Articles
- Page Path
- HOME > Restor Dent Endod > Volume 49(2); 2024 > Article
- Review Article The prevalence of apical periodontitis in patients prior to hematopoietic cell transplantation: a systematic review
-
Letícia Tainá de Oliveira Lemes
 , Carolina Horn Troian-Michel
, Carolina Horn Troian-Michel , Theodoro Weissheimer
, Theodoro Weissheimer , Marcus Vinicius Reis Só
, Marcus Vinicius Reis Só
-
Restor Dent Endod 2024;49(2):e22.
DOI: https://doi.org/10.5395/rde.2024.49.e22
Published online: May 9, 2024
Department of Endodontics, School of Dentistry, Federal University of Rio Grande do Sul (UFRGS), Porto Alegre, RS, Brazil.
- Correspondence to Carolina Horn Troian-Michel, MSc. Department of Endodontics, School of Dentistry, Federal University of Rio Grande do Sul (UFRGS), 2492 Ramiro Barcelos Street, Porto Alegre, RS 90035-003, Brazil. carolinaendo@gmail.com
Copyright © 2024. The Korean Academy of Conservative Dentistry
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 2,099 Views
- 51 Download
Abstract
-
Objectives This systematic review addressed the question: “What is the prevalence of apical periodontitis in patients prior to hematopoietic cell transplantation?”
-
Materials and Methods A systematic search was conducted in MEDLINE/PubMed, Cochrane Library, Scopus, Web of Science, Embase, and Grey Literature Report. Eligibility criteria were based on the condition, content, and population strategy: the condition was the radiographic prevalence of apical periodontitis, the content comprised patients scheduled for hematopoietic stem cell transplantation, and the population consisted of adult and pediatric patients. The revised Risk of Bias in Nonrandomized Studies of Exposure tool was used to assess the quality of studies. The Grading Recommendations Assessments, Development, and Evaluation (GRADE) tool was used to assess the quality of evidence.
-
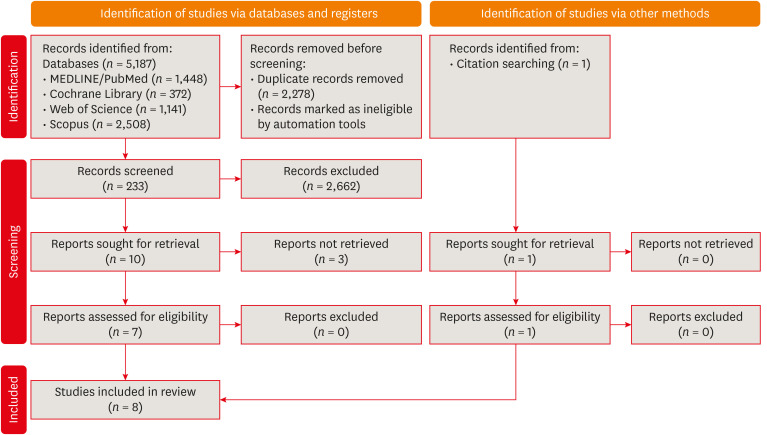
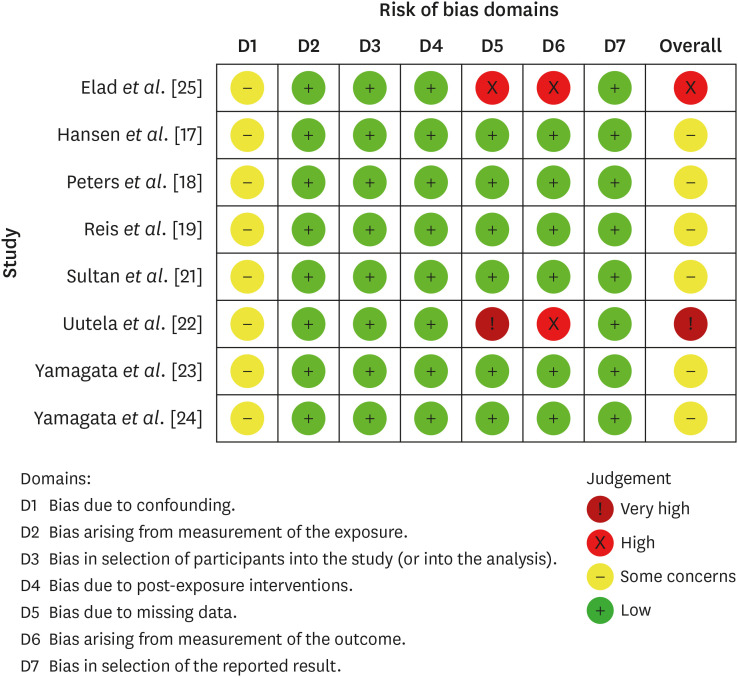
Results Eight studies were included in this review. The average number of patients with apical periodontitis was 15.65% (range, 2.1%–43.34%). One study was classified as having a very high risk of bias, 1 with a high risk of bias, and 6 with some concern for bias. GRADE analysis showed a very low certainty of evidence. Significant limitations concerning the absence of control over confounding variables were identified.
