Abstract
-
Objectives
This study aimed to comparatively assess the histological response of the pulp toward alendronate and Biodentine in a direct pulp capping procedure.
-
Materials and Methods
Twenty-four anterior teeth from 6 New Zealand rabbits were used in this study. Firstly, all rabbits were anesthetized according to their weight. Class V cavities were prepared on the buccal surfaces of anterior teeth. A pin-point exposure of the pulp was then made using a small, sterile round carbide bur and bleeding was arrested with a saline-soaked, sterile cotton pellet. The teeth under study were divided into 2 groups (n = 12). The intentionally exposed pulp was capped with alendronate (Group 1) and Biodentine (Group 2), correspondingly. After 30 days, all rabbits were euthanized; the teeth under study were extracted and taken up for histological analysis.
-
Results
Biodentine showed an intact, very dense dentin bridge formation with a uniform odontoblast (OD) layer pattern and mild or absent inflammatory response whereas specimens capped with alendronate demonstrated a dense dentin bridge formation with non-uniform OD layer pattern and mild to moderate inflammatory response.
-
Conclusions
Biodentine showed more biocompatibility than alendronate. However, alendronate can initiate reparative dentin formation and may be used as an alternative pulp capping agent.
-
Keywords: Alendronate; Pulp capping; Reparative dentin
INTRODUCTION
The pulp - dentin complex is a dynamic tissue as a unit and reparative dentin formation takes place whenever a minor dental injury occurs. This injury could be caused by predominant variables such as carious lesions, iatrogenic processes, or trauma. In direct pulp capping, pulp vitality is preserved by enclosing the exposed pulp surface with a therapeutic agent [
1].
Researchers have long sought an appropriate agent that could promote reparative dentinogenesis and pulpal healing when kept in contact with exposed pulp. An ideal pulp capping agent should be biocompatible and have superior sealing ability thereby preventing bacterial leakage and enabling dense dentin bridge (DB) formation [
2]. Calcium hydroxide was the most preferred material for vital pulp therapy but had certain disadvantages such as pulpal necrosis, poor sealability, and the presence of tunnel defects in the reparative dentin. On histological examination, it was found that the pulpal response to calcium hydroxide had shown an intense inflammatory reaction due to its irritant (highly alkaline) nature. In Biodentine, there was minimal or no inflammation because of its biocompatibility and potential for cell recruitment. Also, the hydroxyapatite crystals at the surface of Biodentine improve sealability, preventing microleakage and pulpal inflammation [
3,
4]. Later, mineral trioxide aggregate (MTA) was developed to overcome these drawbacks [
5]. It had better and accelerated reparative dentin formation, and a greater success rate when compared to calcium hydroxide [
6,
7]. However, its disadvantages include a longer setting time and poor handling characteristics. This led to the evolution of an alternative material that exhibited better mechanical properties [
8].
Biodentine, an improved calcium silicate-based material is popularly used as a second-generation pulp capping material. It is biomimetic in nature, has a shorter setting time, better sealing ability, and the ability to promote bio-mineralization [
9]. Alendronate, a bisphosphonate was also shown to stimulate dentin formation by odontoblasts (ODs) [
10]. It is available for clinical use to treat osteoporosis [
11]. Furthermore, recent studies have expanded its use in replantation of teeth, regeneration of defects in peri-implantitis, prevention of root resorption, and apexification [
12,
13]. Alendronate indirectly decreases bone resorption by inducing osteoclast apoptosis, increasing osteoblast synthesis and maintaining bone balance. It upregulated the expression of osteoblast differentiation–associated genes like osteocalcin, osterix and runt-related transcription factor 2 (RUNX2) [
14]. Alendronate also inhibited the production of osteoclastogenic and inflammatory cytokine interleukin-6, thereby inhibiting bone resorption in periapical lesions of ovariectomized rats [
15]. It enhances bone density and type 1 collagen accumulation [
16]. Igarashi
et al. [
17] analyzed the effects of 3 bisphosphonates on clonal osteoblast-like cell proliferation, alkaline phosphatase (ALP) activity, prostaglandin E2 production, and mineralization. The results revealed that ALP activity increased, prostaglandin E2 synthesis decreased, and mineralization by the cells was accelerated. Thus, it was determined that alendronate improved osteoblastic function at low concentrations. According to Sommercorn
et al. [
10] alendronate promoted dentin formation
in vitro at a concentration of 10
−9 M. Another study by Cengiz
et al. [
18] found that alendronate showed hard tissue formation in pulpotomized rat molars. However, to date, no studies have compared the efficacy of alendronate and Biodentine as pulp capping agents. The purpose of this study was to histologically compare and assess the pulpal response towards alendronate and Biodentine as direct pulp capping material. The hypothesis tested was that the pulpal response to alendronate does not significantly differ from that of Biodentine.
MATERIALS AND METHODS
Rabbit model
For ethical reasons, studies involving pulp capping are initially performed in animal models and the application of pulp capping material is performed under general anesthesia. The study protocol was authorized by the Institutional Animal Ethics Committee (IAEC) in Saveetha Dental College, Saveetha Institute of Medical and Technical Sciences, Chennai, India (approval No. BRULAC/SDCH/SIMATS/IAEC/01-2023/09). Twenty-four teeth from 6 New Zealand rabbits i.e. 1 year-old male white rabbits, were used in this study. All the rabbits were anesthetized using xylazine 10 mg/kg intramuscularly (Xylaxin; Indian Immunologicals, Hyderabad, TS, India) and ketamine 70 mg/kg intraperitonially (iKet injection; Bharat Parenterals Ltd, Vadodara, GJ, India), according to their weight.
