Articles
- Page Path
- HOME > Restor Dent Endod > Volume 49(4); 2024 > Article
- Review Article Comparative evaluation of the biological response of conventional and resin modified glass ionomer cement on human cells: a systematic review
-
Shishir Singh1
 , Gaurav Kulkarni1
, Gaurav Kulkarni1 , R S Mohan Kumar2
, R S Mohan Kumar2 , Romi Jain3
, Romi Jain3 , Ameya M Lokhande1
, Ameya M Lokhande1 , Teena K Sitlaney1
, Teena K Sitlaney1 , Musharraf H F Ansari1
, Musharraf H F Ansari1 , Navin S Agarwal1
, Navin S Agarwal1
-
Restor Dent Endod 2024;49(4):e41.
DOI: https://doi.org/10.5395/rde.2024.49.e41
Published online: November 1, 2024
1Department of Conservative Dentistry and Endodontics, TPCT’s Terna Dental College, Maharashtra, India.
2Department of Conservative Dentistry and Endodontics, Priyadarshini Dental College, Tamilnadu, India.
3Department of Public Health Dentistry, TPCT’s Terna Dental College, Maharashtra, India.
- Correspondence to Shishir Singh, BDS, MDS, PhD. Department of Conservative Dentistry and Endodontics, TPCT’s Terna Dental College, Nerul, Navi Mumbai, Maharashtra 400706. India. drshishirs@gmail.com
Copyright © 2024. The Korean Academy of Conservative Dentistry
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Trial Registration PROSPERO Identifier: CRD42023426021
INTRODUCTION
MATERIALS AND METHODS
1. Inclusion criteria
2. Exclusion criteria
RESULTS
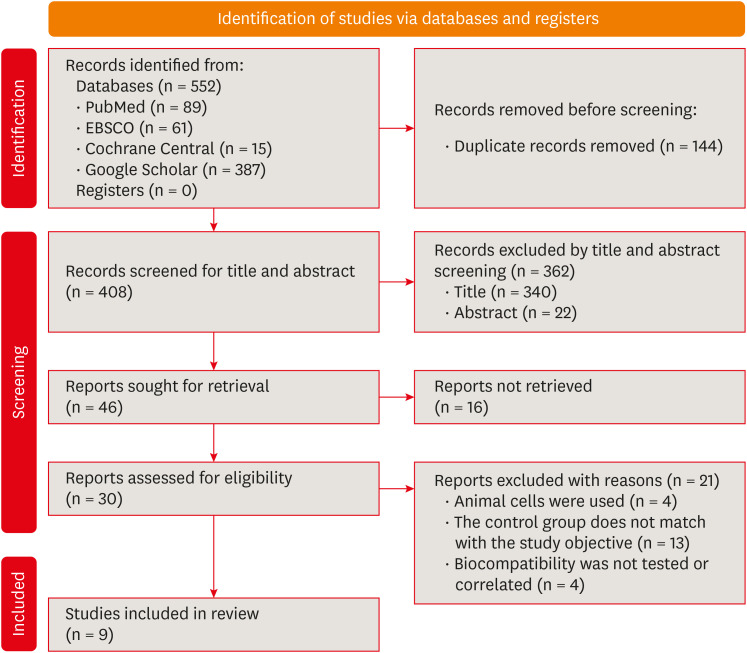
Summary of the inclusion and screening of articles following the PRISMA approach.
General characteristics of the included studies
| Author and year | Study design | Materials tested | Experimental model | Exposure duration | Cell line | Variables studies |
|---|---|---|---|---|---|---|
| Eskandarizadeh et al. (2015) [21] | Non-randomized controlled trial | RMGIC (Vivaglass) GIC (Ionocid) Calcium hydroxide (Dycal) | 30 human premolars | 5 and 30 days | None | Odontoblastic changes, inflammatory response, tertiary dentin formation, and presence of microorganisms |
| Ribeiro et al. (2020) [20] | Non-randomized controlled trial | RMGIC (Riva LC) GIC (Riva SC) Calcium hydroxide (Dycal) | 26 human premolars + 4 controls | 7 and 30 days | None | Inflammatory reaction, tissue disorganization, reactionary dentin formation, and bacteria |
| Mousavinasab et al. (2008) [22] | Non-randomized controlled trial | RMGIC (Vivaglass) GIC (Chem bond Superior) Calcium hydroxide (Dycal) | 55 human premolars | 7, 30 and 60 days | None | Odontoblastic changes, inflammatory cell infiltration, and reactionary dentin formation |
| Leyhausen et al. (1998) [23] | In vitro study (histological studies) | RMGICs (Ionoseal, Vitrebond, Compoglass) and GIC (Ketac Fil) | HGF | 48 hours | Cytotoxicity | Morphology and growth characteristics by PCM |
| Rodriguez et al. (2013) [24] | In vitro study (Histological studies) | RMGIC (Vitrebond) GIC (Ketac Molar) | HGF | 72 hours | Cytotoxicity | Morphological changes by PCM and LDH |
| Mohd Zainal Abidin et al. (2015) [25] | In vitro study (histological studies) | Fuji IX GPExtra | SHED | 72 hours | Cytotoxicity | Cell viability percentage and IC50 |
| Fuji II LC | ||||||
| Koohpeima et al. (2017) [26] | In vitro study (histological studies) | Fuji II | HGF | 24 hours | Cytotoxicity | percentage of cell viability of HGF at 25, 50, 75 and 100% |
| Fuji II LC | ||||||
| de Souza Costa et al. (2003) [27] | In vitro study (histological studies) | Fuji IX, Ketac Molar | Human odontoblast cell line (MDPC-23) | 72 hours | Cytotoxicity | Cell number, cell morphology, cell metabolism |
