Abstract
-
The purpose of this study was to assess the antibacterial effect of sodium dichloroisocyanurate (NaDCC), sodium hypochlorite (NaOCl), and chlorhexidine (CHX) on Enterococcus faecalis and to evaluate and to compare the time-dependant antimicrobial effect of NaDCC with NaOCl and CHX in the root canal in vitro before and after instrumentation.
Extracted human single teeth were prepared by serial instrumentation technique. The samples were autoclaved and contaminated for 3 days with E. faecalis monocultures. The teeth were then divided into 4 groups. Each group was irrigated and inserted with 2% NaOCl, 2% NaDCC, 2% CHX and sterilized saline. After 6, 12, 24, 72h, and 1 week incubation, sterilized paper point was inserted into the root canal. Paper points containing root canal contents were then placed on the agar plate. And then each root canal was prepared with #4 and #5 GG (Gates-Glidden) drill. The debris were collected in the sterilized microtube and the plates were incubated at 37℃ in an increased CO2 atmosphere. After 24h incubation the growth of bacteria around the paper points were measured.
NaOCl and NaDCC solution shows similar antimicrobial effect for E. faecalis at 6, 12, 24, 72h and 1 week. In control group, irrigated with sterilized saline, no antimicrobial effect was observed.
The results are in agreement with other investigators, who have shown the bactericidal property and possibility of NaDCC as a root canal irrigation solution. Thus it seems that NaDCC solutions can be clinically applied into the root canal within 1 week after dilution.
-
Keywords: Sodium dichloroisocyanurate; Sodium hypochlorite; Chlorhexidine; E. faecalis; Root canal irrigants; Antimicrobial effect
I. INTRODUCTION
Bacteria are the main causative factors in the development of periapical inflammation
1). Therefore, the removal of pulpal and dentinal debris and the elimination of viable microorganisms from the root canal system are of vast importance during endodontic therapy.
An effective disinfecting is necessary by augmenting mechanical preparation with antimicrobial irrigants because of the complex anatomy of the root canal system
2). Many kinds of antimicrobial agent were used as an irrigation solution for this purpose. It enhances bacterial elimination and facilitates removal of necrotic tissues and dentine chips from the root canal. It can also prevent the infected hard and soft tissues being packed in the apical portion of the root canal and into the periapical area
3).
Recently,
Enterococcus faecalis has been suggested to be an important etiological agent in endodontic failures.
E. faecalis is a facultative anaerobic gram-positive bacterium and is known to be able to resist intracanal medicaments and to survive as a single organism within the canal system
4).
Chlorine-releasing agents (CRA) are the most commonly used for this purpose
5). The antimicrobial ability of CRA results from the formation of hypochlorous acid (HOCl), when in contact with organic debris. HOCl has been found to disrupt oxidative phosphorylation and other membrane-associated activities of bacteria
6). Also bacterial DNA synthesis (incorporation of [
3H]-thymidine) was significantly decreased by exposure to HOCl
7).
Sodium hypochlorite (NaOCl) is the most widely used irrigating solution in concentrations ranging from 0.5% to 5.25% among CRA. NaOCl is a strong antimicrobial agent and effectively dissolves pulpal remnants and organic components of dentine
8). However, NaOCl is criticized for its high toxicity to the periapical tissues, unpleasant taste and incapability of removing inorganic material
9).
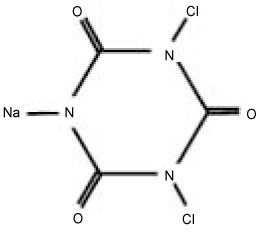
Another CRA is sodium dichloroisocyanurate (NaDCC) used for disinfection of contaminated surfaces and drinking water (
Figure 1). When dissolved, NaDCC is less prone to inactivation by serum than NaOCl
10). In comparison with commercial sodium hypochlorite (NaOCl), sodium dichloroisocyanurate (NaDCC) solution contains the same level of available chlorine and shows similar bactericidal activities with NaOCl. As an effervescent chlorine tablet form, however, NaDCC is known to be fast dissolving, highly convenient, and use-dilutions can be prepared simply, safely and accurately, when and where required than NaOCl
11).