Sample size estimation was done using the nMaster software version 2.0 (Department of Biostatistics, CMC Vellore, Vellore, TN, India; 2016). Considering the difference in the percentage of intact DB formation in Biodentine group and other study groups was 52% [
1], the power of the study at 80% and the alpha error at 5%, the total sample size needed is 24. The 24 teeth were divided into 2 groups, namely alendronate (AL) and Biodentine (BIO), with 12 teeth per group.
Lignocaine (xylocaine 2%, Lignocaine Hydrochloride Injection IP; American Remedies Healthcare Pvt. Ltd., Mumbai, India) was given for local anesthesia. Local and regional anesthetics are an essential supplement to anesthesia. They can completely block the transmission of nociceptive impulses, reducing both intraoperative nociception as well as postoperative pain, while lowering the possibility of side effects linked to systemic drug boluses [
19]. Class V cavities were made on the buccal surfaces of the upper and lower anterior teeth. A small, sterile carbide round bur (ISO No. 001/010; MANI, Utsunomiya, Japan) attached to a slow-speed hand-piece under a coolant was used to make a pin-point pulp exposure of 1 mm (diameter of round bur head) for all the 24 teeth. Bleeding was arrested using a saline-soaked, sterile cotton pellet, and the cavities were disinfected using 2.5% sodium hypochlorite for 30 seconds followed by saline irrigation [
20]. The prepared tooth was isolated with cotton rolls. The 24 specimens were divided into 2 groups, each containing 12 teeth: Group 1, alendronate (AL) and Group 2, Biodentine (BIO), after which the intentionally exposed pulp is capped respectively. Alendronate tablets (sodium alendronate tablets IP 70 mg, osteofos 70; Cipla, Mumbai, India) were powdered using a mortar and pestle. The alendronate powder was mixed with saline on a mixing pad using a plastic mixing spatula to obtain a wet, sand-like consistency, similar to MTA. The Biodentine capsule (Septodont, Saint-Maur-des-Fossés, France) was triturated for thirty seconds as per the manufacturer’s instructions with an amalgamator to achieve a uniform mix of creamy consistency. Then, the prepared mix was directly applied to the exposed pulp using a sterile plastic filling instrument in corresponding groups. A sterile straight explorer tip was used to confirm the initial set of the materials. Restorative glass ionomer cement (Ketac Universal; 3M ESPE, Seefeld, Germany) in putty consistency was mixed and placed over both the experimental pulp capping agents (
Figure 1). At the end of thirty days, all the rabbits were euthanized using CO
2 inhalation, teeth under study were extracted and histological analysis was done
[
6,
21].
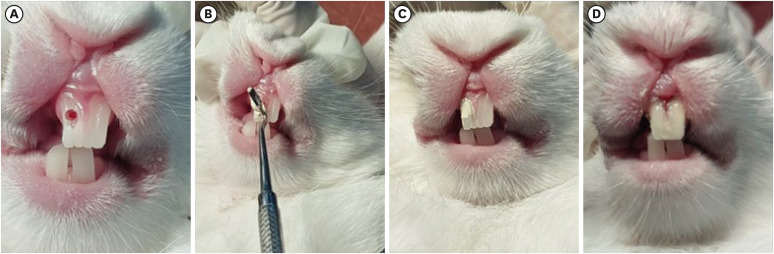
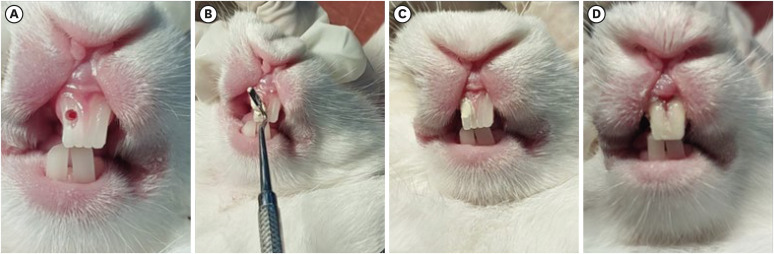
Figure 1
Direct pulp capping procedure performed on rabbit teeth. (A) Mechanical exposure of the pulp was made using a sterile, round carbide bur with adequate coolant. (B) Experimental pulp capping agents i.e. BIO and AL groups applied directly over the exposed pulp using a sterile hand filling instrument. (C) Image showing the agent completely covering the exposed pulp. (D) Restorative Glass ionomer cement is used to cover the pulp capping material.
BIO, Biodentine; AL, alendronate.
Histological evaluation and statistical analysis
The extracted teeth were placed in a sterile, plastic specimen container with 10% formalin for 4 days to allow tissue fixation. The teeth were decalcified in 10% formic acid solution for 4 weeks, sectioned at 5 µm thickness using a microtome (Leica Biosystems, Nußloch, Germany), stained with hemotoxylin-eosin, and examined under an optical microscope (CH 20i; Olympus, Mumbai, MH, India) [
1,
2]. The pulpal exposure area and the tissues around it were taken into account. Pulp response was assessed and shown by the revised criteria of Faraco Junior and Holland (
Table 1) [
22]. A score of 1–4 was given for each section where score 1 indicates the most desired result and score 4 indicates the least.