| Vitrebond, Vitremer, Fuji II LC | ||||||
| Sun et al. (2011) [28] | In vitro study (histological studies) | Fuji II, Fuji II LC, Vitrmer | Human pulp cells, 3T3 mouse fibroblast | 1 and 3 days | Cytotoxicity | Cell number, cell morphology and cell metabolism |
Biocompatibility and cytotoxicity of conventional GIC and RMGIC
| Author and year | Cytotoxicity test used | Results for conventional GICs | Results for resin modified GICs | Author’s conclusion | ||
|---|---|---|---|---|---|---|
| Leyhausen et al. (1998) [23] | DNA Intercalating fluorochrome assay | Ketac Fil Applicap | Vitrebond | Light cure GICs revealed cytotoxic effects when compared to conventional GIC which had no or slight alterations in cell lines. | ||
| - Growth of the primary HGF: Day 1: 107 ± 19.5, Day 9: 103 ± 4 | - Growth of the primary HGF: Day 1: 15.7 ± 13.9, Day 9: 68.8 ± 6.8 | |||||
| Rodriguez et al. (2013) [24] | PCM, LDH assay, EPXMA analysis | Ketac Molar | Vitrebond | Morphological, biochemical, and micro-analytical indicators suggested that RMGIC causes greater alteration that points towards necrosis as compared to conventional GIC. | ||
| - Number of fibroblasts: 52.2% ± 20.4% | - Number of fibroblasts: 3.9% ± 5% | |||||
| - LDH release: 11.04% ± 21.69% | - LDH release: 38.46% ± 7.29% | |||||
| - Intracellular levels (Na): 85.79 ± 58.03 mmol/kg | - Intracellular elements levels (Na): 187.03 ± 113.11 mmol/kg | |||||
| - Intracellular levels (K): 272.72 ± 75.69 mmol/kg | - Intracellular elements levels (K): 186.74 ± 132.06 mmol/kg | |||||
| - Intracellular elements levels (Cl): 115.89 ± 75.69 mmol/kg | - Intracellular elements levels (Cl): 153.12 ± 57.28 mmol/kg | |||||
| Mohd Zainal Abidin et al. (2015) [25] | MTT assay | Fuji IX GPExtra | Fuji II LC | RMGIC exhibited cytotoxic effect on SHED as well as the least favorable cell viability among all the groups. | ||
| IC50 = 45 mg/mL | IC50 = 31.2 5 mg/mL | |||||
| Koohpeima et al. (2017) [26] | MTT assay | Fuji II | Fuji II LC | Study showed that cytotoxic effect of conventional GIC was significantly lesser as compared to other modified GICs. | ||
| - Percentage cell viability: at 25% = 100.80% ± 8.17%, at 50% = 122.64% ± 3.76%, at 75% = 125.15% ± 3.92%, and at 100% = 134.86% ± 0.65% | - Percentage cell viability: at 25% = 98.45% ± 7.24%, at 50% = 102.50% ± 6.16%, at 75% = 5.41% ± 9.16%, at 100% = 112.29% ± 3.85% | |||||
| de Souza Costa et al. (2003) [27] | MTT assay, SEM | Fuji IX, Ketac Molar | Vitrebond, Vitremer, Fuji II LC | Study concluded that Vitremer and Vitrebond (RMGIC) were more cytopathic than Fuji IX GP and Ketac Molar. | ||
| - Reduction in cell metabolism: Fuji IX = 40.3%, Ketac Molar = 42.5% | - Reduction in cell metabolism: Vitrebond = 79.1%, Vitremer = 83.9%, Fuji II LC = 53.75% | |||||
| - Reduction in cell number: Fuji IX = 29.5%, Ketac Molar = 32.5% | - Reduction in cell number: Vitrebond = 74.5%, Vitremer = 75.5%, Fuji II LC = 45.5% | |||||
| Sun et al. (2011) [28] | MTT assay, WST-1 assay | Fuji II | Fuji II LC, Vitremer | Study revealed that Fuji II and Fuji II LC are not cytotoxic to human pulp cells but Vitremer is very cytotoxic. Cytotoxicity was dose-dependent. | ||
| - Cell viability: Day 1: 100.3% ± 6.3%, Day 3: 98.8% ± 7.8% | - Cell viability: Day 1: 88.0% ± 11%, Day 3: 105.9% ± 10.3% | |||||
Histological response of pulp to RMGIC and conventional GIC
