Abstract
-
We investigated the secretion of Interleukin-8 (IL-8) from ginviva and periodontal ligament stimulated with Substance P (SP) and Calcitonin Gene-related Peptide (CGRP). Gingiva (GF), periodontal ligament (PDLF) and pulp (PF) tissues were collected from extracted intact 3rd molars.
Cultured cells were stimulated with different concentrations of SP for 4 hrs, and stimulated with SP, CGRP and Tumor Necrosis Factor-α (TNF-α) for 8 hrs. Then RNase Protection Assay was carried out. ELISA was performed using supernatants of stimulated cells for quantitative analysis of IL-8. Results were assessed using student t-test with significance of P < 0.05.
According to this study, the results were as follows:
IL-8 mRNA was detected in all type of cells studied (PF, GF and PDLF).
IL-8 mRNA expression was not increased after stimulating 4 hrs with SP (10-5M) and SP (10-8M) compared with Mock stimulation in all type of cells studied.
IL-8 mRNA expression was not increased after stimulating 8 hrs with SP (10-4M) and CGRP (10-6M) compared with Mock stimulation in all type of cells studied.
TNF-α(2 ng/ml) increased the expression of IL-8 mRNA in all kind of cells studied.
The secretion of IL-8 from GF was increased 8 hrs after the stimulation with CGRP (10-6M) (p < 0.05).
The secretion of IL-8 from PDLF was increased 8 hrs after the stimulation with SP (10-4M) (p < 0.05).
Calcitonin Gene-related Peptide (CGRP) increased Interleukin-8 (IL-8) which plays an important role in chemotaxis of neutrophil in Calcitonin Gene-related Peptide (CGRP) gingival tissue, whereas Substance P increased the secretion of IL-8 from periodontal ligament.
-
Keywords: Interleukin-8 (IL-8); Substance P (SP); Tumor Necrosis Factor-α (TNF-α); pulp fibroblast (PF); gingival fibroblast (GF); periodontal ligament fibroblast (PDLF)
I. INTRODUCTION
Periodontal disease is the inflammatory disorders of the periodontium ranging from the relatively benign form of gingivitis, in which the inflammation is confined to the marginal tissues, to more aggressive forms such as rapidly progressive periodontitis, in which the disease process leads to loss of connective tissue attachment to the root surfaces, loss of alveolar support, and impaired function of the dentition. Although intense microbiological, immunological, and biochemical studies about the pathogenesis of the various periodontal disease were performed, pathogenesis of several diseases were too complicated to understand. Recently it has come to know that nervous system can regulate immunologic and inflammatory responses
1). One of the potent reasons of individually different response to same irritation, is that neurologic system can modulate immunologic system
2). The nervous system affects the development of inflammatory processes through local release of various neuropeptides
3).
Neutrophils are the most predominant cell population that migrate into inflammatory lesions. Besides their protective role, they are involved in tissue damage under pathologic conditions because of their high contents in neutral and acid proteases and their ability to generate superoxides and other reactive oxygen derivatives
4). Inflammatory neutrophils are recruited into affected tissues through the process of multiple activation events that eventually lead to the release of antimicrobial and inflammatory products.
The recent discovery of a family of mediators called chemokines, which play pivotal roles in regulating inflammatory reactions, has provided an insight into the molecular mechanisms underlying the kinetics of inflammation
5,
6). However, the characterization of chemokine expression in the cells of dental pulp, gingiva and periodontal ligament during inflammation and infection has been sparse
7,
8).
Interleukin-8 (IL-8) is a neutrophil-stimulating factor and displays a wide range of biological effects including chemotaxis and activation of neutrophils
8). It was originally described as a 72 amino acid peptide produced by human blood monocytes stimulated in culture with
E. coli lipopolysaccharide
4). IL-8 is a member of the chemokine C-X-C subfamily that displays potent chmotactic activities for human neutrophils and T lymphocytes
4,
7,
9). In addition to chemotaxis, IL-8 induces neutrophil degranulation, resulting in the release of enzymes that cause tissue destruction
10).
