Articles
- Page Path
- HOME > Restor Dent Endod > Volume 32(2); 2007 > Article
- Original Article Pulp response of mineral trioxide aggregate, calcium sulfate or calcium hydroxide
- Young-Ran Yun1, In-Seok Yang1, Yun-Chan Hwang1, In-Nam Hwang1, Hong-Ran Choi2, Suk-Ja Yoon3, Sun-Hun Kim4, Won-Mann Oh1
-
2007;32(2):-101.
DOI: https://doi.org/10.5395/JKACD.2007.32.2.095
Published online: March 31, 2007
1Department of Conservative Dentistry, DSRI, 2nd stage of BK21, School of Dentistry, Chonnam National University, Korea.
2Department of Oral Pathology, DSRI, 2nd stage of BK21, School of Dentistry, Chonnam National University, Korea.
3Department of Oral and Maxillofacial Radiology, DSRI, 2nd stage of BK21, School of Dentistry, Chonnam National University, Korea.
4Department of Oral Anatomy, DSRI, 2nd stage of BK21, School of Dentistry, Chonnam National University, Korea.
- Corresponding author: Won-Mann Oh. Department of Conservative Dentistry, School of Dentistry, Chonnam National University, 8 Hak-dong, Dong-gu, Gwangju, 501-757, Korea. Tel: 82-62-220-4431, Fax: 82-62-225-8387, wmoh@chonnam.ac.kr
• Received: February 21, 2006 • Revised: June 12, 2006 • Accepted: June 22, 2006
Copyright © 2007 Korean Academy of Conservative Dentistry
- 1,544 Views
- 5 Download
- 12 Crossref
Abstract
-
This study was performed to verify the possibility of MTA and calcium sulfate as a pulp capping agent through comparing the dental pulp response in dogs after capping with MTA, calcium sulfate, and calcium hydroxide.24 teeth of 2 dogs, 8 month old, were used in this study.Under general anesthesia, cervical cavities were prepared and pulp was exposed with sterilized #2 round bur in a high speed handpiece.MTA, calcium hydroxide, and calcium sulfate were applied on the exposed pulp. Then the coronal openings were sealed with IRM and light-cured composite.Two months after treatment, the animals were sacrificed. The extracted teeth were fixed in 10% neutral-buffered formalin solution and were decalcified in formic acid-sodium citrate. They were prepared for histological examination in the usual manner. The sections were stained with haematoxylin and eosin.In MTA group, a hard tissue bridges formation and newly formed odontoblasts layer was observed. There was no sign of pulp inflammatory reaction in pulp tissue.In calcium hydroxide group, there was no odontoblast layer below the dentin bridge. In pulpal tissue, chronic inflammatory reaction with variable intensity and extension occurred in all samples.In calcium sulfate group, newly formed odontoblast layer was observed below the bridge. Mild chronic inflammation with a few neutrophil infiltrations was observed on pulp tissue.These results suggest that MTA is more biocompatible on pulp tissue than calcium hydroxide or calcium sulfate.
I. INTRODUCTION
Direct pulp capping and pulpotomy are well established method of treatment in which the exposed dental pulp is covered with a suitable material that protects the pulp from additional injury and permits healing and repair. Ultimate goal of treating the exposed pulp with an appropriate pulp capping material is to promote the dentinogenic potential of pulp cells1).
Dentin bridge formation can occur under a number of pulp capping materials. Histologically calcium hydroxide based material have been studied and extensively used in a traditional vital pulp therapy over a number of decades2,3). Fitzerald4), Fitzerald et al.5) and Heys et al.6) demonstrated that reparative dentin formation is physiologically induced after pulp capping with calcium hydroxide. Holland et al7) reported three major cause of failure of calcium hydroxide for direct pulp capping procedure: the porosity of the dentinal bridge; calcium hydroxide adheres poorly to dentin; its inability to provide a long term seal against microleakage.
As a root end filling material, MTA (mineral trioxide aggregate), has recently been developed8,9). It's main components are tricalcium silicate, tricalcium aluminate, tricalcium oxide and silicate oxide. The powder is composed of thin particles that are hydrophilic and thus sets in the presence of water. It is used as a retrograde filling material and perforation repair as well as an apexification material and in conservative pulp therapy8-12). Pitt Ford et al.10) pulp capped the teeth of monkeys with MTA and verified the formation of a mineralized tissue bridges in all specimens; only one case showed the pulpal inflammation. Junn et al.11) described that pulps capped with MTA exhibited less inflammation and higher dentin bridge formation than those with calcium hydroxide. Faraco and Holland12) observed the response of dog's dental pulp to MTA and calcium hydroxide cement when used as pulp capping material. A healing process with complete tubular dentin bridge formation and no inflammation in any of the pulp capped with MTA was demonstrated. On the other hand, in the pulp capped with calcium hydroxide, pulpal inflammation was observed in all but three cases.