| Histological events | Material | 5–7 days | 30 days | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No | Mild | Moderate | Severe | No | Mild | Moderate | Severe | ||||
| Ribeiro et al. (2020) [20] | |||||||||||
| Inflammatory response | RMGIC | 1 | 3 | 1 | 0 | 3 | 2 | 0 | 0 | 10 | |
| GIC | 2 | 3 | 0 | 0 | 5 | 0 | 0 | 0 | 10 | ||
| Tissue disorganization | RMGIC | 1 | 4 | 0 | 0 | 3 | 2 | 0 | 0 | 10 | |
| GIC | 2 | 3 | 0 | 0 | 4 | 1 | 0 | 0 | 10 | ||
| Reactionary dentin formation | RMGIC | 4 | 1 | 0 | 0 | 5 | 0 | 0 | 0 | 10 | |
| GIC | 5 | 0 | 0 | 0 | 4 | 1 | 0 | 0 | 10 | ||
| Bacteria | RMGIC | 5 | 0 | 0 | 0 | 3 | 2 | 0 | 0 | 10 | |
| GIC | 5 | 0 | 0 | 0 | 4 | 1 | 0 | 0 | 10 | ||
| Eskandarizadeh et al. (2015) [21] | |||||||||||
| Inflammatory response | RMGIC | 2 | 2 | 1 | 0 | 3 | 2 | 0 | 0 | 10 | |
| GIC | 2 | 2 | 0 | 1 | 3 | 1 | 0 | 1 | 10 | ||
| Odontoblastic changes | RMGIC | 0 | 3 | 2 | - | 2 | 1 | 2 | - | 10 | |
| GIC | 1 | 3 | 1 | - | 3 | 1 | 0 | - | 10 | ||
| Mousavinasab et al. (2008) [22] | |||||||||||
| Inflammatory cell infiltration | RMGIC | 0 | 2 | 6 | 0 | 3 | 2 | 0 | 0 | 13 | |
| GIC | 2 | 1 | 4 | 0 | 2 | 2 | 2 | 0 | 13 | ||
| Odontoblastic changes | RMGIC | 3 | 1 | 4 | 0 | 2 | 1 | 2 | 0 | 13 | |
| GIC | 2 | 2 | 3 | 0 | 2 | 2 | 2 | 0 | 13 | ||
| Reactionary dentin formation | RMGIC | 8 | 0 | 0 | 0 | 2 | 3 | 0 | 0 | 13 | |
| GIC | 7 | 0 | 0 | 0 | 5 | 1 | 0 | 0 | 13 | ||
Risk of bias/quality assessment for non-randomized clinical trials using MINORS tool
| Study | A clearly stated aim | Inclusion of consecutive patients | Prospective collection of data | Endpoints appropriate to the aim of the study | Unbiased assessment of the study endpoint | Follow-up period appropriate to the aim of the study | Loss to follow up less than 5% | Prospective calculation of the study size | Total |
|---|---|---|---|---|---|---|---|---|---|
| Eskandarizadeh et al. (2015) [21] | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 1 | 13 |
| Ribeiro et al. (2020) [20] | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 1 | 14 |
| Mousavinasab et al. (2023) [22] | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 1 | 14 |
Measurement of the risk of bias/quality assessment of in vitro studies (histopathological studies) with the modified ARRIVE and CONSORT scale (acceptability range 21–28).
| Studies | Title | Abstract | Introduction | Introduction | Methods: Study design | Methods: experimental procedures | Method: Sample size | Method: Statistical procedure | Result | Discussion | Potential conflicts | Publication | Total score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leyhausen et al. (1998) [23] | 1 | 2 | 3 | 2 | 2 | 2 | 2 | 3 | 2 | 1 | 1 | 0 | 22 |
| Mohd Zainal Abidin et al. (2015) [25] | 0 | 3 | 2 | 2 | 2 | 3 | 1 | 2 | 2 | 1 | 0 | 1 | 19 |
| Rodriguez et al. (2013) [24] | 1 | 2 | 3 | 1 | 2 | 3 | 2 | 3 | 3 | 1 | 1 | 1 | 23 |
| Koohpeima et al. (2017) [26] | 0 | 3 | 3 | 1 | 1 | 2 | 1 | 3 | 3 | 2 | 1 | 1 | 21 |
| de Souza Costa et al. (2003) [27] | 0 | 2 | 1 | 2 | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 1 | 15 |
| Sun et al. (2011) [28] | 1 | 1 | 2 | 2 | 3 | 1 | 2 | 3 | 2 | 1 | 1 | 21 |
DISCUSSION
CONCLUSIONS
-
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
-
Author Contributions:
Conceptualization: Singh S, Kulkarni G, Mohan Kumar RS, Jain R.
Data curation: Singh S, Kulkarni G, Jain R.
Formal analysis: Jain R, Lokhande AM, Sitlaney TK, Ansari MHF.
