleached and unbleached bovine dentin. Endod Dent Traumatol. 1990;6: 97-103.ArticlePubMed
- 17. Rueggeberg FA, Margeson DH. The effect of oxygen inhibition on an unfilled/filled composite system. J Dent Res. 1990;69: 1652-1658.ArticlePubMedPDF
- 18. Weston HC, Ito S, Wadgaonkar B, Pashley DH. Effects of time and concentration of sodium ascorbate on reversal of NaOCl-induced reduction in bond strengths. J Endod. 2007;33(7):879-881.ArticlePubMed
- 19. Vongphan N, Senawongse1 P, Somsiri W, Harnirattisa C. Effects of sodium ascorbate on microtensile bond strength of total-etching adhesive system to NaOCl treated dentine. J Dent. 2005;33(8):689-695.ArticlePubMed
- 20. O'Keefe KL, Powers JM. Adhesion of resin composite corematerials to dentin. Int J Prosthodont. 2001;14(5):451-456.PubMed
- 21. Kijsamanmith K, Timpawat S, Harnirattisai C, Messer HH. Micro-tensile bond strengths of bonding agents to pulpal floor dentine. Int Endod J. 2002;35(10):833-839.ArticlePubMed
- 22. Son JR, Lee KY, Kang YM, Oh YT, Lee KW, Kim TK. The Effect of Concentration and Application Time of Hydrogen Peroxide on the Microtensile Bond Strength of Resin Restorations to the Dentin at Different Depths. J Korean Acad Conserv Dent. 2009;34(5):406-414.Article
REFERENCES
Figure 5
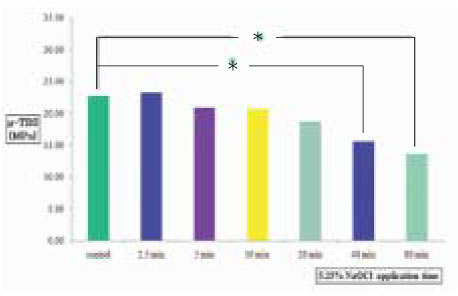
µ-TBS to chamber dentin after the application of certain period of time using 5.25% NaOCl. Asterisk(*) means statistical difference between groups.

Figure 6
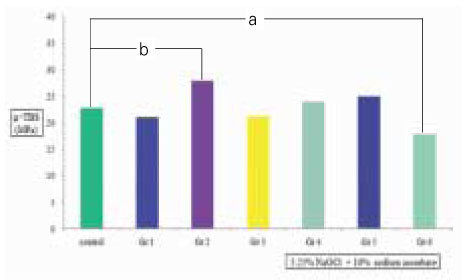
µ-TBS to chamber dentin after the treatment of 5.25% NaOCl +10% sodium ascorbate. Small letter(a,b) means statistical difference between groups.

Figure 7
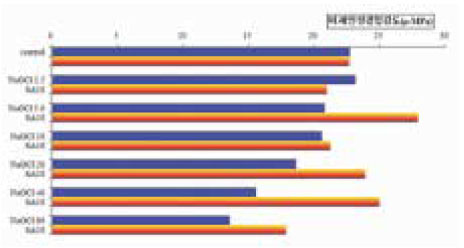
Summarization of µ-TBS to chamber dentin after the treatment of 5.25% NaOCl and 5.25% NaOCl +10% sodium ascorbate.