-
Conclusions With the caveat of the very low quality of evidence in the studies reviewed, there was a low to moderate prevalence of apical periodontitis in patients prior to undergoing hematopoietic cell transplantation.
INTRODUCTION
MATERIALS AND METHODS
- Condition (Co): prevalence of apical periodontitis as assessed radiographically;
- Context (Co): patients who were going to be treated with HSCT;
- Population (PoP): adult and pediatric patients.
1. Risk of bias due to confounding factors: the risk of bias was considered low when all possible confounding factors (e.g., the participants’ age, sex, and dental history) were controlled in the study design or statistical analysis; ‘some concerns’ for risk when confounding factors were partially controlled; high risk when no possible confounding factors were controlled; and very high risk when possible confounding factors were not even discussed.
2. Risk of bias arising from the measurement of exposure: the risk of bias was considered low when all of the participants had the same exposure level or status; some risk when some participants had different exposure levels but those differences did not seem to affect the outcome; high risk when exposure levels were associated with the outcome; and very high risk when exposure levels were not described.
3. Risk of bias in the selection of study participants: the risk of bias was considered low when all eligible participants were included in the study; some risk when participant selection might have affected the outcome; high risk when participant selection did affect the outcome; and very high risk when the selection process was not described.
4. Risk of bias due to postexposure interventions: the risk of bias was considered low when there were no postexposure interventions that might affect the outcome; some risk when postexposure interventions were not likely to affect the outcome; high risk when postexposure interventions could possibly affect the outcome; very high risk when postexposure interventions were directly related to the outcome.
5. Risk of bias due to missing data: the risk of bias was considered low when the outcome was accurately reported for all participants; some risk when some data were missing, but the missing data were not relevant to the outcome of the study; high risk when some relevant data were missing; and very high risk when several relevant data were missing.
6. Risk of bias arising from measurement of the outcome: the risk of bias was considered low when a valid method was used to assess apical periodontitis for all participants; some risk when a valid method was not used, although the method was well described; high risk when a valid method was not used and not well described; and very high risk when the method used was not described.
7. Risk of bias in selection of the reported result: the risk of bias was considered low when all cases of apical periodontitis were accurately reported; some risk when apical periodontitis was reported, but not described; high risk when the authors did not report the prevalence of apical periodontitis; and very high risk when information about apical periodontitis was missing.
1. Risk of bias: looking for design features and study methods that have been shown by empirical evidence to minimize the risk of bias.
2. Inconsistency: determining whether differences underlying the results of the studies are genuine (heterogeneity) or whether the variation in findings is compatible with chance alone (homogeneity).
3. Indirectness: looking for differences between the population of interest and those who have participated in other relevant studies.
4. Imprecision: focusing on the 95% confidence interval around the best estimate of the absolute effect.
5. Other considerations: publication bias, large magnitude of intervention effect, direction of plausible residual confounding, and dose-response gradient.
RESULTS
Flow diagram of the systematic literature search according to Preferred Reporting Items for Systematic reviews and Meta-Analyses 2020 guidelines.