Substance P (SP) and Calcitonin Gene-related Peptide (CGRP) are major sensory neuropeptides, released from the peripheral nerve endings of sensory nerves during inflammation
1,
6,
11). Although the primary function of these two neuropeptides is to induce vasodilatation and consequently to increase blood flow, they can play more direct roles in infiltrations of local inflammatory cells
12). In addition, they can modulate local inflammatory responses by modifying the secretion of various cytokines
1).
The cellular elements inside the periodontal ligament are fibroblasts, endothelial cells, cementoblasts, osteoblasts, and osteoclasts and neutrophiles can be seen predominantly in gingival connective tissues and periodontal ligament space as well
14). Fibroblast which is predominant cell in gingival connective tissue plays an important role in wound healing following the injury such as gingival surgery or pathologic processes through renewal of collagen fibers and other chemical constituents
13,
14).
In the present study, whether these neuropeptides such as SP and CGRP could induce the release of IL-8 in gingiva, periodontal ligament, and pulp during inflammation was evaluated. We investigated responses of these cells cultured from the gingiva, periodontal ligament, and pulp in releasing IL-8 after stimulation with SP or CGRP.
II. MATERIALS AND METHODS
Sample Collection and Cell Culture
Freshly extracted, intact, caries-free third molars (n = 10) were obtained from the patients (15 - 25 years old) in the Department of Oromaxillofacial Surgery at Kyung-Hee Medical Center. Immediately after extraction, teeth were stored in phosphate buffered saline (PBS) and transferred to the laboratory. Gingival tissues were cut around the cemento-enamel junction area, and the periodontal ligaments were obtained by scraping the root surface. Each tooth was grooved longitudinally with a fissure bur at high speed. The tooth was then split with the screw driver, and the entire pulp (coronal and radicular) elevated with cotton pliers, maximum pulpal tissues were obtained.
Repeated washing with PBS, all kinds of tissues obtained from dental pulp, gingiva and periodontal ligament were placed in 48-well plate culture dishes containing Dulbecco's modified Eagle medium (DMEM; Life Technologies/ GIBCO BRL, Gaithersburg, MD, USA) supplemented with 10% fetal bovine serum (FBS) and 100 units/ml Penicillin-G, 100 µg/ml Streptomycin, and 0.25 µg/ml Fungizone (Gemini Bio-Products, Inc., Woodland, CA, USA). The cells from pulp tissue (PF), gingival tissue (GF), and periodontal ligament (PDLF) were grown to confluence and passed at 1 : 2 ratio until used for experiments (passages 5-8 were used).
Once all kind of cells obtained were confluent at passage 5, they were pooled in 100 mm diameter culture dish and cultured until it became confluent for the stimulation with SP and CGRP for RNase Protection Assay. In addition, GF and PDLF were seeded on the 24-well plates, and grown into confluent for Enzyme-Linked Immunosorbent Assay (ELISA).
Stimulation of cells with SP and CGRP
Synthetic human SP and CGRP (Sigma, St. Louis, MO, USA) were prepared in sterile water with 0.1% low-endotoxin bovine serum albumin (BSA, Sigma, St Louis, MO, USA). Cells were seeded in 100 mm culture dish and 24-well plates and grown to confluence before used for experiments. Alpha minimal essential medium containing L-glutamine (Life Technologies/GIBCO BRL, Gaithersburg, MD, USA) without FBS was used 24 hrs before cells were stimulated with neuropeptiedes. Cells seeded in 100 mm culture dish were stimulated with SP (10-8M) or SP (10-5M) or only with medium (Mock stimulation) for 4 hrs, and stimulated with SP (10-4M) or CGRP (10-6M) or TNF-α(2 ng/ml) or only with medium (Mock stimulation) for 8 hrs which will be subjected to RNase Protection Assay.
GF and PDLF seeded on 24-well plate were stimulated SP (10
-4M) or CGRP (10
-6M) or TNF-α (2 ng/ml) or only with medium (Mock stimulation) for 8 hrs for the ELISA. Experiments with pulp cells after stimulation with SP and CGRP were excluded, because those data with PF was already known in the previous study
15,
16).