Investigation: Singh S, Jain R, Lokhande AM, Sitlaney TK, Ansari MHF.
Methodology: Singh S, Kulkarni G, Mohan Kumar RS, Jain R, Lokhande AM, Sitlaney TK, Ansari MHF.
Project administration: Singh S, Jain R, Lokhande AM, Sitlaney TK, Ansari MHF, Agarwal NS.
Software: Jain R, Lokhande AM, Sitlaney TK, Ansari MHF, Agarwal NS.
Supervision: Singh S, Kulkarni G, Mohan Kumar RS, Jain R.
Validation: Singh S, Kulkarni G, Mohan Kumar RS, Agarwal NS.
Visualization: Singh S, Kulkarni G, Mohan Kumar RS, Jain R, Lokhande AM, Sitlaney TK, Ansari MHF, Agarwal NS.
Writing - original draft: Singh S, Kulkarni G, Mohan Kumar RS, Jain R, Lokhande AM, Sitlaney TK, Ansari MHF, Agarwal NS.
Writing - review & editing: Singh S, Kulkarni G, Mohan Kumar RS, Lokhande AM, Sitlaney TK.
- 1. Anusavice KJ. Phillips’ science of dental materials. 11th ed. Philadelphia, PA: WB Saunders; 2003.
- 2. Sidhu SK. Glass-ionomer cement restorative materials: a sticky subject? Aust Dent J 2011;56(Supplement 1):23-30.Article
- 3. Nicholson JW, Czarnecka B. The biocompatibility of resin-modified glass-ionomer cements for dentistry. Dent Mater 2008;24:1702-1708.ArticlePubMed
- 4. Mount GJ. Some physical and biological properties of glass ionomer cement. Int Dent J 1995;45:135-140.PubMed
- 5. Ching HS, Luddin N, Kannan TP, Ab Rahman I, Abdul Ghani NR. Modification of glass ionomer cements on their physical-mechanical and antimicrobial properties. J Esthet Restor Dent 2018;30:557-571.ArticlePubMedPDF
- 6. Genaro LE, Anovazzi G, Hebling J, Zuanon AC. Glass Ionomer cement modified by resin with incorporation of nanohydroxyapatite: in vitro evaluation of physical-biological properties. Nanomaterials (Basel) 2020;10:1412.PubMedPMC
- 7. Rodriguez LC, Saba JN, Chung KH, Wadhwani C, Rodrigues DC. In vitro effects of dental cements on hard and soft tissues associated with dental implants. J Prosthet Dent 2017;118:31-35.PubMed
- 8. Bajantri P, Rodrigues SJ, Shama Prasada K, Pai UY, Shetty T, Saldanha S, et al. Cytotoxicity of dental cements on soft tissue associated with dental implants. Int J Dent 2022;2022:4916464.ArticlePubMedPMCPDF
- 9. Plant CG, Tobias RS, Rippin JW, Brooks JW, Browne RM. A study of the relationship among pulpal response, microbial microleakage, and particle heterogeneity in a glass-ionomer-base material. Dent Mater 1991;7:217-224.ArticlePubMed
- 10. Diemer F, Stark H, Helfgen EH, Enkling N, Probstmeier R, Winter J, et al. In vitro cytotoxicity of different dental resin-cements on human cell lines. J Mater Sci Mater Med 2021;32:4.PubMedPMC
- 11. Geurtsen W, Spahl W, Leyhausen G. Residual monomer/additive release and variability in cytotoxicity of light-curing glass-ionomer cements and compomers. J Dent Res 1998;77:2012-2019.ArticlePubMedPDF
- 12. About I, Camps J, Mitsiadis TA, Bottero MJ, Butler W, Franquin JC. Influence of resinous monomers on the differentiation in vitro of human pulp cells into odontoblasts. J Biomed Mater Res 2002;63:418-423.ArticlePubMed
- 13. Sandberg E, Bergenholtz G, Eklund C, Dahlgren UI. HEMA bound to self-protein promotes auto-antibody production in mice. J Dent Res 2002;81:633-636.ArticlePubMedPDF
- 14. Becher R, Kopperud HM, Al RH, Samuelsen JT, Morisbak E, Dahlman HJ, et al. Pattern of cell death after in vitro exposure to GDMA, TEGDMA, HEMA and two compomer extracts. Dent Mater 2006;22:630-640.PubMed
- 15. Paranjpe A, Bordador LC, Wang MY, Hume WR, Jewett A. Resin monomer 2-hydroxyethyl methacrylate (HEMA) is a potent inducer of apoptotic cell death in human and mouse cells. J Dent Res 2005;84:172-177.ArticlePubMedPDF
- 16. Schweikl H, Schmalz G, Spruss T. The induction of micronuclei in vitro by unpolymerized resin monomers. J Dent Res 2001;80:1615-1620.ArticlePubMedPDF
- 17. Bakopoulou A, Mourelatos D, Tsiftsoglou AS, Giassin NP, Mioglou E, Garefis P. Genotoxic and cytotoxic effects of different types of dental cement on normal cultured human lymphocytes. Mutat Res 2009;672:103-112.ArticlePubMed
- 18. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg 2003;73:712-716.ArticlePubMedPDF
- 19. Ramamoorthi M, Bakkar M, Jordan J, Tran SD. Osteogenic potential of dental mesenchymal stem cells in preclinical studies: a systematic review using modified arrive and consort guidelines. Stem Cells Int 2015;2015:378368.ArticlePubMedPMCPDF
- 20. Ribeiro AP, Sacono NT, Soares DG, Bordini EA, de Souza Costa CA, Hebling J. Human pulp response to conventional and resin-modified glass ionomer cements applied in very deep cavities. Clin Oral Investig 2020;24:1739-1748.ArticlePubMedPDF
- 21. Eskandarizadeh A, Parizi MT, Goroohi H, Badrian H, Asadi A, Khalighinejad N. Histological assessment of pulpal responses to resin modified glass ionomer cements in human teeth. Dent Res J 2015;12:144-149.