Tables & Figures
REFERENCES
Citations
Citations to this article as recorded by 

- Influence of Sodium Hypochorite & EDTA on the Microtensile Bond Strength of Ethanol Wet Bonding
Deok-Joong Kim, Yong-Beom Song, Sang-Hee Park, Hyoung-Sun Kim, Hye-Yoon Lee, Mi-Kyung Yu, Kwang-Won Lee
Journal of Dental Rehabilitation and Applied Science.2013; 29(1): 37. CrossRef
Changes in µ-TBS to pulp chamber dentin after the application of NaOCl & reversal effect by using sodium ascorbate
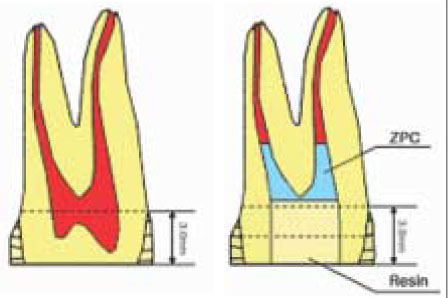
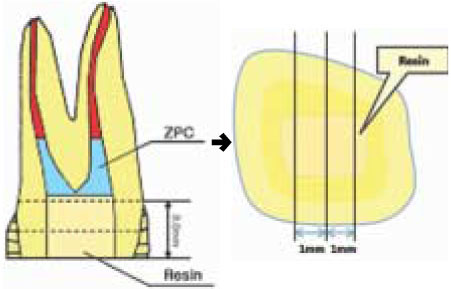
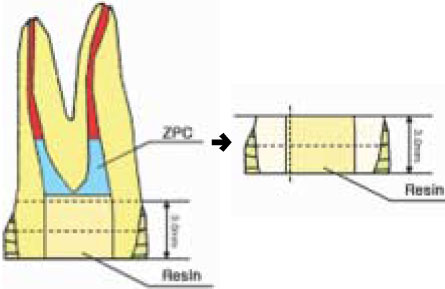
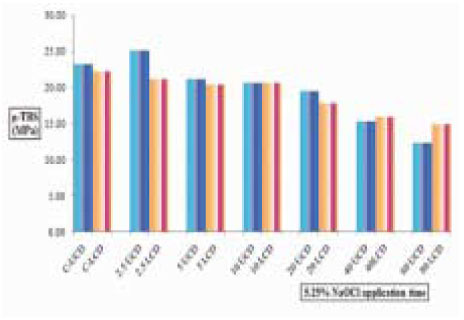
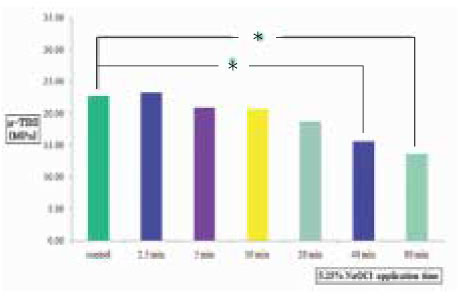
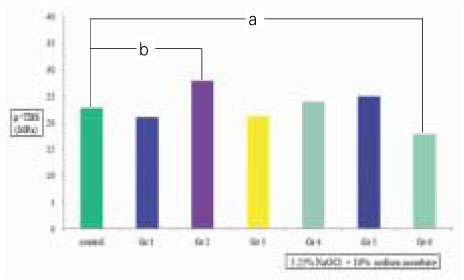
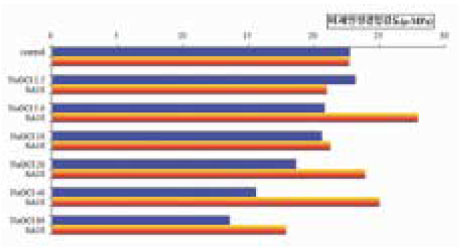
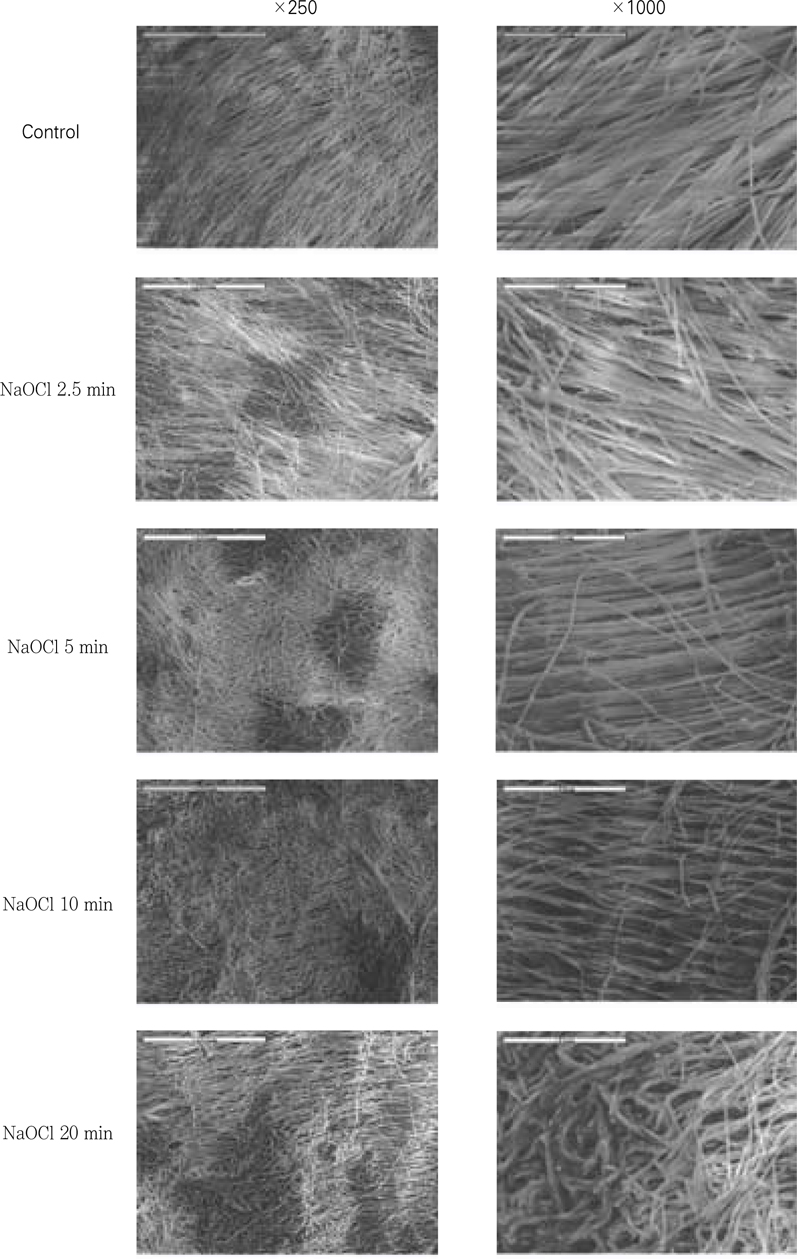
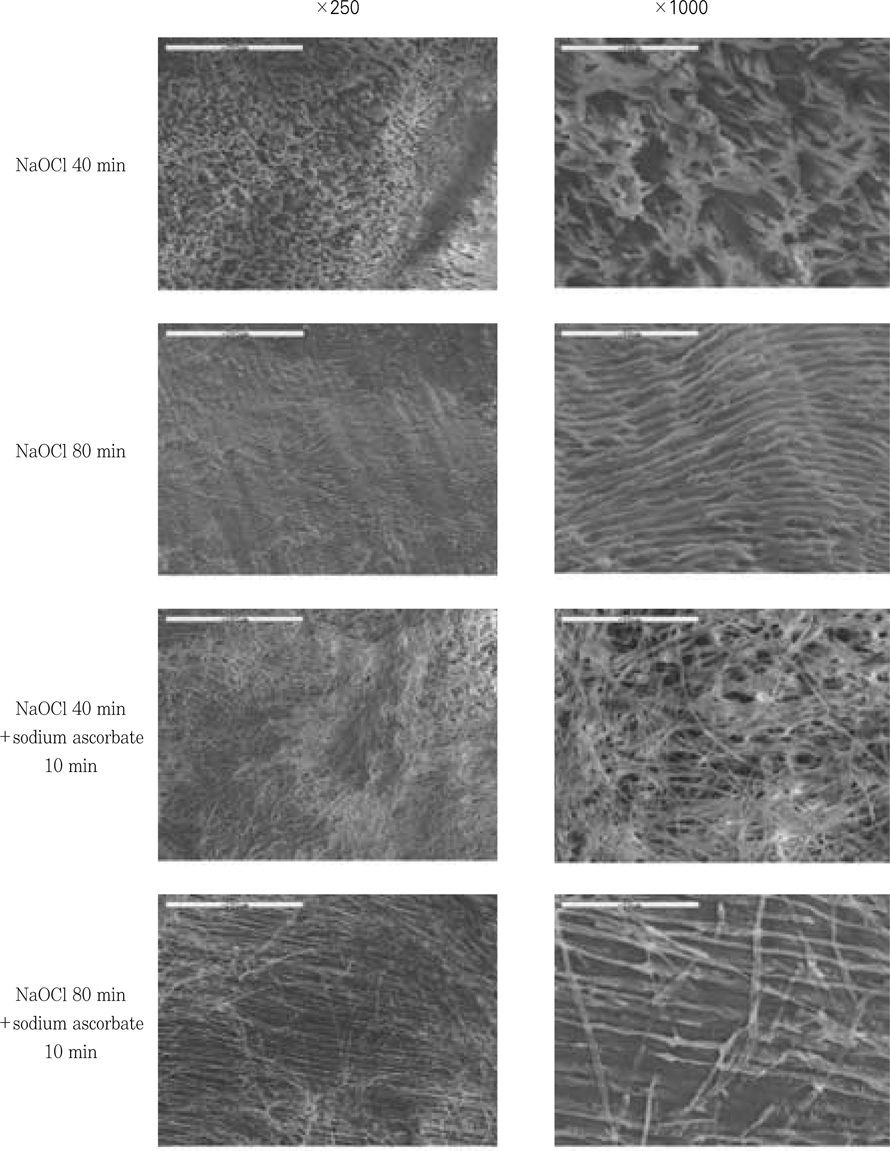