Chlorhexidine (CHX) gluconate has been in use for a long time in dentistry because of its antimicrobial properties, substantivity, and relatively low toxicity
12,
13). It has a wide antimicrobial spectrum and is effective against Gram-positive and Gram-negative bacteria as well as yeasts
14). Therefore, potential use of CHX in endodontics have been under active research over the last few years. Although studies comparing the antimicrobial activity of NaOCl to that of CHX have produced somewhat conflicting results, it seems that CHX, in concentrations between 0.2% and 2%, has similar antibacterial effect in the root canal and in the infected dentine
5).
There have been researches to compare the antibacterial capability of NaOCl to NaDCC and NaOCl to CHX, but no research to compare the materials among these chemicals so far. Hence, the purposes of this experiment were (i) to assess the antibacterial effect of NaDCC, NaOCl, and CHX on E. faecalis; and (ii) to compare the timedependant antimicrobial effect of NaDCC with NaOCl and CHX in the root canal in vitro before and after instrumentation.
II. MATERIALS AND METHODS
Determination of Minimal Inhibitory Concentration (MIC)
The antibacterial effects of NaDCC, NaOCl, and CHX solutions were examined separately.
MIC of each agent was assessed using the broth dilution method. E. faecalis ATCC 292121 obtained from Korean Collection for Type Cultures KCTC 292121 (KCTC, Daejeon, Korea) was grown overnight in Brain Heart Infusion (BHI) broth (Difco Laboratories, Baltimore, Md), adjusted spectrophotometrically to [OD]660 = 1.3 (3.2 × 109 CFU/ml). The bacterial suspensions were supplemented with NaDCC, NaOCl, and CHX solutions in concentrations of 4.0%, 2.0%, 1.0%, 0.5%, 0.25%, 0.125%, 0.0625%, 0.0315%, 0.0157%, 0.0079%, and 0.0039%. After overnight incubation at 37℃, bacterial growth was measured using a spectrophotometer at 660 nm. The MIC of each agent for the tested bacteria was measured in comparison with the controls.
Preparation of specimens
One hundred and twenty-eight freshly extracted, single-rooted human teeth were immersed in 5.25% NaOCl for 24 hours to remove organic debris and to disinfect the surface. The crowns and apices of the teeth were sectioned with a precision cutting device (Accutom P-50, Struers, Copenhagen, Denmark) to produce uniform 10-mm-long specimens. The root canal of each specimen was enlarged throughout with a No.3 Gates-Glidden drill to standardize its internal diameter. The smear layer was removed by immersing the specimens in an ultrasonic bath with 17% Ethylene-diaminetetraacetic acid (EDTA) for 4 minutes, and then immersed again 5.25% NaOCl for 4 minutes.
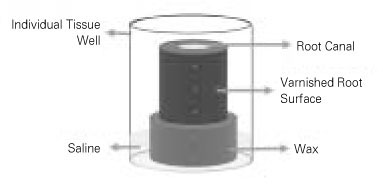
The root specimens were placed in test tubes containing BHI broth, autoclaved for 30 minutes at 121℃, and incubated for 24 hours at 37℃ to confirm the sterility through the absence of turbidity. The sterilized specimens were immersed in an ultrasonic bath of fresh BHI broth for 15 minutes to enhance penetration of the broth into the dentinal tubules. They were blotted with sterile paper points, externally coated with 2 layers of nail varnish, and mounted in 24-well cell culture plates (ref. No. 3524, Corning Cell Wells, Corning Glass Works, Corning, NY, USA) on a base of rope wax (
Figure 2).
After the root specimens were dried with sterile gauze, the lumens of the root were filled with 15 µl of bacterial inoculum and incubated at 37℃ for 72 hours. Saline was added to the tissue wells surrounding the wax base to maintain a humid environment. The lumens were replenished with sterile BHI every 24 hours.
Specimen division
The contaminated teeth were divided into 3 study groups of 40 teeth each and one control group of 8 teeth according to the irrigants used during root canal preparation described as follows:
Group 1: 40 teeth irrigated with 2% NaDCC (Biospot®, Hydra-Chem Limited, Billingshurst, UK) (Table 1)
Group 2: 40 teeth irrigated with 2% NaOCl
Group 3: 40 teeth irrigated with 2% chlorhexidin gluconate liquid (Alphahexidine®, Sungkwang Co. LTD, Bucheon, Korea)
Control: 8 teeth irrigated with saline
Each group was subdivided equally into 4 subgroups depending on the elapsed time after irrigant solution applied (12 hours, 24 hours, 72 hours, and 7 days).