Calcium sulfate (CaSO4) has been widely used as a bone filler which aids in tissue regeneration and excludes epithelium from the site of bone formation. Bahn13) advocated calcium sulfate because it is stable, biocompatible, readily available, easy sterilizable and its rate of resorption coincide with rate of bone growth. Yamazaki et al.14) reported calcium sulfate accelerated the rate of mineralization of new bone by providing a source of calcium ions. In their histological evaluation of defects in alveolar processes of dogs filled with calcium sulfate, Radentz and Collings15) reported that the site filled with calcium sulfate were more mature and were located at higher levels than that of controls.
Despite wide use of calcium hydroxide as pulp capping agent, it is known that calcium hydroxide is not an ideal material for pulp capping agent. This study was performed to verify the possibility of MTA and calcium sulfate as a pulp capping agent through comparing the dental pulp response in dogs after capping.
II. MATERIALS AND METHOD
Twenty four teeth of 2 dogs (10 kg), each 8 months old, were used in this study. General anesthesia was done by intramuscular injection of ketamine (1.5 ml/kg) and then intravenous injection of sodium pentobarbital (0.2 ml/kg), each tooth and oral cavity were scrubbed with 3% H2O2 and povidone and irrigated with saline solution, and then cavities were prepared on the labial surface of 2 mm from gingival margin of canines and molars with sterilized #2 round bur. Standardized pulp exposures (about 0.5 mm in diameter) were done by using a #2 round bur in a high speed handpiece with copious water spray. In incisors, the coronal portion of 2 mm above gingival margin was cut horizontally with sterilized diamond bur in the same manner above and the coronal pulp of 2.5 mm depth was removed with #2 round bur in a high speed handpiece with copious water spray. Bleeding was controlled by irrigation with sterile water and cotton pellets before placing capping materials.
The used materials in this study are calcium hydroxide powder (Oriental Chemical Industries CO. Seoul, Korea), MTA (Densply Tulsa, Tulsa®. OK, U.S.A.) and calcium sulfate (Aldrich Chemical Co. Milwaukee. U.S.A.). The calcium hydroxide powder and calcium sulfate powder were applied on the mechanically exposed pulp with 0.5 mm thickness using messing gun. MTA was mixed with sterile distilled water and applied on 0.5 mm thickness with a messing gun to the exposure site. The opening of every cavity was filled with 0.5 mm thickness layer of IRM (Dentsply caulk, Milford, U.S.A.). Finally, remaining portion of cavity was acid etched with 37% phosphoric acid for 15 sec and restored with light cured composite resin (Palfique Estelite, Tokuyama Dental Corp. Japan) to secure the sealing ability.
Respectively, 8 teeth were treated in each experimental group. At two months after treatment, the animals were sacrificed by administration of an overdose of anesthetic. The extracted teeth were fixed in 10% neutral-buffered formalin solution and decalcified in formic acid-sodium citrate. They were prepared for histological examination in the usual manner. The sections were stained with haematoxylin and eosin.
It was carefully observed about the hard tissue bridge (continuity, morphological aspects), inflammatory reaction, vascular proliferation, the presence of odontoblastic layer and particles of the capping material.
III. RESULTS
In 7 of 8 specimens, exposed sites were closed by a hard tissue bridges (Figure 1, 2). Newly formed dentin bridge was a basophilic dentinal tissue with a few tissue inclusions and irregular dentinal tubules. This mineralized tissue was well connected with lateral dentin of tooth.
At superficial portion of those bridges, a small amount of MTA particles were remained and some slight irregularities with diverse morphology were appeared. Vascular dilatation was prominent. Between dentin bridge and pulp tissue, newly formed odontoblastic layer was shown. There was no sign of pulp inflammatory reaction, except focally vascular proliferation with hyperemia.
Even in case that no hard tissue bridge was found, there was also free of inflammatory cells in the pulp.