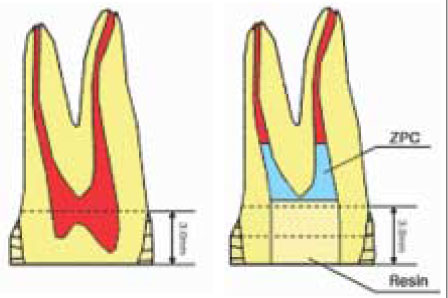
Figure 1
Schematic illustration of specimen preparation for µ-TBS test.
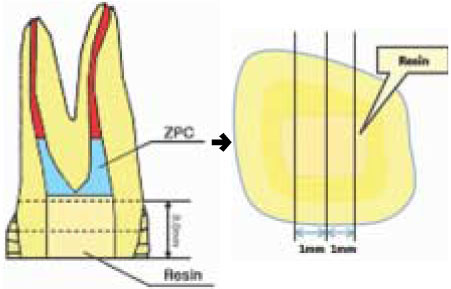
Figure 2
Schematic illustration of stick preparation for µ-TBS test.
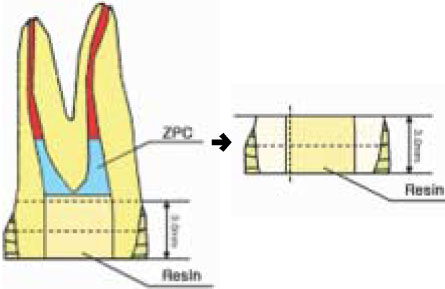
Figure 3
Schematic illustration of specimen preparation for SEM examination.
Figure 4
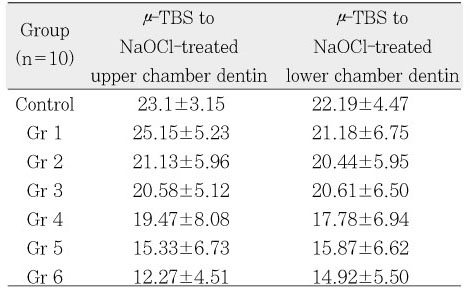
µ-TBS to NaOCl-treated upper and lower chamber dentin.