Antibacterial assessment
Each canal was irrigated with 2 ml irrigant solution and 1 ml sterile saline to flush out the original irrigant. And then each root canal was dried using sterilized paper points.
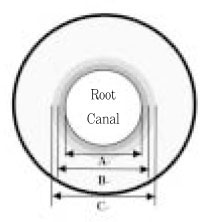
After 12 hours, with the lumens filled with the saline, the first bacteriological sampling was taken using sterile paper point size #80. The paper points were left in the wet canal for 1 minute and then transferred to the tubes containing 2 ml BHI broth. The tubes were then vortexed for 5 minutes and 100 µl aliquot was plated on BHI agar plates. Then same teeth were enlarged with sterile #4 and #5 Gates-Glidden drills to compare before and after instrumentation (
Figure 3). Each specimen was rinsed with sterile saline to collect gross dentin shavings. Serial dilutions (1 : 10, 1 : 100 and 1 : 1000) were made and a 100 µl aliquot of each dilution was placed on BHI plates in duplicate. The BHI agar plates were incubated at 37℃. Microbial growth was verified and the number of bacterial colonies forming units was counted and confirmed by Gram stain in a light microscopic.
III. RESULTS
The MIC values for
E. faecalis were at a concentration of 0.125% for NaDCC and NaOCl solutions. For CHX solution, the MIC was lower concentration of 0.0625% (
Table 2).
NaDCC irrigation solution showed similar antimicrobial effect to NaOCl and CHX for
E. faecalis at 12, 24, 72 h and 7 d (
Table 3).
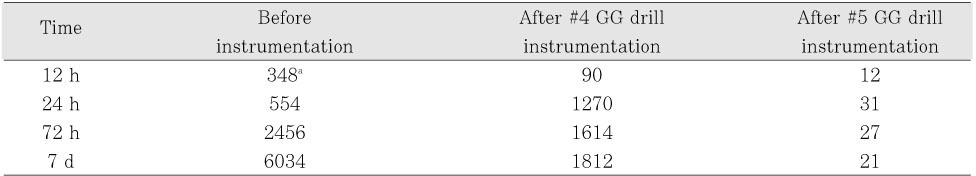
In control group, no antimicrobial effect was observed (
Table 4).
IV. DISCUSSION
In this study
E. faecalis was chosen as the test organism because it is very resistant microorganism and most often associated with persistent apical inflammation in clinical situations
4,
15).
E. faecalis is capable of surviving as a single species within the canal after endodontic therapy, which makes it proper for this study.
Various methodologies can be used to assess the antimicrobial activity of endodontic irrigants and medicaments. Also, the methodology can be a possible explanation for the differences found in the results of studies. Some methodologies allow direct contact of the substances with the microorganisms (as in the agar diffusion test). But the outcome measure of the agar diffusion test seems to be dependent on the ability of the test antimicrobials to diffuse in the agar
16). In others, as in this study, microorganisms located inside the dentinal tubules did not necessarily have direct contact with the antimicrobial substances.
The experimental model used in this study was adapted from that established by Østavik and Haapasalo for
in vitro infection and disinfection of dentinal tubules
17). The model was modified to more closely simulated clinical conditions by using extracted human teeth. It was considered to be more appropriate because of the marked difference in diameter between the canals of bovine and human teeth and thus in the volume of irrigant solution that can be placed in these canals
16). And the cementum was left intact, only the internal lumens were inoculated with a known quantity of the bacteria for a 72-hour period to mimic clinical endodontics
18).
Bacteriolgical sampling is another important step that varies among the different methodologies
7). In this study, the bacteriological sampling was accomplished with a sterile paper point that absorbed the root canal contents. The paper point was then transferred to tubes containing the sterile BHI broth that were plated on the BHI agar plates. The use of paper points has the advantage that it can be performed
in vitro and
in vivo. On the other hand, bacteriological sampling with paper points is limited because only the microorganisms that are in the root canal can be sampled, while the ones that are located inside the dentine tubules are not. So a further sampling procedure was taken by instrumentation of the infected root canal with Gates-Glidden drill. Then the dentin shaving was collected into tubes containing sterile saline. This technique was very sensitive and the amount of dentin powder was not precisely controlled.