- 22. Mousavinasab M, Namazikhah MS, Sarabi N, Jajarm HH, Bidar M, Ghavamnasiri M. Histopathology study on pulp response to glass ionomers in human teeth. J Calif Dent Assoc 2008;36:51-55.ArticlePubMed
- 23. Leyhausen G, Abtahi M, Karbakhsch M, Sapotnick A, Geurtsen W. Biocompatibility of various light-curing and one conventional glass-ionomer cement. Biomaterials 1998;19:559-564.PubMed
- 24. Rodriguez IA, Ferrara CA, Campos-Sanchez F, Alaminos M, Echevarría JU, Campos A. An in vitro biocompatibility study of conventional and resin-modified glass ionomer cements. J Adhes Dent 2013;15:541-546.PubMed
- 25. Mohd Zainal Abidin R, Luddin N, Shamsuria Omar N, Mohamed Aly Ahmed H. Cytotoxicity of fast-set conventional and resin-modified glass ionomer cement polymerized at different times on shed. J Clin Pediatr Dent 2015;39:235-240.ArticlePubMedPDF
- 26. Koohpeima F, Mokhtari MJ, Doozandeh M, Jowkar Z, Yazdanshenas F. Comparison of cytotoxicity of new nanohybrid composite, giomer, glass ionomer and silver reinforced glass ionomer using human gingival fibroblast cell line. J Clin Pediatr Dent 2017;41:368-373.ArticlePubMedPDF
- 27. de Souza Costa CA, Hebling J, Garcia-Godoy F, Hanks CT. In vitro cytotoxicity of five glass-ionomer cements. Biomaterials 2003;24:3853-3858.ArticlePubMed
- 28. Sun J, Weng Y, Song F, Xie D. In vitro responses of human pulp cells and 3T3 mouse fibroblasts to six contemporary dental restoratives. J Biomed Sci Eng 2011;4:18-28.
- 29. Hebling J, Giro EM, Costa CA. Human pulp response after an adhesive system application in deep cavities. J Dent 1999;27:557-564.ArticlePubMed
- 30. de Souza Costa CA, Hebling J, Scheffel DL, Soares DG, Basso FG, Ribeiro AP. Methods to evaluate and strategies to improve the biocompatibility of dental materials and operative techniques. Dent Mater 2014;30:769-784.ArticlePubMed
- 31. Souza PP, Aranha AM, Hebling J, Giro EM, de Souza Costa CA. In vitro cytotoxicity and in vivo biocompatibility of contemporary resin-modified glass-ionomer cements. Dent Mater 2006;22:838-844.PubMed
- 32. Gallorini M, Cataldi A, di Giacomo V. HEMA-induced cytotoxicity: oxidative stress, genotoxicity and apoptosis. Int Endod J 2014;47:813-818.PubMed
- 33. Stanislawski L, Daniau X, Lauti A, Goldberg M. Factors responsible for pulp cell cytotoxicity induced by resin-modified glass ionomer cements. J Biomed Mater Res 1999;48:277-288.ArticlePubMed
- 34. de Souza Costa CA, Ribeiro AP, Giro EM, Randall RC, Hebling J. Pulp response after application of two resin modified glass ionomer cements (RMGICs) in deep cavities of prepared human teeth. Dent Mater 2011;27:e158-e170.ArticlePubMed
- 35. Modena KC, Calvo AM, Sipert CR, Colombini-Ishikiriama BL, Dionísio TJ, Navarro MF, et al. Molecular response of pulp fibroblasts after stimulation with pulp capping materials. Braz Dent J 2020;31:244-251.ArticlePubMed
REFERENCES
Tables & Figures
REFERENCES
Citations