Characteristics and main findings in a sytematic review to assess the prevalence of apical periodontitis prior to HSCT
| Authors | Study design | Number of participants evaluated | Age of participants | Sex of participants | Hematologic diagnoses | Diagnostic method used to diagnose apical periodontitis | Number of participants with apical periodontitis | Age of patients with apical periodontitis | Sex of patients with apical periodontitis | Main findings |
|---|---|---|---|---|---|---|---|---|---|---|
| Elad et al. [25] | Retrospective | Dental evaluation of 46 patients prior to HSCT between 1997 and 1998 (31 allogeneic, 15 autologous) | 6 to 63 years (mean 37 years) | 25 males, 21 females | Acute myelocytic leukemia 14, non-Hodgkins’s lymphoma 9, chronic myelocytic leukemia 9, acute lymphocytic leukemia 6, breast carcinoma 4, multiple myeloma 2, Hodgkin’s disease 1, multiple sclerosis 1 | • Clinical evaluation. | 9 patients (19.56%), 22 teeth | NI | NI | Data indicate a dense distribution of dental needs preceding HSCT, which accentuates the vital need for cooperation between hospital dentists and treating physicians. |
| • Radiographic examination (when necessary) (n = 27) – bite-wing 45.6%, panoramic 39.1%, single periapical X-ray 30.4%, full mouth periapical X-ray 10.9% | ||||||||||
| Hansen et al. [17] | Prospective | 350 patients prior to HSCT (207 autologous, 143 allogeneic) | 8 to 75 years (mean 54 years) | 207 males, 143 females | Multiple myeloma 104, non-Hodgkin’s lymphoma 99, Hodgkin’s lymphoma 28, leukemia 95, other 24 | • Digital periapical radiographs and, when necessary, selected digital periapical radiographs. | 68 patients (19.4%) | NI | NI | Although there was a high percentage of patients that showed moderate and high risk of odontogenic infection before HSCT (58.6%), only 0.57% of patients developed odontogenic complications. |
| Peters et al. [18] | Retrospective cohort | Dental charts of 276 adult patients who underwent BMT protocols between 1987 and 1991 (13 autogenous and 10 allogeneic) | NI | NI | Chronic myelogenous leukemia, non-Hodgkin’s lymphoma, acute myelogenous leukemia, chronic lymphocytic leukemia, myelodysplastic syndrome, and testicular and breast malignant conditions. | • Complete intraoral radiographic survey, panoramic radiograph, and hard and soft tissue examination. | 23 patients (8.33%) with 1 endodontically treated tooth presenting PE-PARL >1.5 mm | 25 to 58 years (mean age 41 years) | 15 males, 8 females | Nontreatment of PE-PARLs did not increase the incidence of infectious complications during BMT (neither increased systemic infection). |
| Reis et al. [19] | Prospective | 33 patients dental evaluated pre-allogeneic HCT in 2018 | 28.4 ± 16.37 years | 20 males, 13 females | Fanconi’s anemia 2, Sickle cell anemia 7, acute lymphocytic leukemia 7, severe aplastic anemia 3, acute myeloid leukemia 8, chronic myeloid leukemia 1, myelodysplastic syndrome 4, non-Hodgkin’s lymphoma 1 | • Clinical and periapical radiographic examination | 5 patients (15%) | NI | NI | Studied population had important incidence of dental pathologies and infectious conditions that could complicate during HCT. |