Mechanical instrumentation is the core method for bacterial reduction in the infected root canal. But the antibacterial effect of mechanical cleansing with sterile saline is reported to be very low and limited
2). Therefore, antibacterial efficiency of instrumentation and irrigation has been on the use of irrigating solutions with strong antibacterial activity as the necessary supplement to mechanical preparation. An ideal root canal irrigant solution must have maximum tissue dissolving and antibacterial effects, but the toxic effect must be minimal
19).
Sodium hypochlorite has been recommended as an irrigant solution in the treatment of infected root canals, because of its well-known bactericidal action. Even though its antibacterial effects are recognized, the exact mechanism of microbial killing is not well elucidated. When NaOCl is added to water, hypochlorous acid (HClO), which contains active chlorine, a strong oxidizing agent, is formed. Substantial evidence suggests that chlorine exerts its antibacterial effect by the irreversible oxidation of -SH groups of essential enzymes, disrupting the metabolic functions of the bacterial cell. Chlorine may also combine with cypoplasmic components to form N-chloro compounds, toxic complexes which destroy the microorganisms. However, the first contact oxidation reactions of chlorine with bacteria may lead to the rapid killing of bacterial cells even prior to the formation of N-chloro compounds in the cytoplasm
7).
Several investigators have suggested the use of chlorhexidine gluconate as an equally valuable root canal irrigant. It has ability to be absorbed and released by dental tissues, would disinfect the tissues and then maintain a root canal devoid of microorganisms by sustained release of CHX gluconate into the root canals
20). CHX gluconate is a cationic bisguanide which combines to the cell wall of the microorganism and causes leakage of intra cellular components. At low concentrations of CHX, small-molecular-weight substances will leak out, resulting in a bacteriostatic effect. At higher concentrations, CHX has a bactericidal effect owing to precipitation and/or coagulation of the cytoplasm, probably caused by protein cross-linking.
In this study, the MIC values of NaDCC and NaOCl were found to be equal. The results are similar to those reported by Heling et al
6). They evaluated MIC values of NaDCC and NaOCl for
Streptococcus sobrinus,
Streptococcus salivarius,
Enterococcus faecalis, and
Streptococcus mutans. They found that the MIC values for four bacteria were at a concentration of 0.157% for both agents and the MIC for
S. mutans incubated in NaOCl was at a higher concentration (0.315%) than for NaDCC (0.157%). The MIC value of CHX was at much higher concentration (0.063%) than for NaDCC and NaOCl in this study. These results indicate that the antibacterial effect of CHX is higher than NaDCC and NaOCl.
The antibacterial effect of NaOCl is time-dependent. Antibacterial effect was observed in the presence of 0.5% NaOCl only after 15 minutes
21). And using infected root canals
in vitro, 0.5% and 2% NaOCl significantly reduced bacteria at a 100 µm depth of the dentin tubules
22). CHX gluconate has substantive antimicrobial activity in the root canal as long as 48 hours to 72 hours after instrumentation
12). Dametto et al
23) found that the substantive antimicrobial activity of 2% CHX is more effective than 5.25% NaOCl in keeping a low
E. faecalis CFU count 7 days after biomechanical instrumentation. They showed 5.25% NaOCl and 2% CHX had strong antimicrobial activity after an immediate instrumentation, but generalized increasing in the CFU count of the specimens treated with 5.25% NaOCl was investigated 7 days after the instrumentation.
Coates
10) demonstrated that tablets of NaDCC were stable for prolonged periods of time and NaDCC products always presented minimal levels of available chlorine specified although, concentrated NaOCl products sometimes did not due to inherent instability. And Lee et al
24) revealed that the antimicrobial effects of NaOCl and NaDCC were increased when their concentrations were increased. And the cytotoxicity of NaDCC was rather higher than same concentration of NaOCl solution until 1 week after. But one day after dilution, antimicrobial effect of NaDCC was slightly higher than NaOCl, while, there was no difference in 1 week dilution solution.
In this study, 2.0% NaOCl and 2.0% CHX have similar antimicrobial activity both after the immediate and 7-day instrumentation. On the other hand, the bacterial CFU was found in one each canal of the first and second instrumentation after 7 days with the 2.0% of NaDCC. However these results showed no significant difference compared to the other two agents.