Complete hard tissue bridge were observed in 5 cases. All of this bridge showed continuity with the lateral dentin.
In some case, this newly formed bridge extended into dental pulp and was formed with dentinal tissue with a few tissue inclusions and irregular dentinal tubules. But there was no odontoblastic layer below the dentin bridge. In pulpal tissue, chronic inflammatory reaction with variable intensity occurred in all cases. A vascular proliferation was appeared with hyperemia (Figure 3).
In 6 of 8 specimens, the mechanically exposed sites were closed by well formed dentin bridges. At superficial portion of those bridges, a small amount of calcium sulfate powder particle was observed and some slight irregularities with diverse morphology were appeared in dentinal bridge. In the pulpal tissue, below the bridge, newly formed odontoblastic layer were demonstrated. And mild chronic inflammation with a few neutrophil infiltrations was observed (Figure 4).
IV. DISCUSSION
The nature of hard tissue formed under pulp capping materials is not unknown. Dentin bridge often contains multiple perforations and imperfections7). The hard tissue that forms has been described as a dentin-like, bone-like, and reparative dentin bridge. Kakehashi et al16) reported the dentin formation over mechanically exposed pulps without any filling materials in germ-free environment, therefore demonstrating the healing ability of the pulp given the proper environment.
MTA has been found to induce dentinogenesis when used as a pulp capping agent. However, entire process by which this occurs has not been explained. MTA induced a greater frequency of dentin-bridge formation, less pulp inflammation, and bridges compared with calcium hydroxide11). When human osteosarcoma cells17), or osteoblasts18,19) were cultured in the presence of MTA, the cell growth was good. MTA further offered a biologically active substrate for cell attachment, while increased levels of alkaline phosphatase, osteocalcin, and interleukin-6 and -8 were measured. In vitro studies, the antibacterial effects of MTA are comparable to those of calcium hydroxide8). Pitt Ford et al.10) have documented that MTA placed in mechanically exposed pulps of monkeys stimulated pulp healing with minimal inflammatory reactions and dentinal bridge formation.
A significant finding of this study was the verification of a hard tissue bridge under pulps capped with MTA that resembles normal dentin or tertiary dentin in morphology. Thus, the results of this study suggest that MTA may provide a healing environment for regeneration of pulp and dentin. Between bridges and pulp tissues, newly formed odontoblastic layer was shown.
The present study demonstrated that pulp capping with MTA induces cytological and functional changes in pulp cells, resulting in formation of reparative dentin at the surface of mechanically exposed dental pulp. The results of this study showed similar to the findings of Pitt Ford et al.10) and Tziafas et al.20). The results of this study indicate that MTA is an effective pulp capping material, able to stimulate hard tissue bridge formation during wound healing and biocompatible to pulp tissue.
Calcium sulfate is a main component of plaster of Paris. Calcium sulfate has been used for filling bone cavities. It has been found to resorb rapidly in vivo and rapidly replaced by new bone21,22). It has been used in vivo as a vehicle for the release of antibiotics23,24), bone morphogenetic protein14). However, colonization of this substrate by osteoblastic cells and cellular mechanism involved in its resorption are still unknown.
Calcium sulfate accelerates the rate of mineralization of new bone by providing a ready source of calcium ions to aid the early mineralization process13,14).
In this study, the mechanically exposed sites capped with calcium sulfate powder were closed by well formed dentin bridges in 6 of 8 specimens. Some slight irregularities with diverse morphology were appeared in dentinal bridge. In the pulpal tissue, below the bridge, newly formed odontoblast layer was demonstrated. Mild chronic inflammation with a few neutrophil infiltrations was also observed. These findings indicate that calcium sulfate also can be used as an effective pulp-capping material, able to stimulate hard tissue bridge formation during wound healing like MTA or calcium hydroxide.
In view of the results of the present study, MTA seemed to superior to calcium hydroxide and calcium sulfate for pulp capping on mechanically exposed pulp of dog teeth. However further research about MTA and calcium sulfate is warranted on larger samples for clinical use.
V. CONCLUSION
In MTA group, a hard tissue bridge formation and newly formed odontoblasts layer was observed. There was no sign of inflammatory reaction in pulp tissue except focally vascular proliferation with hyperemia.
In calcium hydroxide powder group, hard tissue bridge was observed. But there was no odontoblastic layer below the dentin bridge. In pulpal tissue, chronic inflammatory reaction with variable intensity and extension occurred in all samples.