- Thermal Aging-Induced Alterations in Surface and Interface Topography of Bio-Interactive Dental Restorative Materials Assessed by 3D Non-Contact Profilometry
Zehra Güner, Gökçe Keçeci, Sadık Olguner, Hakan Çandar, Ayşenur Güngör Borsöken, Lezize Sebnem Turkun
Coatings.2026; 16(1): 53. CrossRef - Advanced Platelet-Rich Fibrin Plus Sealed Exclusively with Glass Ionomer Cement: Setting a New Standard for Healing, Aesthetics and Predictive Modelling in Regenerative Endodontics
Dubravka Turjanski, Dragutin Lisjak, Petra Bučević Sojčić, Jelena Valpotić, Tea Borojević Renić, Kristina Goršeta, Domagoj Glavina
Materials.2025; 18(18): 4421. CrossRef - The conventional glass ionomers – A forgotten paradigm
Shishir Singh
Journal of Conservative Dentistry and Endodontics.2024; 27(12): 1201. CrossRef
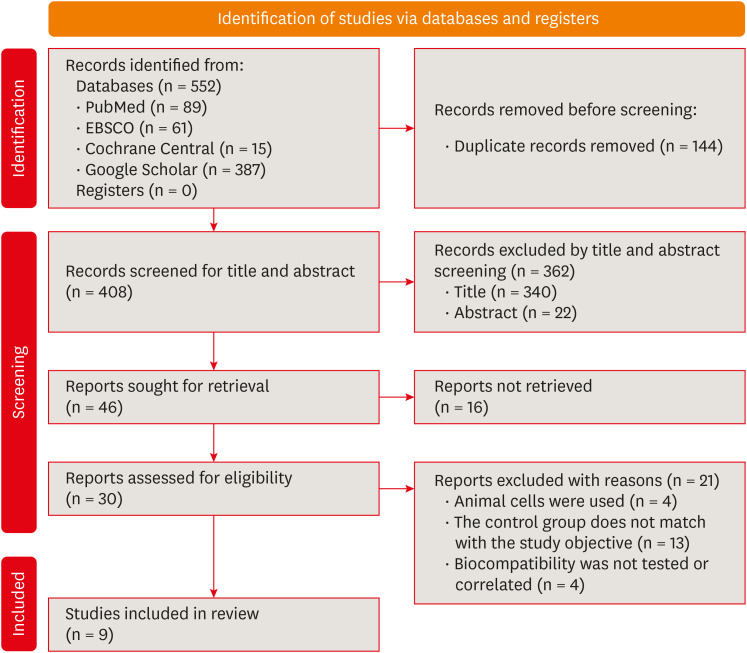
Figure 1
General characteristics of the included studies
| Author and year | Study design | Materials tested | Experimental model | Exposure duration | Cell line | Variables studies |
|---|---|---|---|---|---|---|
| Eskandarizadeh | Non-randomized controlled trial | RMGIC (Vivaglass) GIC (Ionocid) Calcium hydroxide (Dycal) | 30 human premolars | 5 and 30 days | None | Odontoblastic changes, inflammatory response, tertiary dentin formation, and presence of microorganisms |
| Ribeiro | Non-randomized controlled trial | RMGIC (Riva LC) GIC (Riva SC) Calcium hydroxide (Dycal) | 26 human premolars + 4 controls | 7 and 30 days | None | Inflammatory reaction, tissue disorganization, reactionary dentin formation, and bacteria |
| Mousavinasab | Non-randomized controlled trial | RMGIC (Vivaglass) GIC (Chem bond Superior) Calcium hydroxide (Dycal) | 55 human premolars | 7, 30 and 60 days | None | Odontoblastic changes, inflammatory cell infiltration, and reactionary dentin formation |
| Leyhausen | RMGICs (Ionoseal, Vitrebond, Compoglass) and GIC (Ketac Fil) | HGF | 48 hours | Cytotoxicity | Morphology and growth characteristics by PCM | |
| Rodriguez | RMGIC (Vitrebond) GIC (Ketac Molar) | HGF | 72 hours | Cytotoxicity | Morphological changes by PCM and LDH | |
| Mohd Zainal Abidin | Fuji IX GPExtra | SHED | 72 hours | Cytotoxicity | Cell viability percentage and IC50 | |
| Fuji II LC | ||||||
| Koohpeima | Fuji II | HGF | 24 hours | Cytotoxicity | percentage of cell viability of HGF at 25, 50, 75 and 100% | |
| Fuji II LC | ||||||
| de Souza Costa | Fuji IX, Ketac Molar | Human odontoblast cell line (MDPC-23) | 72 hours | Cytotoxicity | Cell number, cell morphology, cell metabolism | |
| Vitrebond, Vitremer, Fuji II LC | ||||||
| Sun | Fuji II, Fuji II LC, Vitrmer | Human pulp cells, 3T3 mouse fibroblast | 1 and 3 days | Cytotoxicity | Cell number, cell morphology and cell metabolism |
RMGIC, resin modified glass ionomer cement; GIC, glass ionomer cement; IC50, half maximal inhibitory concentration; HGF, human gingival fibroblast; PCM, phase contrast microscopy; LDH, lactate dehydrogenase release; SHED, stem cell of human exfoliated deciduous teeth.