These results demonstrate that NaDCC could be considered as a root canal irrigant in endodontic treatment. It was considered that this study was performed under in vitro conditions, more researches on the tissue dissolubility, the direct effect on the periapical tissue and the antibacterial effect against other bacteria as well as the in vivo conditions should be undertaken to establish the possible application of NaDCC in the endodontic therapy.
V. CONCLUSION
The results are in agreement with other investigators, who have shown the bactericidal property and possibility of NaDCC as a root canal irrigation solution. Thus it seems that NaDCC solutions can be clinically applied into the root canal within 1 week after dilution. Further studies need to be undertaken to establish the possible application of NaDCC in clinical endodontic therapy.
REFERENCES
- 1. Kakehashi S, Stanley HR, Fizgerald RJ. The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral Surg Oral Med Oral Pathol. 1965;20: 340-349.ArticlePubMed
- 2. Byström A, Sundqvist G. Bacteriologic evaluation of the efficacy of mechanical root canal instrumentation in endodontic therapy. Scand J Dent Res. 1981;89: 321-328.ArticlePubMed
- 3. Haapasalo M, Endal U, Zandi H, Jeffrey M. Eradication of endodontic infection by instrumentation and irrigation solutions. Endod Topics. 2005;10: 77-102.Article
- 4. Sundqvist G, Fiigdor D, Persson S, Sjögren U. Microbiologic analysis of teeth with failed endodontic treatment and the outcome of conservative re-treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85: 86-93.ArticlePubMed
- 5. Heling I, Chandler NP. Antimicrobial effect of irrigant combinations within dentinal tubules. Int Endod J. 1998;31: 8-14.ArticlePubMed
- 6. Barrette WC Jr, Hannum DM, Wheeler WD, Hurst JK. General mechanism for the bacterial toxicity of hypochlorous acid: abolition of ATP production. Biochemistry. 1989;28: 9172-9178.ArticlePubMed
- 7. McKenna SM, Davies KJ. The inhibition of bacterial growth by hypochlorous acid. Possible role in the bactericidal activity of phagocytes. Biochem J. 1988;254: 685-692.ArticlePubMedPMCPDF
- 8. Siqueira JF Jr, Machado AG, Silveira RM, Lopes HP, de Uzeda M. Evaluation of the effectiveness of sodium hypochlorite used with three irrigation methods in the elimination of Enterococcus faecalis from the root canal, in vitro. Int Endod J. 1997;30: 279-282.ArticlePubMed
- 9. McComb D, Smith DC, Beagrie GS. The results of in vivo endodontic chemomechanical instrumentation--a scanning electron microscopic study. J Br Endod Soc. 1976;9: 11-18.ArticlePubMed
- 10. Coates D. Comparison of sodium hypochlorite and sodium dichloroisocyanurate disinfectants: neutralization by serum. J Hosp Infect. 1988;11: 60-67.ArticlePubMed
- 11. Coates D. A comparison of sodium hypochlorite and sodium dichloroisocyanurate products. J Hosp Infect. 1985;6: 31-40.Article
- 12. Russell AD, Day MJ. Antibacterial activity of chlorhexidine. J Hosp Infect. 1993;25: 229-238.ArticlePubMed