The serum was not used in the medium to prevent the increase in the base-line level of IL-8 secretion from these cells. Based on the preliminary experiments, the serum induced significant levels of IL-8 and from pulp. Varying doses of neuropeptides or 0.1% BSA (Mock stimulation) were added to the cell cultures. Recombinant human TNF-α (R&D Systems, Minneapolis, MN, USA), a known IL-8 agonist, was used as a positive control for IL-8 induction.
Extraction of Total RNA
After cells were stimulated with SP for 4 hrs, and with SP and CGRP, and TNF-α for 8 hrs, supernatants were discarded. All kinds of cells stimulated were collected scraping with cell scraper (Fisher Scientific, Pittsburgh, PA, USA).
5 - 10 µg of total RNA was extracted from all kinds of cells stimulated using the RNeasy mini kit following the manufacturer's instructions (Qiagen, LRS Laboratories, Inc., Seoul, Korea).
RNase Protection Assay
The total RNA extracted were subjected to RNase protection assay as specified by the manufacturer, using the human cytokine-5 probe set (BD Biosciences, San Diego, CA, USA) and BD RiboQuant Ribonuclease Protection Assay Systems (BD Biosciences, San Diego, CA, USA). The resulting protected RNAs were resolved on 5% denaturing polyacrylamide gels and exposed to X-ray films.
Enzyme Linked Immunosorbent Assay (ELISA) for IL-8
After GF and PDLF were stimulated with SP (10-4M), CGRP (10-6M) and TNF-α (2 ng/ml) and only with medium(Mock stimulation), whose total volume were 300 µl in each well for 8 hrs, supernatants were collected and stored at -80℃.
Standard ELISA for IL-8 was performed as described previously
7) using 2 µg/ml of polyclonal goat anti-human IL-8 (R&D Systems, Minneapolis, MN, USA) as capturing antibodies, 1 g/ml polyclonal rabbit anti-human IL-8 (Endogen Inc., Cambridge, MA, USA) as detecting antibodies, and 0.1 g/ml horseradish peroxidase (HRPO)-labeled polyclonal goat anti-rabbit immunoglobulin G (Biosource International, Camarillo, CA, USA) as a secondary antibody. Subsequently fresh developing buffer containing substrate of optimal concentrations of TMB (3,3',5,5'-Tetramethyl benzidine; Sigma, St. Louis, MO, USA), H
2O
2 and sodium acetate, pH 6.0 was added and the developing reaction was stopped with 1.2 M/L sulfuric acid. Absorbance at 450 nm was determined with a microplate reader(Bio-Tek Instrument, Inc., Laguna Hills, CA, USA) and concentrations were derived using the Delta Soft III software (Bio-Tek Instrument, Inc., Laguna Hills, CA, USA). Known concentrations of recombinant human (rh) IL-8 (Endogen Inc., Cambridge, MA, USA) was used to establish a standard curve for determining the concentrations of experimental samples.
The Student t-test was used to assess the significance of differences between data. The differences were considered significant when the probability (P) value was < 0.05.
III. RESULTS
RNase Protection Assay
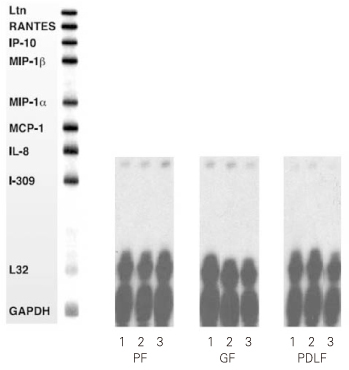
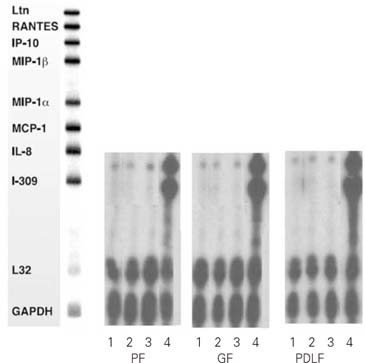
IL-8 mRNA was detected in all kinds of cells studied (PF, GF, PDLF) 4hrs after stimulation with Mock, SP (10
-5M), and SP (10
-8M) as shown in
Figure 1. But, other cytokines (Ltn, RANTES, IP-10, MIP-1β, MIP-1α, MCP-1, and I-309) included in the human cytokine-5 probe set (BD Biosciences, San Diego, CA, USA) were not detected after Mock stimulation and after SP stimulation as well. The expression of IL-8 mRNA was not increased 4 hrs after stimulation with SP (10
-5M) and SP (10
-8M) compared with Mock stimulation in all kinds of cells studied (
Figure 1).