Table 1Scoring criteria for pulpal response
|
Criteria |
Sore |
|
1 |
2 |
3 |
4 |
|
Continuity of DB |
Intact DB |
Nonuniform DB involving > 1/2 of area of pulp exposure |
Signs of initial DB involving < 1/2 of the exposure site |
No DB evident |
|
Thickness of DB |
Very dense: 0.25 mm |
Dense: 0.1–0.25 mm |
Spare: < 0.1 mm |
No DB evident |
|
Intensity of pulpal inflammation |
Absence or sparsely located inflammatory cells |
Mild inflammation: < 10 cells |
Moderate inflammation: 10–25 cells |
Severe inflammation: > 25 cells |
|
OD layer pattern |
Uniform cell pattern |
Inconsistent cell pattern (OD and OD-like cells) |
Sparsely distributed OD-like cells |
No cells seen |
Statistical Package for Social Sciences (SPSS) for Windows version 22.0, released in 2013 (IBM Corp., Armonk, NY, USA) was used to perform the statistical analyses. Descriptive analysis includes the expression of histological parameters in terms of frequency and proportions for each group. Pearson’s χ
2 test was performed to compare data between the 2 experimental groups for all the 4 parameters mentioned in
Table 1. A
p value of < 0.05 was considered to be significant for the test.
RESULTS
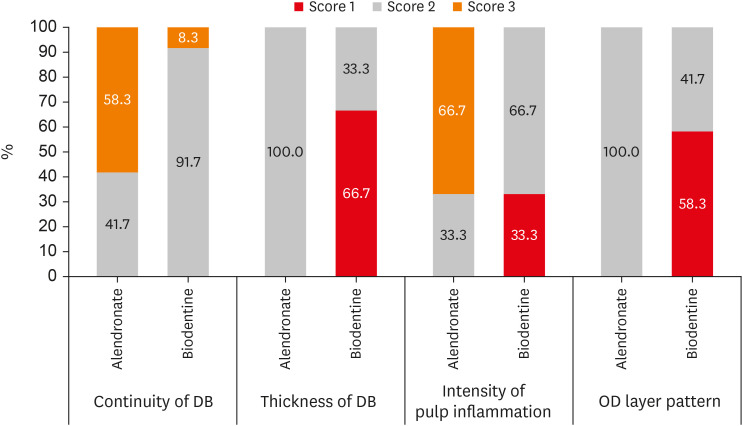
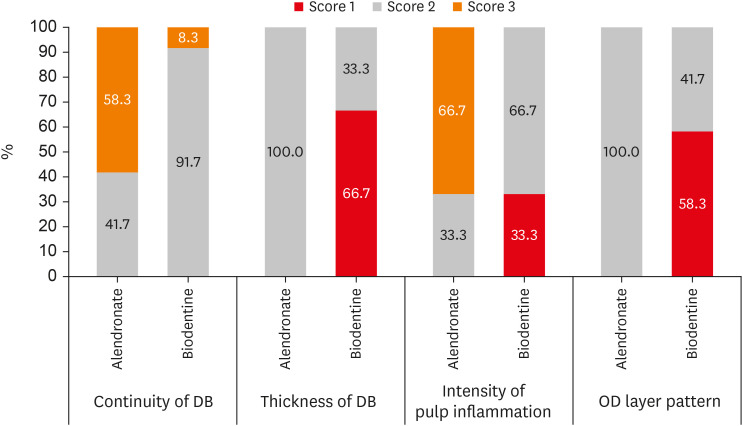
The histological evaluation demonstrated that 91.7% of specimens in the BIO group showed non-uniform DB involving greater than half area of pulp exposure, that is, score 2 as compared to the AL group where 41.7% of the specimens showed score 2 and 58.3% of the specimens showed signs of initial DB involving less than half of the exposure site, score 3. BIO group showed better DB formation than AL group which is statistically significant (p = 0.009).
The 66.7% of the specimens treated with Biodentine showed very dense DB, greater than 0.25 mm thick, that is, score 1 as compared to the AL group, which predominantly showed a thickness of density ranging between 0.1–0.25 mm, score 2 (100.0%). More dense DB formation was seen in BIO group than in AL group (
p = 0.001) (
Figure 2).