| Sultan et al. [21] | Retrospective | Records of 92 patients pre allogeneic HCT from 2007 to 2011 | 24 to 66 years (mean 48 years) | 44 males, 48 females | Acute myeloid leukemia | • Dental radiographs (full mouth series and panoramic), caries charting, pulp vitality testing in teeth with large restorations, and periodontal status assessment | 10 patients (10.87%) | NI | NI | Bacteremia with a potential oral source occurred in 12/92 patients (13%); of these, 11/12 (92%) patients developed bacteremia during HCT. |
| Uutela et al. [22] | Prospective cross‐sectional study | 143 adults allogeneic HSCT recipient patients from 2008 and 2016 | 21 to 58 years (mean 44.8 years) | 70 males, 73 females | Acute lymphoblastic leukemia 29, acute myeloid leukemia 49, chronic lymphocytic leukemia 8, chronic myeloid leukemia 5, myelodysplastic syndrome 10, Hodgkin’s lymphoma 3, myeloproliferative neoplasm 8, non-Hodgkin’s lymphoma 18, plasma cell dyscrasia 10, other diseases 3 | • Panoramic radiograph and DMFT index | 1 patient had fistula, 2 patients had symptomatic periapical process (2.1%) | NI | NI | Oral examinations prior to HSCT showed a higher prevalence of oral disorders in HSCT recipients than in healthy controls. |
| Yamagata et al. [23] | Prospective trial | Dental evaluation of 41 patients pre HSCT from 1998 to 2004 (allogeneic or autologous - not specified) | 17 to 58 years (mean 41.3 years) | 22 males, 19 females | Chronic myeloid leukemia 14, malignant lymphoma 7, acute myeloid leukemia 4, non-Hodgkin’s lymphoma 4, myelodysplastic syndrome 4, multiple myeloma 3, acute lymphoblastic leukemia 3, other malignancies 2 | • The dental status of all patients was evaluated by clinical and radiographic examination, including panoramic and occasional periapical films for symptomatic teeth. | 19 patients (46.34%), 43 teeth | NI | NI | Among 43 teeth with asymptomatic periapical periodontitis before HSCT, only 12 (apical radiolucencies larger than 5 mm) were treated (extraction or endodontic treatment). No conversions to an acute stage or infectious complications occurred in any patient. |
| Yamagata et al. [24] | Retrospective | Dental evaluation of 30 children prior to HSCT from 2000 to 2003 (allogeneic or autologous – not specified) | 2 to 18 years | 18 boys, 12 girls | Acute lymphocytic leukemia 20, acute myelocytic leukemia 2, other malignancies 8. | • Clinical examination and panoramic and/or dental radiographic evaluations. | 2 children (permanent teeth) (6.66%) | NI | NI | As a dental management program was adopted before HSCT, no odontogenic infections occurred during the immunosuppressive period. |
The quality assessment of included studies according to the Cochrane Collaboration common scheme for bias and the Risk of Bias in Non-randomized Studies - of Exposure tool.
Quality of evidence assessment for the studies included in a systematic review
DISCUSSION
CONCLUSIONS
-
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
-
Author Contributions:
Conceptualization: de Oliveira Lemes LT.
Data curation: Troian-Michel CH, Reis Só MV.
Formal analysis: Troian-Michel CH.
Investigation: de Oliveira Lemes LT, Troian-Michel CH.
Methodology: de Oliveira Lemes LT.
Supervision: Reis Só MV.
Visualization: Troian-Michel CH.
Writing - original draft: de Oliveira Lemes LT, Troian-Michel CH.
Writing - review & editing: Weissheimer T, Reis Só MV.