IL-8 mRNA was detected in all kinds of cells studied (PF, GF, PDLF) 8hrs after stimulation with Mock, SP (10
-4M), and CGRP (10
-6M) as shown in
Figure 2. The expression of IL-8 mRNA was not increased 8 hrs after stimulation with SP (10
-4M) or CGRP (10
-6M) compared with Mock stimulation in all type of cells studied.
On the other hand, TNF-α (2 ng/ml) remarkably increased the expression of IL-8 mRNA in all kind of cells studied (
Figure 2). In addtion, I-309 which was not detected 8 hrs after Mock stimulation was distinctively expressed 8 hrs after stimulation with TNF-α (2 ng/ml) in all kinds of cells studied (
Figure 2).
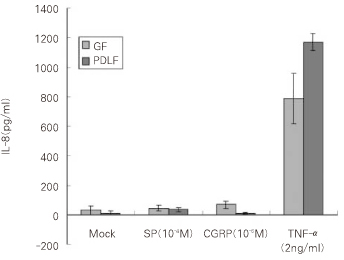
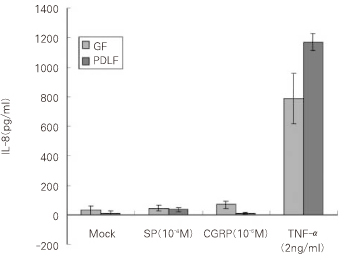
As a result of quantitative analysis from the supernatent of the cells after the stimulation with SP (SP10
-4M) and CGRP (10
-6M), and TNF-α (2 ng/ml) as a positive control, The secretion of IL-8 from GF was increased mildly up to 44.2 pg/ml (1.5-fold) 8 hrs after stimulation with SP (10
-4M) and was increased significantly to 68.5 pg/ml (2.3-fold) 8 hrs after stimulation with CGRP (10
-6M) compared with Mock stimulation (30.1 pg/ml) as shown in
Figure 3 (p < 0.05). IL-8 from GF was significantly increased (788.3 pg/ml) 8 hrs after stimulation with TNF-α (2 ng/ml) (p < 0.05).
PDLF stimulated with SP (10-4M) for 8 hrs increased IL-8 (35.7 pg/ml) compared with Mock stimulation (12.3 pg/ml) significantly (p < 0.05). There was no induction of IL-8 from PDLF (10.2 pg/ml) after the stimulation with CGRP (10-6M) in comparison with Mock stimulation. As same as in GF, the secretion of IL-8 from PDLF was significantly increased up to approximately 100-fold (1170.7 pg/ml) after TNF-α stimulation (2 ng/ml) (p < 0.05).
IV. DISCUSSION
In teeth and tooth supporting tissues, SP and CGRP are shown to exert vasoactive functions including increase in blood flow and interstitial tissue pressur
15,
16), regulation of local inflammation
17), guiding of immune cells
18) and enhancement of cell proliferation
19).
Other cell types from different tissues release IL-8 during stimulation with neuropeptides
20-
22) and substantial evidence has shown that neuropeptides can stimulate the production of proinflammatory cytokines in various cell types. SP induces skin keratinocytes to release IL-1 and mast cells to produce tumor necrosis factor-α (TNF-α). CGRP stimulates human dermal microvascular endothelial cells to secrete IL-8, whereas SP induces human dermal microvascular endothelial cells to express vascular cell adhesion molecule-1
21). Furthermore, SP induces IL-8 production by osteoarthritis fibroblasts, whereas CGRP increases IL-8 and IL-6 secretion from rheumatoid arthritis fibroblasts
22).
Neutrophil recruitment depends on chemotactic agonists that are synthesized and released at the site of inflammation. Chemotactic agonists may be derived from the host or infecting microorganisms.