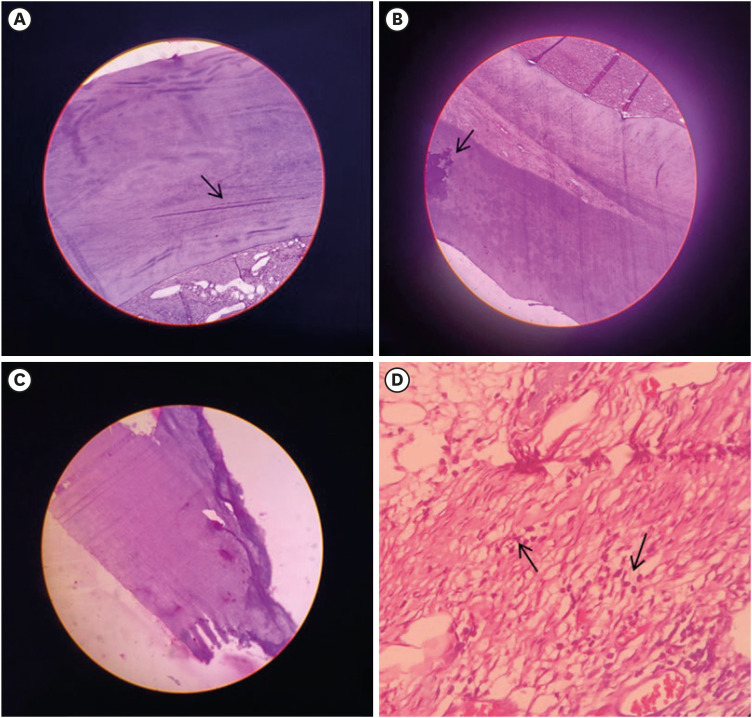
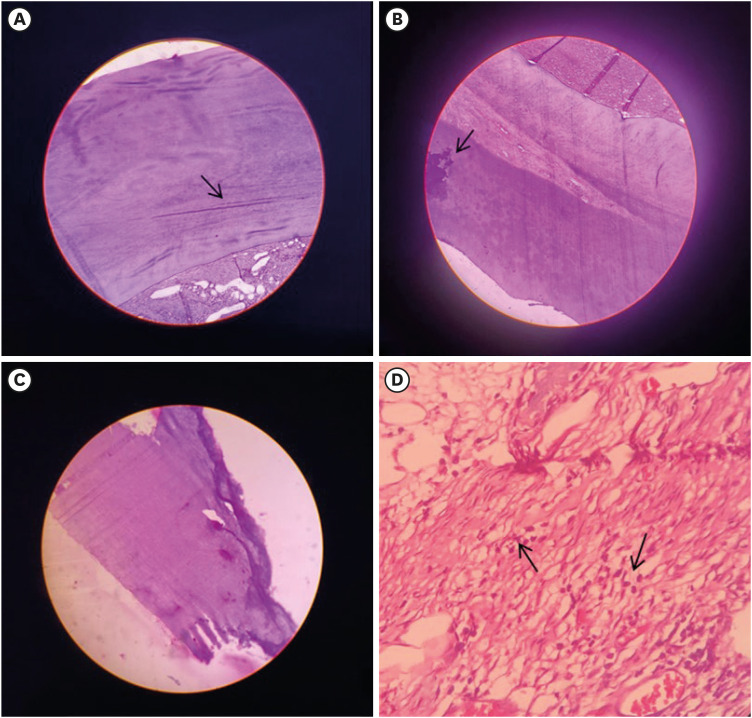
Figure 2
Image showing H&E-stained decalcified section at 10× magnifications. (A) AL group exhibited a mineralized component consisting of numerous microtubules (arrow) suggestive of dentin found along with collagenized stroma consisting of collagen fibers. (B) Mineralized component with eosinophilic zones resembling flecks of calcification (arrow) was seen in the AL group. (C) BIO group exhibited signs of non-uniform dentin bridge formation in a greater part of the exposed pulp region that is very dense in thickness. (D) BIO group exhibited mineralized components found in association with cellular connective tissue components (H&E staining 40×). The stroma shows collagen fibers lined by fibroblasts and a few, scattered inflammatory cells (arrow).
H&E, hematoxylin-eosin; AL, alendronate; BIO, Biodentine.
The BIO group showed an inflammatory cell count of fewer than 10 cells, score 2 (66.7%), which was followed by 33.3% specimens with absence or sparsely located inflammatory cells, score 1 as compared to the AL group, which predominantly showed moderate inflammation with an inflammatory cell count between 10 to 25 cells, score 3 (66.7%), followed by mild inflammation, score 2 (33.3%). A statistically significant difference of moderate inflammatory response was seen in the alendronate than BIO group which showed no or mild inflammatory response (p = 0.001).
The 58.3% of the specimens in the BIO group showed uniform cell patterns, score 1 as compared to the AL group, in which all the specimens showed inconsistent cell patterns (OD and OD-like cells) with score 2. A more uniform OD cell pattern was seen histologically in BIO group than in AL group with statistical significance (
p = 0.002) (
Table 2).
Table 2 Comparison of different study parameters between 2 groups using Pearson’s χ2 test
|
Variable |
Scores |
Alendronate |
Biodentine |
p value |
|
Continuity of DB |
Score 2 |
5 (41.7%) |
11 (91.7%) |
0.009*
|
|
Score 3 |
7 (58.3%) |
1 (8.3%) |
|
Thickness of DB |
Score 1 |
0 (0.0%) |
8 (66.7%) |
0.001*
|
|
Score 2 |
12 (100.0%) |
4 (33.3%) |
|
Intensity of pulp inflammation |
Score 1 |
0 (0.0%) |
4 (33.3%) |
0.001*
|
|
Score 2 |
4 (33.3%) |
8 (66.7%) |
|
Score 3 |
8 (66.7%) |
0 (0.0%) |
|
OD layer pattern |
Score 1 |
0 (0.0%) |
7 (58.3%) |
0.002*
|
|
Score 2 |
12 (100.0%) |
5 (41.7%) |
A graphical presentation of the quality of DB formation, degree of pulpal inflammation, and OD cell pattern is given in
Figure 3.