Biocompatibility and cytotoxicity of conventional GIC and RMGIC
| Author and year | Cytotoxicity test used | Results for conventional GICs | Results for resin modified GICs | Author’s conclusion | ||
|---|---|---|---|---|---|---|
| Leyhausen | DNA Intercalating fluorochrome assay | Ketac Fil Applicap | Vitrebond | Light cure GICs revealed cytotoxic effects when compared to conventional GIC which had no or slight alterations in cell lines. | ||
| - Growth of the primary HGF: Day 1: 107 ± 19.5, Day 9: 103 ± 4 | - Growth of the primary HGF: Day 1: 15.7 ± 13.9, Day 9: 68.8 ± 6.8 | |||||
| Rodriguez | PCM, LDH assay, EPXMA analysis | Ketac Molar | Vitrebond | Morphological, biochemical, and micro-analytical indicators suggested that RMGIC causes greater alteration that points towards necrosis as compared to conventional GIC. | ||
| - Number of fibroblasts: 52.2% ± 20.4% | - Number of fibroblasts: 3.9% ± 5% | |||||
| - LDH release: 11.04% ± 21.69% | - LDH release: 38.46% ± 7.29% | |||||
| - Intracellular levels (Na): 85.79 ± 58.03 mmol/kg | - Intracellular elements levels (Na): 187.03 ± 113.11 mmol/kg | |||||
| - Intracellular levels (K): 272.72 ± 75.69 mmol/kg | - Intracellular elements levels (K): 186.74 ± 132.06 mmol/kg | |||||
| - Intracellular elements levels (Cl): 115.89 ± 75.69 mmol/kg | - Intracellular elements levels (Cl): 153.12 ± 57.28 mmol/kg | |||||
| Mohd Zainal Abidin | MTT assay | Fuji IX GPExtra | Fuji II LC | RMGIC exhibited cytotoxic effect on SHED as well as the least favorable cell viability among all the groups. | ||
| IC50 = 45 mg/mL | IC50 = 31.2 5 mg/mL | |||||
| Koohpeima | MTT assay | Fuji II | Fuji II LC | Study showed that cytotoxic effect of conventional GIC was significantly lesser as compared to other modified GICs. | ||
| - Percentage cell viability: at 25% = 100.80% ± 8.17%, at 50% = 122.64% ± 3.76%, at 75% = 125.15% ± 3.92%, and at 100% = 134.86% ± 0.65% | - Percentage cell viability: at 25% = 98.45% ± 7.24%, at 50% = 102.50% ± 6.16%, at 75% = 5.41% ± 9.16%, at 100% = 112.29% ± 3.85% | |||||
| de Souza Costa | MTT assay, SEM | Fuji IX, Ketac Molar | Vitrebond, Vitremer, Fuji II LC | Study concluded that Vitremer and Vitrebond (RMGIC) were more cytopathic than Fuji IX GP and Ketac Molar. | ||
| - Reduction in cell metabolism: Fuji IX = 40.3%, Ketac Molar = 42.5% | - Reduction in cell metabolism: Vitrebond = 79.1%, Vitremer = 83.9%, Fuji II LC = 53.75% | |||||
| - Reduction in cell number: Fuji IX = 29.5%, Ketac Molar = 32.5% | - Reduction in cell number: Vitrebond = 74.5%, Vitremer = 75.5%, Fuji II LC = 45.5% | |||||
| Sun | MTT assay, WST-1 assay | Fuji II | Fuji II LC, Vitremer | Study revealed that Fuji II and Fuji II LC are not cytotoxic to human pulp cells but Vitremer is very cytotoxic. Cytotoxicity was dose-dependent. | ||
| - Cell viability: Day 1: 100.3% ± 6.3%, Day 3: 98.8% ± 7.8% | - Cell viability: Day 1: 88.0% ± 11%, Day 3: 105.9% ± 10.3% | |||||
Values are in mean ± standard deviation.
GIC, glass ionomer cement; RMGIC, resin modified glass ionomer cement; HGF, human gingival fibroblast; PCM, phase contrast microscopy; LDH, lactate dehydrogenase release; EPXMA, Electron Probe Microanalyzer; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, IC50, half maximal inhibitory concentration; SHED, stem cell human exfoliated deciduous teeth; SEM, scanning electron microscopy; WST-1, Water-Soluble Tetrazolium 1.