IL-8 stimulates neutrophils to transport CD11/CD18 (LFA-1 or Mac) integrin from the neutrophil cytoplasm to cell surface
23). This facilitates neutrophil migration into the tissue. Neutrophil migration may be induced by a haptotactic gradient of IL-8 either on the endothelial cell surface or in the extracellular matrix
23,
24).
IL-8 can be induced by IL-1α, IL-1β and tumor necrosis factor-α (TNF-α) in many different cells
4). It was also derived
in vitro from dermal fibroblasts, keratinocytes, endothelial cells, hepatocytes and synovial cells in response to inflammatory mediators such as IL-1β and TNF-α
25).
As mentioned above, various cell types from different tissues release IL-8 during stimulation with neuropeptides. In some cells, both SP an CGRP increase the secretion of IL-8. Cultured human pulp cells stimulated with SP have been reported to increase IL-8 secretion in the previous study
12).
Our data demonstrated that cultured primary human gingival cells having a fibroblast-like phenotype increased the secretion of IL-8 significantly in response to CGRP, but mildly to SP, whereas cultured periodontal ligament cells increased the secretion of IL-8 significantly in response to SP, but mildly to CGRP. Our findings suggest that SP and CGRP have a more direct role in the initiation of inflammatory cell infiltration in gingiva and periodontal ligament. Our report indicated that SP and CGRP stimulate the chemotaxis of leukocytes, which may have been in part a result of this IL-8 induction effect.
In the previouse study
20), the basal level of IL-8 from the cultured primary pulp cells were between 100 pg/ml and 200 pg/ml after Mock stimulation, nevertheless endothelial cells (ECV 304 cells) were around 20 pg/ml. In this study, the basal level of IL-8 from the cultured primary gingival cells (30.1 pg/ml) and periodontal ligament cells (10.2 pg/ml) were lower than pulp cells after Mock stimulation.
From the immunohistochemical studies, in healthy gingival tissues SP was found in the connective tissues between the collagenous elements, whereas in inflamed tissue SP was markedly increased particularly around the blood vessels as well as in close association with much of the inflammatory cell infiltrate
2). IL-8 was elevated in chronically inflammed gingiva, and the major IL-8 was detected only in the epithelial cell layer, which means that IL-8 may play a crucial role in the recruitment and activation of neutrophils and T lymphocytes in periodontitis
8). The neural modulation of inflammatory events in the gingiva, a site in which continuous tissue reactivity is generated in response to the accumulation of dental plaque, is of particular interest in light of the recognized potential for stress to serve as modifying factor in inflammatory lesions which affect the periodontium, which is accumulating evidence that interactions between the nervous system, immune cells, and cells such as fibroblasts are critical to the development and persistence of a variety of inflammatory disorders.
V. CONCLUSION
According to this study, the results were as follows:
IL-8 mRNA was detected in all type of cells studied (PF, GF and PDLF).
IL-8 mRNA expression was not increased after stimulating 4 hrs with SP (10-5M) and SP (10-8M) compared with Mock stimulation in all type of cells studied.
IL-8 mRNA expression was not increased after stimulating 8 hrs with SP (10-4M) and CGRP (10-6M) compared with Mock stimulation in all type of cells studied.
TNF-α(2 ng/ml) increased the expression of IL-8 mRNA in all kind of cells studied.
The secretion of IL-8 from GF was increased 8 hrs after the stimulation with CGRP (10-6M) (p < 0.05).
The secretion of IL-8 from PDLF was increased 8 hrs after the stimulation with SP (10-4M) (p < 0.05).
The present study has demonstrated that Calcitonin Gene-related Peptide (CGRP) increased Interleukin-8 (IL-8) which plays an important role in chemotaxis of neutrophil in gingival tissue, whereas Substance P increased the secretion of IL-8 from periodontal ligament.