Figure 3
Distribution of different study parameters between 2 groups [21].
DB, dentin bridge; OD, odontoblast.
DISCUSSION
The tooth pulp is made up of immune cells, inflammatory cells and stem cells [
23]. Recruitment of these stem cells to the wound site occurs following damage to the pulp. They can promote mineralization by proliferating and differentiating into OD-like cells [
24]. The host’s immune system and the differentiating ODs have an intrinsic relationship. To recover from an injury, this intricately intertwined or linked mechanism between OD cells and inflammatory cells needs a suitable environment that is offered by pulp capping agents [
25].
Various studies have used rabbits or rodents as a reliable model to assess pulpal response to pulp capping materials, both histologically and physiologically. Normally, incisors are the teeth under study as they allow better isolation, greater accessibility to perform cavity preparation and mechanical exposure for placement of the capping agent onto the pulp. However, the incisors of rabbits are constantly growing which sets them apart from human teeth. Despite this, rabbit incisors have been proven to be a useful model that may be used to examine the potential reaction of the human pulp [
26,
27,
28]. In this study, a duration of 30 days was taken to assess the effect of the pulp capping agents on pulp tissues. An interval of 2–4 weeks is sufficient for rabbit studies as rapid growth of reparative dentine was observed. This is because, in healthy rabbits, incisor teeth are open-rooted and continuously growing [
2]. Moreover, in a study by Tran
et al. [
6] evaluating the capacity of tricalcium silicate-based cements to induce pulp healing in a rat pulp injury model, homogeneous DB formation occurred at the site of injury by 30 days. Dentinal tubules were present and the cells secreting the DB had odontoblastic characteristics. In another study by Cengiz
et al. [
18] evaluating the potential of alendronate to stimulate hard tissue formation in pulpotomized rat molars, 30-day alendronate specimens showed ODs and new dentin formation with continued dentin deposition on the lateral walls of the root canal.
Biodentine, also known as “dentine in a capsule,” is a biologically active dentine substitute that was introduced to overcome the disadvantages of calcium hydroxide and MTA. It enables a clinician to attain biomimetic mineralization within a deep carious lesion. Moreover, it stimulates tissue regeneration, and exhibits good mechanical properties, and favorable pulp response [
29]. Since Biodentine releases calcium ions, it results in successful pulp therapies and has been demonstrated to have the ability to encourage biomineralization [
30,
31]. The specimens treated with Biodentine showed a score of 1 for DB formation. This is due to its capability to stimulate the release of transforming growth factor-β. This growth factor helps in the migration and differentiation of pulp cells to the target area [
32]. The increased stimulation of mineralization markers like ALP, osteopontin (OPN), and RUNX2 during the pulp response to Biodentine may be responsible for the mineralization foci seen around the pulp capping material [
31]. Similarly, Jung
et al. [
33] assessed Biodentine’s ability to induce mineralization and found that there was an increase in the expression of dentin sialophosphoprotein and dentin phosphoprotein 1. Tran
et al. [
6] noted that the dentin formed was tubular with well-arranged OD and OD-like cells. Most of the specimens with Biodentine capping had a score of 1 for pulpal inflammation. This could be attributed to Biodentine’s ability to influence the response of transient receptor potential ankyrin 1 (TRPA1) in OD-like cells, by initiating p38 mitogen-activated protein kinase signals. According to El Karim
et al. [
34], Biodentine was able to lessen the expression of TRPA1 produced by tumor necrosis factor-α, a channel that activates the signaling pathways for pain and inflammation.
Alendronate inhibited bone resorption and promoted new bone formation [
35,
36,
37]. It is currently the most potent bisphosphonate available to treat osteoporosis. This is because alendronate becomes attached to the hydroxyapatite crystals in bone and binds to resorbed surfaces, especially those under active osteoclastic resorption. Chemically, it is linked to inorganic pyrophosphate, an endogenous regulator responsible for bone turnover [
38,
39]. Normal bone tissue grows throughout treatment and the bone matrix gets deposited with alendronate. It has been locally administered in animals for alveolar bone resorption after mucoperiosteal flap surgery [
40,
41,
42]. Sommercorn
et al. [
10] found that alendronate had the potential to stimulate odontoblastic activity resulting in dentin formation. In concurrence with the above study, alendronate showed a score of 2 for DB formation. Rothbarth
et al. [
43] examined the effects of alendronate on the luxated molars of young rats. Immunolabelling of the surface of Howship’s lacunae showed the presence of OPN in the root dentin of luxated alendronate specimens. OPN, a non-collagenous protein is produced during dentinogenesis by ODs but only trace amounts are found in the dentinal matrix following mineralization.
In this study, alendronate encouraged dentin formation in ODs, which have similar features to osteoblasts [
18]. Score 2 and score 3, that is mild to moderate inflammation of the pulp was seen in all the alendronate specimens. This could be because of its acidic nature [
44]. None of the specimens in our study showed score 4, severe inflammation. While inflammation promotes healing and DB formation, it should not lead to necrosis or apoptosis [
1]. However, more research is needed to determine the exact mechanism by which alendronate forms DB.