In calcium sulfate powder group, dentin bridge formation was shown. Newly formed odontoblastic layer was observed. And mild chronic inflammation with a few neutrophil infiltrations was observed on pulp tissue.
These results suggest that MTA is more biocompatible on pulp tissue than calcium hydroxide or calcium sulfate. MTA and calcium sulfate are clinically suitable for pulp capping agents on mechanically exposed pulp.
- 1. Schröder U. Effects of calcium-hydroxide-containing pulp capping agents on pulp cell migration, proliferation and differentiation. J Dent Res. 1985;64 Spec No: 541-548.PubMed
- 2. Tronstad L. Reaction of the exposed pulp to Dycal treatment. Oral Surg Oral Med Oral Pathol. 1979;38: 945-953.Article
- 3. Schröder U, Granath LE. Early reaction of intact human teeth to calcium hydroxide following experimental pulpotomy and its significance to the development of hard tissue barrier. Odontol Revy. 1971;22: 179-188.PubMed
- 4. Fitzgerald M. Cellular mechanisms of dentinal bridge repair using 3H-thymidine. J Dent Res. 1979;58: 2198-2206.ArticlePubMedPDF
- 5. Fitzgerald M, Chiego JD Jr, Heys R. Autoradiographic analysis of odontoblast replacement following pulp exposure in primate teeth. Arch Oral Biol. 1990;35: 707-715.ArticlePubMed
- 6. Heys DR, Fitzerald M, Heys RJ, Chiego JD Jr. Healing of primate dental pulps capped with Teflon. Oral Surg Oral Med Oral Pathol. 1990;69: 227-237.ArticlePubMed
- 7. Holland R, de Souza V, de Mello W, et al. Permeability of the hard tissue bridge formed after pulpotomy with calcium hydroxide. A historical study. J Am Dent Assoc. 1979;99: 472-475.PubMed
- 8. Torabinejad M, Hong CU, McDonald F, Pitt Ford TR. Physical and chemical properties of a new root-end filling material. J Endod. 1995;21: 349-353.ArticlePubMed
- 9. Torabinejad M, Chivian N. Clinical applications of mineral trioxide aggregate. J Endod. 1999;25: 197-205.ArticlePubMed
- 10. Ford TR, Torbinejad M, Abedi HR, Backland LK, Kariyawasam SP. Using mineral trioxide aggregate as a pulp-capping material. J Am Dent Assoc. 1996;127: 1491-1494.ArticlePubMed
- 11. Junn DJ, McMillian P, Backland LK, Torbinejad M. Quantitative assessment of dentin bridge formation following pulp capping with mineral trioxide aggregate. J Endod. 1998;24: 278. (abstract).
- 12. Faraco IM Jr, Holland R. Response of the observe the response of dog's dental pulp to mineral trioxide aggregate (MTA) and calcium hydroxide cement. Dent Traumatol. 2001;17: 163-166.PubMed
- 13. Bahn SL. Plaster: a bone substitute. Oral Surg Oral Med Oral Pathol. 1966;21: 672-679.ArticlePubMed
- 14. Yamazaki Y, Oida S, Akimoto Y. Response of mouse femoral muscle to an implant of a composite of bone morphogenetic protein and plaster of Paris. Clin Orthop. 1988;234: 240-249.
- 15. Radentz WH, Collings CK. The implantation of plaster of Paris in the alveolar process of the dog. J Periodontol. 1965;36: 357-364.ArticlePubMed
- 16. Kakehashi S, Stanley HR, Fitzgerald RJ. The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral Surg Oral Med Oral Pathol. 1965;20: 340.ArticlePubMed
- 17. Mitchell PJC, Pitt Ford TR, Torabinejad M, McDonald F. Osteoblast biocompatibility of mineral trioxide aggregate. Biomaterials. 1999;20: 167-173.ArticlePubMed
- 18. Koh ET, McDonald F, Pitt Ford TR, Torabinejad M. Cellular response to mineral trioxide aggregate. J Endod. 1998;24: 543-547.ArticlePubMed
- 19. Koh ET, Torabinejad M, Pitt Ford TR, Brady K, McDonald F. Mineral trioxide aggregate stimulates a biological response in human osteoblasts. J Biomed Mater Res. 1997;37: 432-439.ArticlePubMed
- 20. Tziafas D, Pantelidou O, Alvanou A, Belibasakis G, Papadimitriou S. The dentinogenic effect of mineral trioxide aggregate (MTA) in short-term capping experiments. Int Endod J. 2002;35: 245-254.ArticlePubMed
- 21. Peltier LF. The use of plaster of Paris to fill defects in bone. Clin Orthop. 1961;21: 1-31.PubMed
- 22. Peltier LF, Jones RH. Treatment of unicameral bone cyst by curretage and packing with plaster of Paris pellets. J Bone Joint Surg Am. 1978;60: 820-822.PubMed
- 23. Ricci JL, Rosenblum SF, Brezenoff L, Blumenthal NC. Stimulation of bone ingrowth into an implantable chamber through the use of rapidly resorbing calcium sulfate hemihydrate. 1992;Berlin: Fourth World Biomaterials Congress.