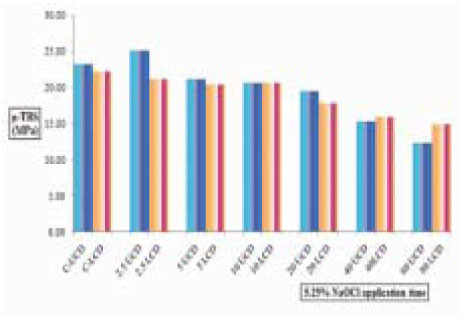
Figure 5
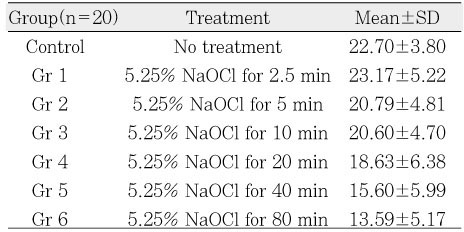
µ-TBS to chamber dentin after the application of certain period of time using 5.25% NaOCl. Asterisk(*) means statistical difference between groups.
Figure 6
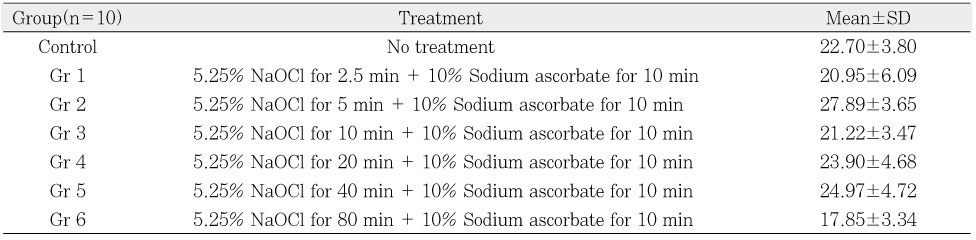
µ-TBS to chamber dentin after the treatment of 5.25% NaOCl +10% sodium ascorbate. Small letter(a,b) means statistical difference between groups.