Histological response of pulp to RMGIC and conventional GIC
| Histological events | Material | 5–7 days | 30 days | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No | Mild | Moderate | Severe | No | Mild | Moderate | Severe | ||||
| Ribeiro | |||||||||||
| Inflammatory response | RMGIC | 1 | 3 | 1 | 0 | 3 | 2 | 0 | 0 | 10 | |
| GIC | 2 | 3 | 0 | 0 | 5 | 0 | 0 | 0 | 10 | ||
| Tissue disorganization | RMGIC | 1 | 4 | 0 | 0 | 3 | 2 | 0 | 0 | 10 | |
| GIC | 2 | 3 | 0 | 0 | 4 | 1 | 0 | 0 | 10 | ||
| Reactionary dentin formation | RMGIC | 4 | 1 | 0 | 0 | 5 | 0 | 0 | 0 | 10 | |
| GIC | 5 | 0 | 0 | 0 | 4 | 1 | 0 | 0 | 10 | ||
| Bacteria | RMGIC | 5 | 0 | 0 | 0 | 3 | 2 | 0 | 0 | 10 | |
| GIC | 5 | 0 | 0 | 0 | 4 | 1 | 0 | 0 | 10 | ||
| Eskandarizadeh | |||||||||||
| Inflammatory response | RMGIC | 2 | 2 | 1 | 0 | 3 | 2 | 0 | 0 | 10 | |
| GIC | 2 | 2 | 0 | 1 | 3 | 1 | 0 | 1 | 10 | ||
| Odontoblastic changes | RMGIC | 0 | 3 | 2 | - | 2 | 1 | 2 | - | 10 | |
| GIC | 1 | 3 | 1 | - | 3 | 1 | 0 | - | 10 | ||
| Mousavinasab | |||||||||||
| Inflammatory cell infiltration | RMGIC | 0 | 2 | 6 | 0 | 3 | 2 | 0 | 0 | 13 | |
| GIC | 2 | 1 | 4 | 0 | 2 | 2 | 2 | 0 | 13 | ||
| Odontoblastic changes | RMGIC | 3 | 1 | 4 | 0 | 2 | 1 | 2 | 0 | 13 | |
| GIC | 2 | 2 | 3 | 0 | 2 | 2 | 2 | 0 | 13 | ||
| Reactionary dentin formation | RMGIC | 8 | 0 | 0 | 0 | 2 | 3 | 0 | 0 | 13 | |
| GIC | 7 | 0 | 0 | 0 | 5 | 1 | 0 | 0 | 13 | ||
RMGIC, resin modified glass ionomer cement; GIC, glass ionomer cement.
Risk of bias/quality assessment for non-randomized clinical trials using MINORS tool
| Study | A clearly stated aim | Inclusion of consecutive patients | Prospective collection of data | Endpoints appropriate to the aim of the study | Unbiased assessment of the study endpoint | Follow-up period appropriate to the aim of the study | Loss to follow up less than 5% | Prospective calculation of the study size | Total |
|---|---|---|---|---|---|---|---|---|---|
| Eskandarizadeh | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 1 | 13 |
| Ribeiro | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 1 | 14 |
| Mousavinasab | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 1 | 14 |
MINOTRS, Methodological Index For Non-Randomized Studies.
Measurement of the risk of bias/quality assessment of in vitro studies (histopathological studies) with the modified ARRIVE and CONSORT scale (acceptability range 21–28).
| Studies | Title | Abstract | Introduction | Introduction | Methods: Study design | Methods: experimental procedures | Method: Sample size | Method: Statistical procedure | Result | Discussion | Potential conflicts | Publication | Total score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leyhausen | 1 | 2 | 3 | 2 | 2 | 2 | 2 | 3 | 2 | 1 | 1 | 0 | 22 |
| Mohd Zainal Abidin | 0 | 3 | 2 | 2 | 2 | 3 | 1 | 2 | 2 | 1 | 0 | 1 | 19 |
| Rodriguez | 1 | 2 | 3 | 1 | 2 | 3 | 2 | 3 | 3 | 1 | 1 | 1 | 23 |
| Koohpeima | 0 | 3 | 3 | 1 | 1 | 2 | 1 | 3 | 3 | 2 | 1 | 1 | 21 |
| de Souza Costa | 0 | 2 | 1 | 2 | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 1 | 15 |
| Sun | 1 | 1 | 2 | 2 | 3 | 1 | 2 | 3 | 2 | 1 | 1 | 21 |
ARRIVE, Animal Research: Reporting of In Vivo Experiments; CONSORT, Consolidated Standards of Reporting Trials.
RMGIC, resin modified glass ionomer cement; GIC, glass ionomer cement; IC50, half maximal inhibitory concentration; HGF, human gingival fibroblast; PCM, phase contrast microscopy; LDH, lactate dehydrogenase release; SHED, stem cell of human exfoliated deciduous teeth.
Values are in mean ± standard deviation.
GIC, glass ionomer cement; RMGIC, resin modified glass ionomer cement; HGF, human gingival fibroblast; PCM, phase contrast microscopy; LDH, lactate dehydrogenase release; EPXMA, Electron Probe Microanalyzer; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, IC50, half maximal inhibitory concentration; SHED, stem cell human exfoliated deciduous teeth; SEM, scanning electron microscopy; WST-1, Water-Soluble Tetrazolium 1.
RMGIC, resin modified glass ionomer cement; GIC, glass ionomer cement.
MINOTRS, Methodological Index For Non-Randomized Studies.
ARRIVE, Animal Research: Reporting of In Vivo Experiments; CONSORT, Consolidated Standards of Reporting Trials.

 KACD
KACD
 ePub Link
ePub Link Cite
Cite