- 24. Rauschmann MA, Wichelhaus TA, Stirnal V, Dingeldein E, Zichner L, Schnettler R, Alt V. Nanocrystalline hydroxyapatite and calcium sulphate as biodegradable composite carrier material for local delivery of antibiotics in bone infections. Biomaterials. 2005;26: 2677-2684.ArticlePubMed
REFERENCES
Figure 1
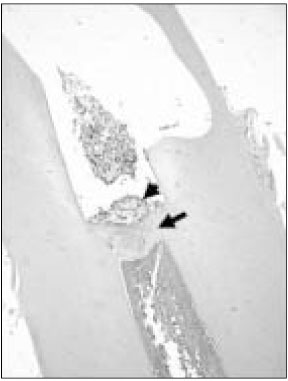
Histological section of maxillary canine of dog after pulp capping with MTA (Haematoxylin and eosin stain: original magnification × 40). Black arrow indicates newly formed hard tissue. Black arrow head indicates MTA particle.

Figure 2
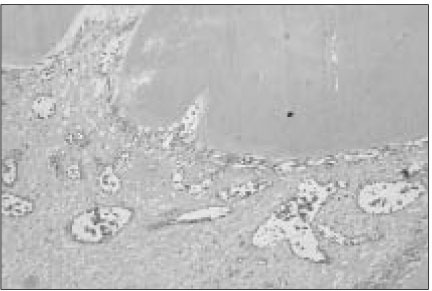
Histological section of mandibular canine of dog after pulp capping with MTA (Haematoxylin and eosin stain: original magnification × 200). Between dentin bridges and pulp tissues, newly odontoblastic layer were well developed.

Tables & Figures
REFERENCES
Citations
Citations to this article as recorded by 

- Effects of the exposure site on histological pulpal responses after direct capping with 2 calcium-silicate based cements in a rat model
Panruethai Trongkij, Supachai Sutimuntanakul, Puangwan Lapthanasupkul, Chitpol Chaimanakarn, Rebecca Wong, Danuchit Banomyong
Restorative Dentistry & Endodontics.2018;[Epub] CrossRef - Conservative approach of a symptomatic carious immature permanent tooth using a tricalcium silicate cement (Biodentine): a case report
Cyril Villat, Brigitte Grosgogeat, Dominique Seux, Pierre Farge
Restorative Dentistry & Endodontics.2013; 38(4): 258. CrossRef - Comparison of gene expression profiles of human dental pulp cells treated with mineral trioxide aggregate and calcium hydroxide
Yong-Beom Kim, Won-Jun Shon, Woocheol Lee, Kee-Yeon Kum, Seung-Ho Baek, Kwang-Shik Bae
Journal of Korean Academy of Conservative Dentistry.2011; 36(5): 397. CrossRef - Pulp response of beagle dog to direct pulp capping materials: Histological study
Ji-Hyun Bae, Young-Gyun Kim, Pil-Young Yoon, Byeong-Hoon Cho, Yong-Hoon Choi
Journal of Korean Academy of Conservative Dentistry.2010; 35(1): 5. CrossRef - Gene expression profiling in human dental pulp cells treated with mineral trioxide aggregate
Yong-Beom Kim, Won-Jun Shon, WooCheol Lee, Kee-Yeon Kum, Seung-Ho Baek, Kwang-Shik Bae
Journal of Korean Academy of Conservative Dentistry.2010; 35(3): 152. CrossRef - Biocompatibility of experimental mixture of mineral trioxide aggregate and glass ionomer cement
Min-Jae Oh, Yu-Na Jeong, In-Ho Bae, So-Young Yang, Bum-Jun Park, Jeong-Tae Koh, Yun-Chan Hwang, In-Nam Hwang, Won-Mann Oh