Figure 7
Summarization of µ-TBS to chamber dentin after the treatment of 5.25% NaOCl and 5.25% NaOCl +10% sodium ascorbate.
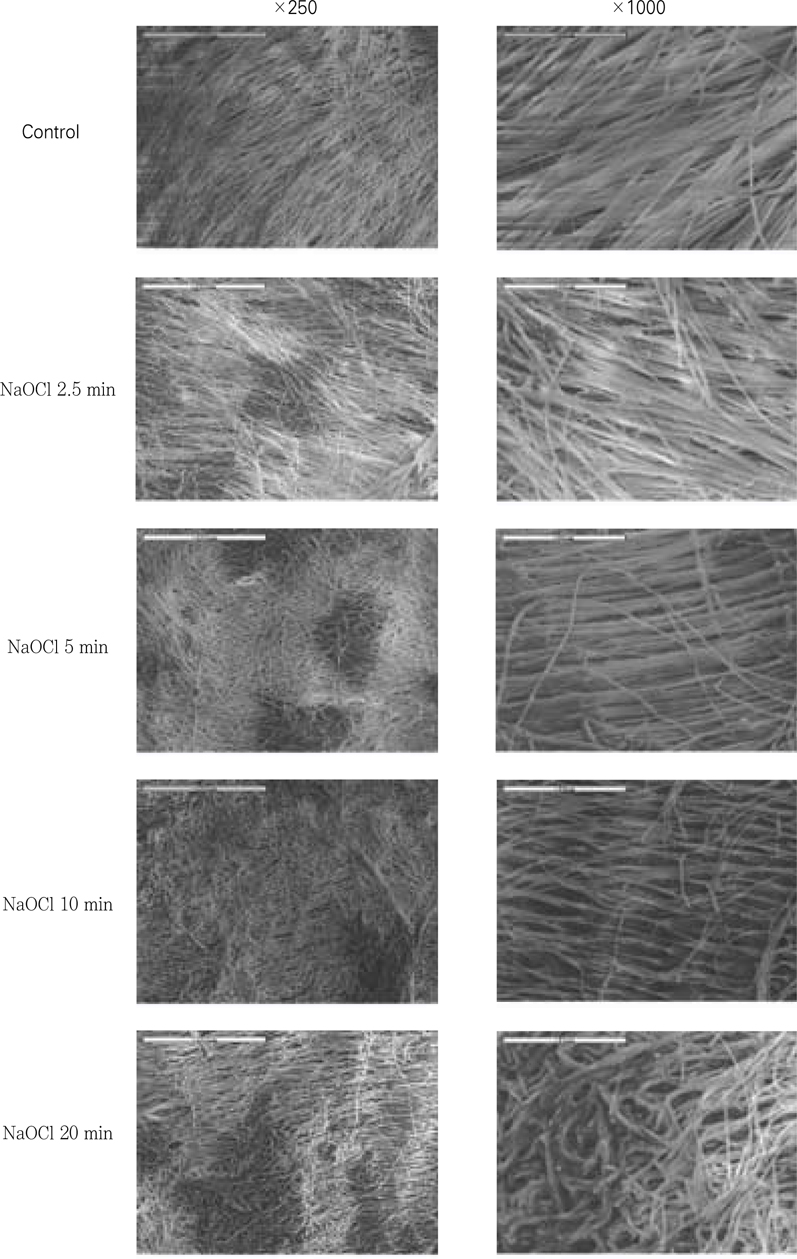
Figure 8
SEM examination after the application of certain period of time using 5.25% NaOCl.
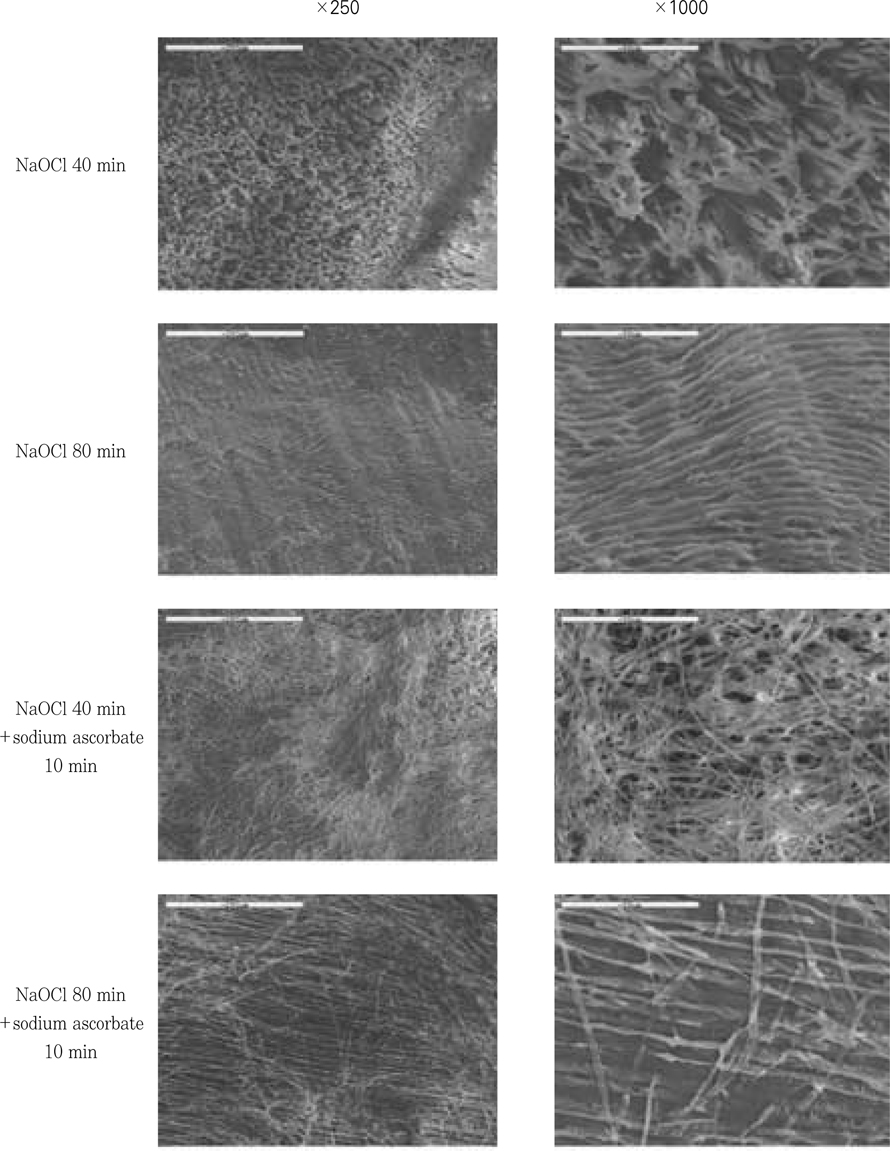
Figure 9
SEM examination after the treatment of 5.25% NaOCl and 5.25% NaOCl +10% sodium ascorbate.