SUPPLEMENTARY MATERIAL
- 1. Sureda A, Bader P, Cesaro S, Dreger P, Duarte RF, Dufour C, et al. Indications for allo- and auto-SCT for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2015. Bone Marrow Transplant 2015;50:1037-1056.ArticlePubMedPDF
- 2. Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med 2006;354:1813-1826.ArticlePubMed
- 3. Elad S, Raber-Durlacher JE, Brennan MT, Saunders DP, Mank AP, Zadik Y, et al. Basic oral care for hematology-oncology patients and hematopoietic stem cell transplantation recipients: a position paper from the joint task force of the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO) and the European Society for Blood and Marrow Transplantation (EBMT). Support Care Cancer 2015;23:223-236.ArticlePubMedPDF
- 4. Lucas VS, Roberts GJ, Beighton D. Oral health of children undergoing allogeneic bone marrow transplantation. Bone Marrow Transplant 1998;22:801-808.ArticlePubMedPDF
- 5. Tibúrcio-Machado CS, Michelon C, Zanatta FB, Gomes MS, Marin JA, Bier CA. The global prevalence of apical periodontitis: a systematic review and meta-analysis. Int Endod J 2021;54:712-735.ArticlePubMedPDF
- 6. Haapasalo M, Udnæs T, Endal U. Persistent, recurrent, and acquired infection of the root canal system post‐treatment. Endod Topics 2003;6:29-56.Article
- 7. Nair PN. Pathogenesis of apical periodontitis and the causes of endodontic failures. Crit Rev Oral Biol Med 2004;15:348-381.PubMed
- 8. Gomes BP, Herrera DR. Etiologic role of root canal infection in apical periodontitis and its relationship with clinical symptomatology. Braz Oral Res 2018;32(Supplement 1):e69.ArticlePubMed
- 9. Graber CJ, de Almeida KN, Atkinson JC, Javaheri D, Fukuda CD, Gill VJ, et al. Dental health and viridans streptococcal bacteremia in allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transplant 2001;27:537-542.ArticlePubMedPDF
- 10. Page MJ, Higgins JP, Sterne JA. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane handbook for systematic reviews of interventions version 6.2. updated February 2021]. cited June 7, 2023]. Available from: https://training.cochrane.org/handbook.
- 11. Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid-Based Healthc 2015;13:147-153.ArticlePubMed
- 12. Munn Z, Stern C, Aromataris E, Lockwood C, Jordan Z. What kind of systematic review should I conduct? A proposed typology and guidance for systematic reviewers in the medical and health sciences. BMC Med Res Methodol 2018;18:5.ArticlePubMedPMCPDF
- 13. ROBINS-E Development Group. Risk of Bias in Non-randomized Studies - of Exposure (ROBINS-E). Launch version, 1 June 2022. updated June 1, 2022]. cited June 7, 2023]. Available from: https://www.riskofbias.info/welcome/robins-e-tool.
- 14. Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011;64:383-394.ArticlePubMed
- 15. Bogusławska-Kapała A, Struzycka I, Hałaburda K. Current attitudes to the elimination of infection foci from the oral cavity of adult patients qualified for allogeneic hematopoietic stem cell transplantation. Pol Merkuriusz Lek 2013;35:305-308.
- 16. Donker AE, van Merkesteyn JP, Bredius RG, van Weel-Sipman MH. Value of panoramic radiographs in paediatric pre-bone marrow transplantation oral evaluation. Int J Oral Maxillofac Surg 2002;31:170-172.ArticlePubMed