- 13. White RR, Hays GL, Janer LR. Residual antimicrobial activity after canal irrigation with chlorhexidine. J Endod. 1997;23: 229-231.ArticlePubMed
- 14. Russell AD. Activity of biocides against mycobacteria. Soc Appl Bacteriol Symp Ser. 1996;25: 87S-101S.ArticlePubMed
- 15. Goldman M, Pearson AH. Postdebridement bacterial flora and antibiotic sensitivity. Oral Surg Oral Med Oral Pathol. 1969;28: 897-905.PubMed
- 16. Basrani B, Tjaderhane L, Santos JM, Pascon E, Grad H, Lawrence HP, Friedman S. Efficacy of chlorhexidine- and calcium hydroxide-containing medicaments against Enterococcus faecalis in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96: 618-624.ArticlePubMed
- 17. Haapasalo M, Østavik D. In vitro Infection and Disinfection of Dentinal Tubules. J Dent Res. 1987;66: 1375-1379.ArticlePubMedPDF
- 18. Eddy RS, Joyce AP, Roberts S, Buxton TB, Liewehr F. An in vitro evaluation of the antibacterial efficacy of chlorine dioxide on E. faecalis in bovine incisors. J Endod. 2005;31: 672-675.ArticlePubMed
- 19. Kuruvilla JR, Kamath MP. Antimicrobial activity of 2.5% sodium hypochlorite and 0.2% chlorhexidine gluconate separately and combined, as endodontic irrigants. J Endod. 1998;24: 472-476.ArticlePubMed
- 20. Ringel AM, Patterson SS, Newton CW, Miller CH, Mulhern JM. In vivo evaluation of chlorhexidine gluconate solution and sodium hypochlorite solution as root canal irrigants. J Endod. 1982;8: 200-204.ArticlePubMed
- 21. Harrison JW, Hand RE. The effect of dilution and organic matter on the anti-bacterial property of 5.25% sodium hypochlorite. J Endod. 1981;7: 128-132.ArticlePubMed
- 22. Vahdaty A, Pitt Ford TR, Wilson RF. Efficacy of chlorhexidine in disinfecting dentinal tubules in vitro. Endod Dent Traumatol. 1993;9: 243-248.ArticlePubMed
- 23. Dametto FR, Ferraz CC, De Almeida Gomes BP, Zaia AA, Teixeira FB, De Souza-Filho FJ. In vitro assessment of the immediate and prolonged antimicrobial action of chlorhexidine gel as an endodontic irrigant against Enterococcus faecalis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99: 768-772.ArticlePubMed
- 24. Lee WC, Kang BS, Kim CH, Son HH. Evaluation of sodium dichloroisocyanurate as a root canal irrigation solution; Cl- concentration, pH, cytotoxicity and antimicrobial effect in vitro. J Korean Acad Conserv Dent. 2003;28: 425-430.Article
Figure 1Molecular structure of Sodium dichloroisocyanurate
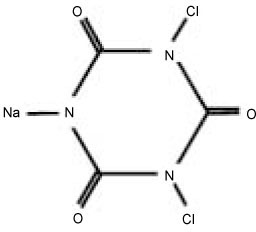
Figure 2Schematic illustration of incubating tooth block
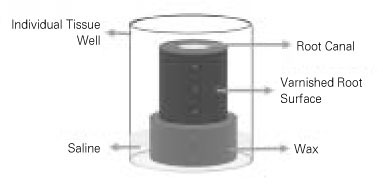
Figure 3Schematic illustration of sampling of dentin.
Table 1

Table 2MIC values (%) for E. faecalis incubated in diluted solutions of NaDCC, NaOCl and CHX

Table 3Number (%) of canals with bacterial growth before and after instrumentation and irrigation
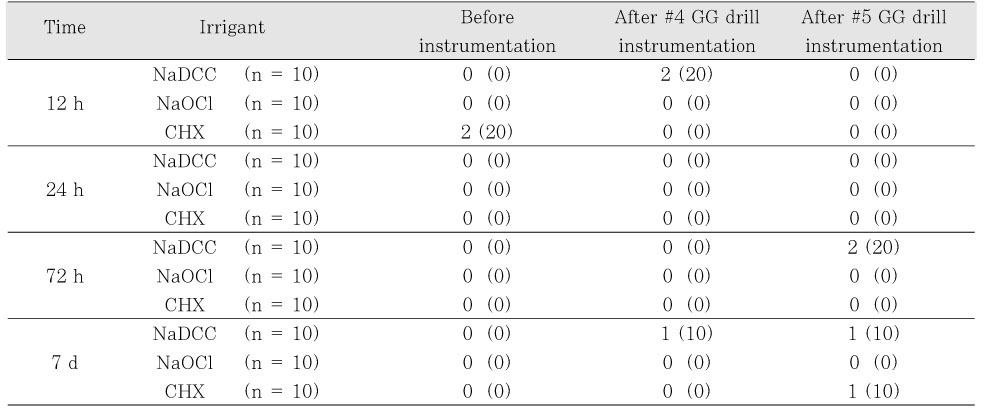
Table 4Mean bacterial CFU counts of control group before, after #4 and #5 GG drill instrumentation


 KACD
KACD







 ePub Link
ePub Link Cite
Cite