Journal of Korean Academy of Conservative Dentistry.2010; 35(5): 359. CrossRef - Biocompatibility of bioaggregate cement on human pulp and periodontal ligament (PDL) derived cells
Choo-Ryung Chung, Euiseong Kim, Su-Jung Shin
Journal of Korean Academy of Conservative Dentistry.2010; 35(6): 473. CrossRef - Physical and chemical properties of experimental mixture of mineral trioxide aggregate and glass ionomer cement
Yu-Na Jeong, So-Young Yang, Bum-Jun Park, Yeong-Joon Park, Yun-Chan Hwang, In-Nam Hwang, Won-Mann Oh
Journal of Korean Academy of Conservative Dentistry.2010; 35(5): 344. CrossRef - The effect of several root-end filling materials on MG63 osteoblast-like cells
Jeong-Ho Lee, Won-Jun Shon, WooCheol Lee, Seung-Ho Baek
Journal of Korean Academy of Conservative Dentistry.2010; 35(3): 222. CrossRef - Effects of condensation techniques and canal sizes on the microleakage of orthograde MTA apical plug in simulated canals
Deuk-Lim Nam, Jeong-Kil Park, Bock Hur, Hyeon-Cheol Kim
Journal of Korean Academy of Conservative Dentistry.2009; 34(3): 208. CrossRef - Comparison of biocompatibility of four root perforation repair materials
Min-Kyung Kang, In-Ho Bae, Jeong-Tae Koh, Yun-Chan Hwang, In-Nam Hwang, Won-Mann Oh
Journal of Korean Academy of Conservative Dentistry.2009; 34(3): 192. CrossRef - A bioactivity study of Portland cement mixed with β-glycerophosphosphate on human pulp cell
Young-Hwan Oh, Young-Joo Jang, Yong-Bum Cho
Journal of Korean Academy of Conservative Dentistry.2009; 34(5): 415. CrossRef
Pulp response of mineral trioxide aggregate, calcium sulfate or calcium hydroxide
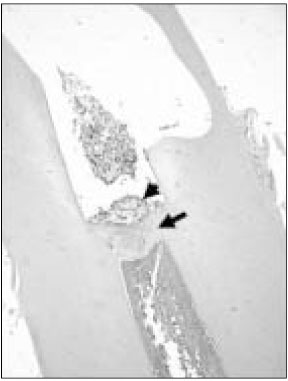
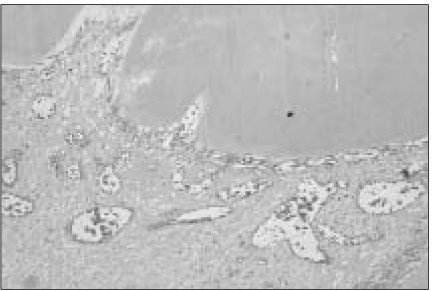
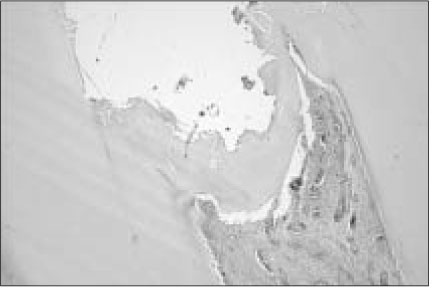

Figure 1
Histological section of maxillary canine of dog after pulp capping with MTA (Haematoxylin and eosin stain: original magnification × 40). Black arrow indicates newly formed hard tissue. Black arrow head indicates MTA particle.
Figure 2
Histological section of mandibular canine of dog after pulp capping with MTA (Haematoxylin and eosin stain: original magnification × 200). Between dentin bridges and pulp tissues, newly odontoblastic layer were well developed.
Figure 3
Histological section of mandibular molar of dog after pulp capping with Calcium hydroxide (Haematoxylin and eosin stain: original magnification × 100).
Figure 4
Histological section of mandibular molar of dog after pulp capping with CaSO4 (Haematoxylin and eosin stain: original magnification × 200).
Figure 1
Figure 2
Figure 3
Figure 4
Pulp response of mineral trioxide aggregate, calcium sulfate or calcium hydroxide

 KACD
KACD

 ePub Link
ePub Link Cite
Cite