Statistically, the BIO group showed minimal inflammation when compared to the AL group. The DB formation was intact in most of the specimens of the BIO group whereas it was non-uniform for the AL group. The thickness of the DB was very dense for BIO group than AL group. According to Laurent
et al. [
32], Biodentine particles were trapped in the newly formed foci and mineralization in the form of osteodentin was seen. Tricalcium silicate is one of the primary ingredients of Biodentine, and the presence of silicon and calcium ions may stimulate cell proliferation and differentiation [
31]. The OD layer pattern was more uniform in the BIO group when compared to the AL group. The null hypothesis was rejected since the pulpal response to alendronate differed from that of Biodentine. It must be taken into consideration that even with significantly different values between the 2 groups, none of the specimens showed loss of vitality or an absence of dentin-bridge. Thus, the results of this study are in accordance with a study by Cengiz
et al. [
18] who stated that alendronate is able to preserve pulp vitality and promote hard tissue formation.
Direct pulp capping procedure is a potentially effective treatment option for teeth with pulpal exposure. Since alendronate can form reparative dentin, it can be used as a viable alternative for pulp capping procedures. However, alendronate needs certain modifications to improve its biocompatibility, handling, mechanical properties, and antibacterial activity.
Dental research frequently uses animal models for vital pulp therapy as the cellular structures and tooth morphology of animals are similar to those of humans. Nonetheless, the majority of research has been carried out on sound, uninfected teeth, which makes it challenging to accurately evaluate the inflammatory shift following vital pulp therapy [
45]. One of the drawbacks of this study was the use of sterilized teeth. In a clinical setting, direct pulp capping is usually done on a carious tooth. Therefore, it is possible that the study’s results may not be applicable to decayed teeth. Some of the other limitations were that rabbit incisor teeth are open-rooted and growing consistently. Thus, the pulp capping materials could not be placed for evaluation for more than 4 weeks. The grainy consistency of alendronate was difficult to handle. Also, alendronate does not have any antibacterial effect of its own. Future studies can consider performing vital pulp therapy in caries-induced pulpitis models and using various vehicles like poly lactic-co-glycolic acid. Other bioactive materials such as hydroxyapatite and nanoparticles may be added with alendronate to enhance its bioactivity, antibacterial efficacy, and mechanical properties.
Although there are studies in which alendronate has been used in concentrations of 10−9 M and 900 µg, there is no literature evidence of an ideal dosage to be used for vital pulp therapy in humans. Further research could be carried out to determine its ideal dose for stimulation of ODs and its effect on human dental pulp.
CONCLUSION
Biodentine was found to be more biocompatible when compared to alendronate. However, considering that alendronate promoted mineralization and showed an intact or non-uniform, dense DB formation, it could serve as a pulp capping agent in direct pulp capping procedures.
ACKNOWLEDGEMENT
The authors thank Dr. G. Mydhili, animal expert, Department of Anatomy, Saveetha Dental College, SIMATS for her support in animal care and Dr. Deekshitha, Department of Oral and Maxillofacial Pathology, Saveetha Dental College, SIMATS for her guidance with the histological examination.
-
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
-
Author Contributions:
Conceptualization: Kerena JC, Boopathi T, Manimaran S.
Data curation: Kerena JC.
Formal analysis: Kerena JC.
Funding acquisition: Kerena JC.
Investigation: Kerena JC.
Methodology: Kerena JC.
Project administration: Boopathi T.
Resources: Kerena JC.
Software: Kerena JC.
Supervision: Boopathi T, Manimaran S.
Validation: Boopathi T, Manimaran S.
Visualization: Kerena JC, Boopathi T, Manimaran S.
Writing - original draft: Kerena JC, Boopathi T, Manimaran S.
Writing - review & editing: Kerena JC, Boopathi T, Manimaran S, Sebeena M, Karthick K, Deepa NT.