- 17. Hansen HJ, Estilo C, Owosho A, Solano AK, Randazzo J, Huryn J, et al. Dental status and risk of odontogenic complication in patients undergoing hematopoietic stem cell transplant. Support Care Cancer 2021;29:2231-2238.ArticlePubMedPDF
- 18. Peters E, Monopoli M, Woo SB, Sonis S. Assessment of the need for treatment of postendodontic asymptomatic periapical radiolucencies in bone marrow transplant recipients. Oral Surg Oral Med Oral Pathol 1993;76:45-48.ArticlePubMed
- 19. Reis TC, Bortolotti F, Innocentini LMAR, Ferrari TC, Ricz HMA, Cunha RLG, et al. Assessment of oral health condition in recipients of allogeneic hematopoietic cell transplantation. Hematol Transfus Cell Ther 2022;44:549-554.ArticlePubMedPMC
- 20. Skallsjö K, von Bültzingslöwen I, Hasséus B, Johansson JE, Öhman J, Raber-Durlacher JE, et al. Oral health in patients scheduled for hematopoietic stem cell transplantation in the Orastem study. PLoS One 2023;18:e0285615.ArticlePubMedPMC
- 21. Sultan AS, Zimering Y, Petruzziello G, Alyea EP 3rd, Antin JH, Soiffer RJ, et al. Oral health status and risk of bacteremia following allogeneic hematopoietic cell transplantation. Oral Surg Oral Med Oral Pathol Oral Radiol 2017;124:253-260.ArticlePubMed
- 22. Uutela P, Passweg J, Halter J, Weiger R, Waltimo T, Mauramo M. Common oral diseases in allogeneic haematopoietic stem cell transplantation (HSCT) recipients pre-HSCT. Eur J Haematol 2019;102:351-356.ArticlePubMedPDF
- 23. Yamagata K, Onizawa K, Yanagawa T, Hasegawa Y, Kojima H, Nagasawa T, et al. A prospective study to evaluate a new dental management protocol before hematopoietic stem cell transplantation. Bone Marrow Transplant 2006;38:237-242.ArticlePubMedPDF
- 24. Yamagata K, Onizawa K, Yoshida H, Yamagata K, Kojima Y, Koike K, et al. Dental management of pediatric patients undergoing hematopoietic stem cell transplant. Pediatr Hematol Oncol 2006;23:541-548.ArticlePubMed
- 25. Elad S, Garfunkel AA, Or R, Michaeli E, Shapira MY, Galili D. Time limitations and the challenge of providing infection-preventing dental care to hematopoietic stem-cell transplantation patients. Support Care Cancer 2003;11:674-677.ArticlePubMedPDF
- 26. Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso-Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence--study limitations (risk of bias). J Clin Epidemiol 2011;64:407-415.ArticlePubMed
- 27. Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence--inconsistency. J Clin Epidemiol 2011;64:1294-1302.ArticlePubMed
- 28. Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence--indirectness. J Clin Epidemiol 2011;64:1303-1310.ArticlePubMed
- 29. Murad MH, Mustafa RA, Schünemann HJ, Sultan S, Santesso N. Rating the certainty in evidence in the absence of a single estimate of effect. Evid Based Med 2017;22:85-87.PubMedPMC
- 30. Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso-Coello P, et al. GRADE guidelines: 9. Rating up the quality of evidence. J Clin Epidemiol 2011;64:1311-1316.ArticlePubMed
REFERENCES
Tables & Figures
REFERENCES
Citations

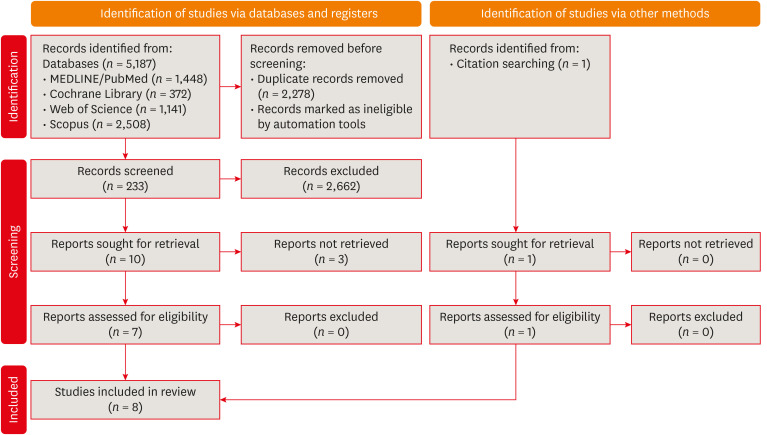
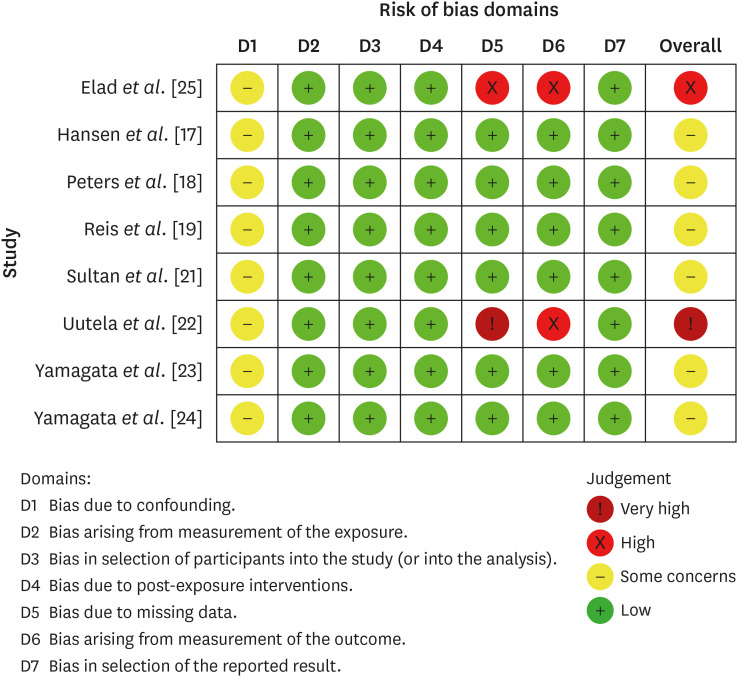
Figure 1
Figure 2
Characteristics and main findings in a sytematic review to assess the prevalence of apical periodontitis prior to HSCT
| Authors | Study design | Number of participants evaluated | Age of participants | Sex of participants | Hematologic diagnoses | Diagnostic method used to diagnose apical periodontitis | Number of participants with apical periodontitis | Age of patients with apical periodontitis | Sex of patients with apical periodontitis | Main findings |