REFERENCES
- 1. Kiss M, Kemeny L, Gyulai R, Michel G, Husz S, Kovacs R, Dobozy A, Ruzicka T. Effects of the neuropeptides substance P, calcitonin gene-related peptide and α-melanocyte-stimulating hormone on the IL-8/IL-8 receptor system in a cultured human keratinocyte cell line and dermal fibroblasts. Inflammation. 1999;23: 557-567.ArticlePubMedPDF
- 2. Bartold PM, Kylstra A, Lawson R. Substance P. An Immunohistochemical and Biochemical Study in Human Gingival Tissues. A Role for Neurogenic Inflammation? J Periodontol. 1994;65: 1113-1121.ArticlePubMedPDF
- 3. Rameshwar P, Gascon P. Induction of negative hematopoietic regulators by neurokinin-A in bone marrow stroma. Blood. 1996;88: 98-106.ArticlePubMedPDF
- 4. Baggiolini M, Walz A, Kunkel SL. Neutrophil-activating peptide-1/Interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989;84: 1045-1049.ArticlePubMedPMC
- 5. Kent LW, Dyken RA, Rahemtulla F, Allison AC, Michalek SM. Effects of in vitro passage of healthy human gingival fibroblasts on cellular morphology and cytokine expression. Arch Oral Biol. 1996;41: 263-270.PubMed
- 6. Guo CJ, Lai JP, Luo HM, Douglas SD, Ho WZ. Substance P up-regulates macrophage inflammatory protein-1β expression in human T lymphocytes. J Neuroimmunol. 2002;131: 160-167.ArticlePubMedPMC
- 7. Huang GTJ, Potente AP, Kim JW, Chugal N, Zhang X. Increased interleukin-8 espression in inflamed human dental pulps. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88: 214-220.PubMed
- 8. Fitzgerald JE, Kreutzer DL. Localization of Interleukin-8 in human gingival tissues. Oral Microbiol Immunol. 1996;10: 297-303.Article
- 9. Larsen CG, Anderson AO, Appella E, Oppenheim JJ, Matsushima K. The neutrophil-activating protein (NAP-1) is also chemotactic for T lymphocytes. Science. 1989;243: 1464-1466.ArticlePubMed
- 10. Peveri P, Walz A, Dewald B, Baggiolini M. A novel neutrophil activating factor produced by human mononuclear phagocytes. J Exp Med. 1988;167: 1547-1559.ArticlePubMedPMCPDF
- 11. Cuesta MC, Quintero L, Pons H, Suarez-Roca H. Substance P and calcitonin gene-related peptide increase IL-1β, IL-6 and TNFα secretion from human peripheral blood mononuclear cells. Neurochem Int. 2002;40: 301-306.ArticlePubMed
- 12. Park SH, Hsiao GYW, Huang GTJ. Role of substance P and calcitonin gene-related peptide in the regulation of interleukin-8 and monocyte chemotactic protein-1 expression in human dental pulp. Int Endod J. 2004;37: 185-192.ArticlePubMed
- 13. Bordin S, Narayanan S, Reddy J, Cleveland D, Page RC. Fibroblast subtypes in the periodontium. J Periodont Res. 1984;19: 642-644.Article
- 14. McCulloch CAG, Bordin S. Role of fibroblast subpopulations in periodontal physiology and pathology. J Periodont Res. 1991;26: 144-154.Article
- 15. Heyeraas KJ, Berggreen E. Interstitial fluid pressure in normal and inflamed pulp. Crit Rev Oral Biol Med. 1999;10(3):328-336.ArticlePubMedPDF
- 16. Berggreen E, Heyeraas KJ. Effect of the sensory neuropeptide antagonists h-CGRP((8-37)) and SR 140.33 on pulpal and gingival blood flow in ferrets. Arch Oral Biol. 2000;45(7):537-542.ArticlePubMed