REFERENCES
- 1. Khazane MK, Mahalaxmi S, Vidhya S. Histologic evaluation of dentin bridge formation by pachymic acid and Biodentine in human tooth culture model. Endodontology 2022;34:32-37.Article
- 2. Aljandan B, AlHassan H, Saghah A, Rasheed M, Ali AA. The effectiveness of using different pulp-capping agents on the healing response of the pulp. Indian J Dent Res 2012;23:633-637.ArticlePubMed
- 3. Kaul S, Kumar A, Jasrotia A, Gorkha K, Kumari S, Jeri SY. Comparative analysis of Biodentine, calcium hydroxide, and 2% chlorhexidine with resin-modified glass ionomer cement as indirect pulp capping materials in young permanent molars. J Contemp Dent Pract 2021;22:511-516.ArticlePubMed
- 4. Nowicka A, Lipski M, Parafiniuk M, Sporniak-Tutak K, Lichota D, Kosierkiewicz A, et al. Response of human dental pulp capped with Biodentine and mineral trioxide aggregate. J Endod 2013;39:743-747.ArticlePubMed
- 5. Sangwan P, Sangwan A, Duhan J, Rohilla A. Tertiary dentinogenesis with calcium hydroxide: a review of proposed mechanisms. Int Endod J 2013;46:3-19.ArticlePubMed
- 6. Tran XV, Gorin C, Willig C, Baroukh B, Pellat B, Decup F, et al. Effect of a calcium-silicate-based restorative cement on pulp repair. J Dent Res 2012;91:1166-1171.ArticlePubMedPDF
- 7. Hilton TJ, Ferracane JL, Mancl L. Northwest Practice-based Research Collaborative in Evidence-based Dentistry (NWP). Comparison of CaOH with MTA for direct pulp capping: a PBRN randomized clinical trial. J Dent Res 2013;92(7 Supplement):16S-22S.PubMed
- 8. Asgary S, Eghbal MJ, Parirokh M, Ghanavati F, Rahimi H. A comparative study of histologic response to different pulp capping materials and a novel endodontic cement. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2008;106:609-614.ArticlePubMed
- 9. Andrei M, Vacaru RP, Coricovac A, Ilinca R, Didilescu AC, Demetrescu I. The effect of calcium-silicate cements on reparative dentinogenesis following direct pulp capping on animal models. Molecules 2021;26:2725.ArticlePubMedPMC
- 10. Sommercorn LM, Di Fiore PM, Dixit SN, Koerber A, Lingen MW, Veis A. Effect of alendronate on immature human dental root explants. J Endod 2000;26:133-137.ArticlePubMed
- 11. Russell RG. Bisphosphonates: the first 40 years. Bone 2011;49:2-19.ArticlePubMed
- 12. Shibata T, Komatsu K, Shimada A, Shimoda S, Oida S, Kawasaki K, et al. Effects of alendronate on restoration of biomechanical properties of periodontium in replanted rat molars. J Periodontal Res 2004;39:405-414.ArticlePubMed
- 13. Meraw SJ, Reeve CM, Wollan PC. Use of alendronate in peri-implant defect regeneration. J Periodontol 1999;70:151-158.ArticlePubMed
- 14. Ma X, Xu Z, Ding S, Yi G, Wang Q. Alendronate promotes osteoblast differentiation and bone formation in ovariectomy-induced osteoporosis through interferon-β/signal transducer and activator of transcription 1 pathway. Exp Ther Med 2018;15:182-190.ArticlePubMed
- 15. Silva RA, Sousa-Pereira AP, Lucisano MP, Romualdo PC, Paula-Silva FW, Consolaro A, et al. Alendronate inhibits osteocyte apoptosis and inflammation via IL-6, inhibiting bone resorption in periapical lesions of ovariectomized rats. Int Endod J 2020;53:84-96.PubMed
- 16. Chavarry NG, Perrone D, Farias ML, Dos Santos BC, Domingos AC, Schanaider A, et al. Alendronate improves bone density and type I collagen accumulation but increases the amount of pentosidine in the healing dental alveolus of ovariectomized rabbits. Bone 2019;120:9-19.ArticlePubMed
- 17. Igarashi K, Hirafuji M, Adachi H, Shinoda H, Mitani H. Effects of bisphosphonates on alkaline phosphatase activity, mineralization, and prostaglandin E2 synthesis in the clonal osteoblast-like cell line MC3T3-E1. Prostaglandins Leukot Essent Fatty Acids 1997;56:121-125.ArticlePubMed
- 18. Cengiz SB, Batirbaygil Y, Onur MA, Atilla P, Asan E, Altay N, et al. Histological comparison of alendronate, calcium hydroxide and formocresol in amputated rat molar. Dent Traumatol 2005;21:281-288.ArticlePubMed
- 19. Grubb T, Lobprise H. Local and regional anaesthesia in dogs and cats: descriptions of specific local and regional techniques (Part 2). Vet Med Sci 2020;6:218-234.ArticlePubMedPMCPDF
- 20. Ballal NV, Duncan HF, Wiedemeier DB, Rai N, Jalan P, Bhat V, et al. 4-year pulp survival in a randomized trial on direct pulp capping. J Endod 2024;50:4-9.ArticlePubMed
- 21. Kim J, Song YS, Min KS, Kim SH, Koh JT, Lee BN, et al. Evaluation of reparative dentin formation of ProRoot MTA, Biodentine and BioAggregate using micro-CT and immunohistochemistry. Restor Dent Endod 2016;41:29-36.ArticlePubMedPMCPDF
- 22. Faraco Junior IM, Holland R. Histomorphological response of dogs’ dental pulp capped with white mineral trioxide aggregate. Braz Dent J 2004;15:104-108.ArticlePubMed
- 23. Kim SG, Zheng Y, Zhou J, Chen M, Embree MC, Song K, et al. Dentin and dental pulp regeneration by the patient’s endogenous cells. Endod Topics 2013;28:106-117.ArticlePubMedPMC
- 24. Galler KM, Weber M, Korkmaz Y, Widbiller M, Feuerer M. Inflammatory response mechanisms of the dentine-pulp complex and the periapical tissues. Int J Mol Sci 2021;22:1480.ArticlePubMedPMC
- 25. Aguilar P, Linsuwanont P. Vital pulp therapy in vital permanent teeth with cariously exposed pulp: a systematic review. J Endod 2011;37:581-587.ArticlePubMed
- 26. Hu CC, Zhang C, Qian Q, Tatum NB. Reparative dentin formation in rat molars after direct pulp capping with growth factors. J Endod 1998;24:744-751.ArticlePubMed
- 27. Orhan EO, Maden M, Senguüven B. Odontoblast-like cell numbers and reparative dentine thickness after direct pulp capping with platelet-rich plasma and enamel matrix derivative: a histomorphometric evaluation. Int Endod J 2012;45:317-325.ArticlePubMed
- 28. Likitpongpipat N, Sangmaneedet S, Klanrit P, Noisombut R, Krisanaprakornkit S, Chailertvanitkul P. Promotion of dental pulp wound healing in New Zealand white rabbits’ teeth by Thai propolis product. J Vet Dent 2019;36:17-24.ArticlePubMedPDF
- 29. About I. Biodentine™: properties and clinical applications. Cham: Springer Nature; 2022.