Figure 1
Figure 2
Figure 3
Figure 4
Figure 5
Figure 6
Figure 7
Figure 8
Figure 9
Changes in µ-TBS to pulp chamber dentin after the application of NaOCl & reversal effect by using sodium ascorbate
Specimen preparation according to the application time of 5.25% NaOCl to the chamber dentin.
Specimen preparation according to the application of sodium ascorbate to the NaOCl-treated chamber dentin.
µ-TBS to NaOCl-treated upper & lower chamber dentin
µ-TBS to chamber dentin after the application of certain period of time using 5.25% NaOCl
µ-TBS to chamber dentin after the application of sodium ascorbate to the NaOCl-treated chamber dentin.
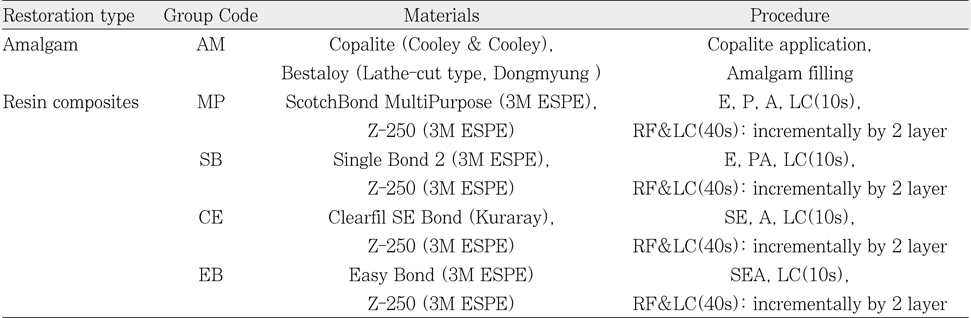
Table 1
Specimen preparation according to the application time of 5.25% NaOCl to the chamber dentin.
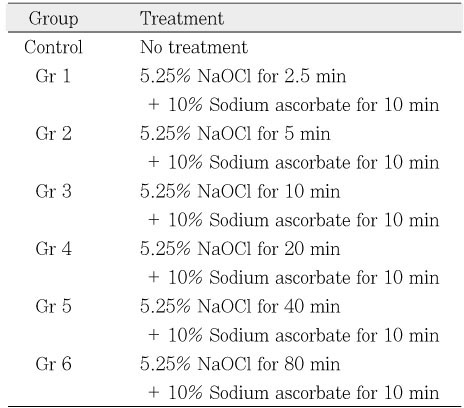
Table 2
Specimen preparation according to the application of sodium ascorbate to the NaOCl-treated chamber dentin.
Table 3
µ-TBS to NaOCl-treated upper & lower chamber dentin
Table 4
µ-TBS to chamber dentin after the application of certain period of time using 5.25% NaOCl
Table 5
µ-TBS to chamber dentin after the application of sodium ascorbate to the NaOCl-treated chamber dentin.

 KACD
KACD
 ePub Link
ePub Link Cite
Cite