- 17. Fristad I, Kvinnsland IH, Jonsson R, Heyeraas KJ. Effect of intermittent long-lasting electrical tooth stimulation on pulpal blood flow and immunocompetent cells: a hemodynamic and immunohistochemical study in young rat molars. Exp Neurol. 1997;146(1):230-239.ArticlePubMed
- 18. Fristad I, Heyeraas KJ, Kwinnsland IH, Jonsson R. Recruitment of immunocompetent cells after dentinal injuries in innervated and denervated young rat molars: an innunohistochemcal study. J Histochem Cytochem. 1995;43(9):871-879.ArticlePubMedPDF
- 19. Bongenhielm U, Haegerstrand A, Theodorsson E, Fried K. Effect of neuropeptides on growth of cultivated rat molar pulp fibroblasts. Regul Pept. 1995;60(2-3):91-98.PubMed
- 20. Patel T, Park SH, Lin LM, Chiappelli F, Huang GTJ. Substance P induces interleukin-8 secretion from human dental pulp cells. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96: 478-485.ArticlePubMed
- 21. Ansel JC, Armstrong CA, Song I, Quinlan KL, Olerud JE, Caughman SW, Bunnett NW. Interaction of the skin and nervous system. J Investig Dermatol Symp Proc. 1997;2(1):23-26.PubMed
- 22. Raap T, Justen HP, Miller LE, Cutolo M, Scholmerich J, Straub RH. Neurotransmitter modulation of interleukin 6(IL-6) and IL-8 secretion of synovial fibroblasts in patients with rheumatoid arthritis compared to osteoarthritis. J Rheumatol. 2000;27: 2558-2565.PubMed
- 23. Detmers PA, Lo SK, Olsen-Egbert E, Walz A, Baggiolini M, Cohn ZA. Neutrophil-activating protein 1/interleukin 8 stimulates the binding activity of the leukocyte adhesion receptor CD11b /CD18 on human neutrophils. J Exp Med. 1990;171: 1155-1162.ArticlePubMedPMCPDF
- 24. Rot A. Neutrophil attractant/activation protein-1(interleukin-8) induces in vitro neutrophil migration by haptotactic mechanism. Eur J Immunol. 1993;23: 303-306.ArticlePubMed
- 25. Takashiba S, Takigawa M, Takahashi K, Myokai F, Nishimura F, Chihara T, Kurihara H, Nomura Y, Murayama Y. Interleukin-8 is a major neutrophil chemotactic factor derived from cultured human gingival fibroblasts stimulated with interleukin-1β or tumor necrosis factor alpha. Infection and Immunity. 1992;60: 5253-5258.PubMedPMC
Figure 1
RNase Protection Assay 4hrs after stimulation of the cells with MOCK and various dose of Substance P.
1. MOCK stimulation with medium only
2. Stimulation with Substance P (10-5M)
3. Stimulation with Substance P (10-8M)
PF: Pulp Fibroblasts
GF: Gingival Fibroblasts
PDLF: Periodontal Ligament Fibroblasts
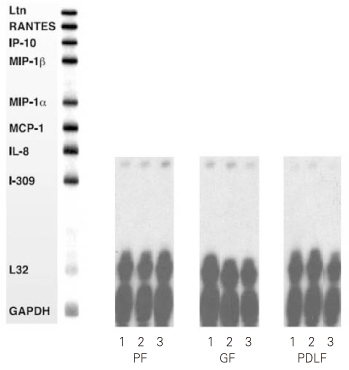
Figure 2
RNase Protection Assay 8hrs after stimulation of the cells with Mock and Substance P, CGRP, and TNF-α.
1. MOCK stimulation with medium only
2. Stimulation with Substance P (10-4M)
3. Stimulation with Calcitonin Gene Related Peptides (CGRP, 10-6M)
4. Stimulation with TNF-α (2 ng/ml)
PF: Pulp Fibroblasts
GF: Gingival Fibroblasts
PDLF: Periodontal Ligament Fibroblasts
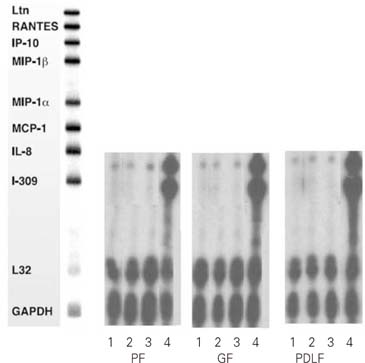
Figure 3
IL-8 secretion from the cells 8hrs after stimulation with SP (10-4M), CGRP (10-6M) and TNF-α (2 ng/ml).
GF: Gingival Fibroblasts
PDLF: Periodontal Ligament Fibroblasts




 KACD
KACD

 ePub Link
ePub Link Cite
Cite