- 30. Minic S, Florimond M, Sadoine J, Valot-Salengro A, Chaussain C, Renard E, et al. Evaluation of pulp repair after Biodentine™ full pulpotomy in a rat molar model of pulpitis. Biomedicines 2021;9:784.ArticlePubMedPMC
- 31. Daltoé MO, Paula-Silva FW, Faccioli LH, Gatón-Hernández PM, De Rossi A, Bezerra Silva LA. Expression of mineralization markers during pulp response to Biodentine and mineral trioxide aggregate. J Endod 2016;42:596-603.ArticlePubMed
- 32. Laurent P, Camps J, About I. Biodentine™ induces TGF-β1 release from human pulp cells and early dental pulp mineralization. Int Endod J 2012;45:439-448.PubMed
- 33. Jung JY, Woo SM, Lee BN, Koh JT, Nör JE, Hwang YC. Effect of Biodentine and Bioaggregate on odontoblastic differentiation via mitogen-activated protein kinase pathway in human dental pulp cells. Int Endod J 2015;48:177-184.ArticlePubMed
- 34. El Karim IA, McCrudden MT, McGahon MK, Curtis TM, Jeanneau C, Giraud T, et al. Biodentine reduces tumor necrosis factor alpha-induced TRPA1 expression in odontoblast like cells. J Endod 2016;42:589-595.ArticlePubMed
- 35. Tsuchimoto M, Azuma Y, Higuchi O, Sugimoto I, Hirata N, Kiyoki M, et al. Alendronate modulates osteogenesis of human osteoblastic cells in vitro
. Jpn J Pharmacol 1994;66:25-33.PubMed
- 36. Sahni M, Guenther HL, Fleisch H, Collin P, Martin TJ. Bisphosphonates act on rat bone resorption through the mediation of osteoblasts. J Clin Invest 1993;91:2004-2011.ArticlePubMedPMC
- 37. Vitté C, Fleisch H, Guenther HL. Bisphosphonates induce osteoblasts to secrete an inhibitor of osteoclast-mediated resorption. Endocrinology 1996;137:2324-2333.ArticlePubMed
- 38. Selby P. Alendronate treatment for osteoporosis: a review of the clinical evidence. Osteoporos Int 1996;6:419-426.ArticlePubMedPDF
- 39. Masarachia P, Weinreb M, Balena R, Rodan GA. Comparison of the distribution of 3H-alendronate and 3H-etidronate in rat and mouse bones. Bone 1996;19:281-290.ArticlePubMed
- 40. De Almeida J, Ervolino E, Bonfietti LH, Novaes VC, Theodoro LH, Fernandes LA, et al. Adjuvant therapy with sodium alendronate for the treatment of experimental periodontitis in rats. J Periodontol 2015;86:1166-1175.ArticlePubMed
- 41. Binderman I, Adut M, Yaffe A. Effectiveness of local delivery of alendronate in reducing alveolar bone loss following periodontal surgery in rats. J Periodontol 2000;71:1236-1240.ArticlePubMed
- 42. Pradeep AR, Kanoriya D, Singhal S, Garg V, Manohar B, Chatterjee A. Comparative evaluation of subgingivally delivered 1% alendronate versus 1.2% atorvastatin gel in treatment of chronic periodontitis: a randomized placebo-controlled clinical trial. J Investig Clin Dent 2017;8:e12215.ArticlePDF
- 43. Rothbarth CP, Bradaschia-Correa V, Ferreira LB, Arana-Chavez VE. Effects of the bisphosphonate alendronate on molars of young rats after lateral luxation. Dent Traumatol 2014;30:415-422.ArticlePubMed
- 44. Ke J, Dou H, Zhang X, Uhagaze DS, Ding X, Dong Y. Determination of pKa values of alendronate sodium in aqueous solution by piecewise linear regression based on acid-base potentiometric titration. J Pharm Anal 2016;6:404-409.ArticlePubMedPMC
- 45. Huang H, Okamoto M, Watanabe M, Matsumoto S, Moriyama K, Komichi S, et al. Development of rat caries-induced pulpitis model for vital pulp therapy. J Dent Res 2023;102:574-582.ArticlePubMedPDF
 , Sekar Manimaran
, Sekar Manimaran , Joseline Charles Kerena
, Joseline Charles Kerena , Mathew Sebeena
, Mathew Sebeena , Kumaravadivel Karthick
, Kumaravadivel Karthick , Natesan Thangaraj Deepa
, Natesan Thangaraj Deepa





 KACD
KACD
 ePub Link
ePub Link Cite
Cite