|---|---|---|---|---|---|---|---|---|---|---|
| Elad | Retrospective | Dental evaluation of 46 patients prior to HSCT between 1997 and 1998 (31 allogeneic, 15 autologous) | 6 to 63 years (mean 37 years) | 25 males, 21 females | Acute myelocytic leukemia 14, non-Hodgkins’s lymphoma 9, chronic myelocytic leukemia 9, acute lymphocytic leukemia 6, breast carcinoma 4, multiple myeloma 2, Hodgkin’s disease 1, multiple sclerosis 1 | • Clinical evaluation. | 9 patients (19.56%), 22 teeth | NI | NI | Data indicate a dense distribution of dental needs preceding HSCT, which accentuates the vital need for cooperation between hospital dentists and treating physicians. |
| • Radiographic examination (when necessary) ( | ||||||||||
| Hansen | Prospective | 350 patients prior to HSCT (207 autologous, 143 allogeneic) | 8 to 75 years (mean 54 years) | 207 males, 143 females | Multiple myeloma 104, non-Hodgkin’s lymphoma 99, Hodgkin’s lymphoma 28, leukemia 95, other 24 | • Digital periapical radiographs and, when necessary, selected digital periapical radiographs. | 68 patients (19.4%) | NI | NI | Although there was a high percentage of patients that showed moderate and high risk of odontogenic infection before HSCT (58.6%), only 0.57% of patients developed odontogenic complications. |
| Peters | Retrospective cohort | Dental charts of 276 adult patients who underwent BMT protocols between 1987 and 1991 (13 autogenous and 10 allogeneic) | NI | NI | Chronic myelogenous leukemia, non-Hodgkin’s lymphoma, acute myelogenous leukemia, chronic lymphocytic leukemia, myelodysplastic syndrome, and testicular and breast malignant conditions. | • Complete intraoral radiographic survey, panoramic radiograph, and hard and soft tissue examination. | 23 patients (8.33%) with 1 endodontically treated tooth presenting PE-PARL >1.5 mm | 25 to 58 years (mean age 41 years) | 15 males, 8 females | Nontreatment of PE-PARLs did not increase the incidence of infectious complications during BMT (neither increased systemic infection). |
| Reis | Prospective | 33 patients dental evaluated pre-allogeneic HCT in 2018 | 28.4 ± 16.37 years | 20 males, 13 females | Fanconi’s anemia 2, Sickle cell anemia 7, acute lymphocytic leukemia 7, severe aplastic anemia 3, acute myeloid leukemia 8, chronic myeloid leukemia 1, myelodysplastic syndrome 4, non-Hodgkin’s lymphoma 1 | • Clinical and periapical radiographic examination | 5 patients (15%) | NI | NI | Studied population had important incidence of dental pathologies and infectious conditions that could complicate during HCT. |
| Sultan | Retrospective | Records of 92 patients pre allogeneic HCT from 2007 to 2011 | 24 to 66 years (mean 48 years) | 44 males, 48 females | Acute myeloid leukemia | • Dental radiographs (full mouth series and panoramic), caries charting, pulp vitality testing in teeth with large restorations, and periodontal status assessment | 10 patients (10.87%) | NI | NI | Bacteremia with a potential oral source occurred in 12/92 patients (13%); of these, 11/12 (92%) patients developed bacteremia during HCT. |
| Uutela | Prospective cross‐sectional study | 143 adults allogeneic HSCT recipient patients from 2008 and 2016 | 21 to 58 years (mean 44.8 years) | 70 males, 73 females | Acute lymphoblastic leukemia 29, acute myeloid leukemia 49, chronic lymphocytic leukemia 8, chronic myeloid leukemia 5, myelodysplastic syndrome 10, Hodgkin’s lymphoma 3, myeloproliferative neoplasm 8, non-Hodgkin’s lymphoma 18, plasma cell dyscrasia 10, other diseases 3 | • Panoramic radiograph and DMFT index | 1 patient had fistula, 2 patients had symptomatic periapical process (2.1%) | NI | NI | Oral examinations prior to HSCT showed a higher prevalence of oral disorders in HSCT recipients than in healthy controls. |
| Yamagata | Prospective trial | Dental evaluation of 41 patients pre HSCT from 1998 to 2004 (allogeneic or autologous - not specified) | 17 to 58 years (mean 41.3 years) | 22 males, 19 females | Chronic myeloid leukemia 14, malignant lymphoma 7, acute myeloid leukemia 4, non-Hodgkin’s lymphoma 4, myelodysplastic syndrome 4, multiple myeloma 3, acute lymphoblastic leukemia 3, other malignancies 2 | • The dental status of all patients was evaluated by clinical and radiographic examination, including panoramic and occasional periapical films for symptomatic teeth. | 19 patients (46.34%), 43 teeth | NI | NI | Among 43 teeth with asymptomatic periapical periodontitis before HSCT, only 12 (apical radiolucencies larger than 5 mm) were treated (extraction or endodontic treatment). No conversions to an acute stage or infectious complications occurred in any patient. |
| Yamagata | Retrospective | Dental evaluation of 30 children prior to HSCT from 2000 to 2003 (allogeneic or autologous – not specified) | 2 to 18 years | 18 boys, 12 girls | Acute lymphocytic leukemia 20, acute myelocytic leukemia 2, other malignancies 8. | • Clinical examination and panoramic and/or dental radiographic evaluations. | 2 children (permanent teeth) (6.66%) | NI | NI | As a dental management program was adopted before HSCT, no odontogenic infections occurred during the immunosuppressive period. |
ANC, absolute neutrophil count; PE-PARL, postendodontic asymptomatic periapical radiolucency; BMT, bone marrow transplant; HCT, hematopoietic cell transplantation; HSCT, hematopoietic stem cell transplantation; AlloHCT, allogeneic hematopoietic cell transplantation; DMFT, decayed, missing, and filled teeth; NI, not included.
Quality of evidence assessment for the studies included in a systematic review
| Certainty assessment | ||||||
|---|---|---|---|---|---|---|
| Number of studies | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Overall certainty of evidence |
| 8 Observational studies | Seriousa | Seriousb | Not serious | Not serious | None | ⊕◯◯◯ |
| Very low | ||||||
aOne domain showed ‘some concern’ for all studies; 2 studies showed high/very high risk of bias.
bThere was heterogeneity in the results that could not be explained by the information given in the studies.
ANC, absolute neutrophil count; PE-PARL, postendodontic asymptomatic periapical radiolucency; BMT, bone marrow transplant; HCT, hematopoietic cell transplantation; HSCT, hematopoietic stem cell transplantation; AlloHCT, allogeneic hematopoietic cell transplantation; DMFT, decayed, missing, and filled teeth; NI, not included.
aOne domain showed ‘some concern’ for all studies; 2 studies showed high/very high risk of bias.
bThere was heterogeneity in the results that could not be explained by the information given in the studies.

 KACD
KACD
 ePub Link
ePub Link Cite
Cite

