Abstract
-
Objectives
Previous in vitro studies determined the whitening effects of bleaching products on stained resin composite surfaces. This in vitro study aimed to verify the effectiveness of a whitening system on composite resin previously subjected to pigmentation, specifically examining the depth of whitening effectiveness within the material structure.
-
Materials and Methods
A commercially available nano-filled composite resin was used. Specimens were stained using a coffee-based solution and a 10% carbamide peroxide-based gel was employed as the whitening agent. The pigment’s penetration and the effect of the bleaching gel were evaluated by measuring color (CieLab values) from the outer edge to the inner part of the specimens. Color measurements were taken at 14 points, starting from 0.1 mm from the external perimeter up to 3.0 mm.
-
Results
Analysis of variance tests showed a statistically significant difference between the Control Group (CG), Pigmentation Group, and Whitening Group. The whitening agent was effective up to 1.5 mm in depth, with Whiteness index (W) values not statistically different from those of CG up to 0.5 mm in depth.
-
Conclusions
Whitening agents on nano-filled resin composite previously pigmented appear effective in restoring the W to values similar to the original, particularly in the superficial layers of the sample.
-
Keywords: Bleaching agents; Color; Composite resins, Food coloring agents; Tooth bleaching; Tooth discoloration
INTRODUCTION
Tooth whitening procedures have become popular, common, and desired techniques for improving teeth appearance. Recently, the effectiveness of whitening systems has also been tested on composite resins. In order to assess the whitening effects of both “at-home” and “in-office” tooth bleaching systems on discolored composite resin surfaces, various previous
in vitro studies have been conducted [
1,
2,
3,
4,
5].
Esthetic failures, particularly discolorations and color mismatches, are common reasons for the replacement of composite resin dental restorations [
6,
7,
8,
9]. According to the literature, extrinsic or intrinsic factors may induce pigmentation of resin-based materials [
10,
11,
12,
13,
14]. Intrinsic factors include the pigmentation of the resin material itself, caused by modification of the resin matrix and of the matrix-fillers interface [
15]. Change or oxidation in the amine accelerator in the structure of the polymer matrix or in unreacted pendant methacrylate groups seems to be responsible for chemical discoloration [
10,
16,
17,
18]. Color stability of composite resin materials seems to be related to other characteristics as well: filler type, size and volume, type of monomer and matrix, water sorption and degree of polymerization [
19,
20,
21,
22,
23]. Extrinsic factors consist of contamination from exogenous sources, causing discoloration by absorption of pigments. Surface discoloration by pigments is related to oral hygiene, eating habits and smoking [
3,
12]. Coffee, turmeric and red wine are the foods potentially more harmful in terms of pigmenting power, followed by mate and tea [
13,
14].
Once the discoloration has occurred, to restore the color of the restoration to the original one, some options can be considered. Polishing procedures are fast to perform and not invasive. However, sometimes they could be ineffective since the pigments penetrate deeply into the composite structure and an excessive amount of material would have to be removed to regain the original color [
24]. It is, therefore, often necessary to resort to a total or partial replacement of the restoration.
To overcome problems related to discoloration, dental bleaching is known as an effective and safe method to remove stains on dental tissue [
25,
26,
27,
28]. Several authors evaluated their efficacy on dental composites too. In general, the effectiveness of the whitening system was evaluated, limited to the surface of the material [
2,
3,
4,
5]. The whitening procedure results in color change that depends on the staining solution and the material nature [
5]. However, it is not clear how the whitening process takes place. Some authors hypothesized that it is a simple superficial cleansing effect that causes the color change of the composite surface after the application of the bleaching products, not an intrinsic whitening effect [
29,
30].
Taking these aspects into consideration, the purpose of this in vitro study was to verify the effectiveness of a whitening system on composite resin previously subjected to pigmentation, and compare the data obtained with a non-pigmented Control Group (CG). In particular to understand whether the whitening product is effective in the composite sub-superficial layers and how deeply.
The research hypothesis examined in this study was whether there would be statistically significant differences in color among 3 groups: composite resin subjected to pigmentation, composite resin subjected to pigmentation treated with subsequent bleaching, and resin composite in the CG (no pigmentation, no bleaching), across various depth layers of the tested specimens.
MATERIALS AND METHODS
A commercially available nano-filled composite resin (Filtek Supreme XTE, 3M ESPE, St. Paul, MN, USA) was utilized in this laboratory study (
Table 1).
Table 1 Characteristics of the nano-filled composite resin used in the test
|
Commercial name and manufacturer |
Filler content (vol%) |
Matrix composition |
Filler composition |
|
Filtek Supreme XTE (3M ESPE) |
63 |
Bis-GMA, UDMA, TEGDMA, PEGDMA, Bis-EMA |
Combination of non-agglomerated/non-aggregated 20-nm silica filler, non-agglomerated/non-aggregated 4 to 11 nm zirconia filler, and aggregated zirconia/silica cluster filler |
Eighty resin composite discs were fabricated, following the methods described below: 10 were included in the CG, and the remaining 70 underwent pigmentation protocol. Of these, 35 constituted the Pigmentation Group (PG) and another 35 subsequently underwent whitening treatment (Whitening Group, WG).
Figure 1 represents the preparation and analysis process of the various groups. The sample size was estimated considering an effect size equal to 1 clinically significant, with a 2-sided 5% significance level (Bonferroni correction α = 0.0036) and a power of 80%. An additional 15% of specimens were included to compensate for any dropout.
Figure 1
Schematic representation of specimen preparation and analysis.
CG, Control Group; PG, Pigmentation Group; WG, Whitening Group.
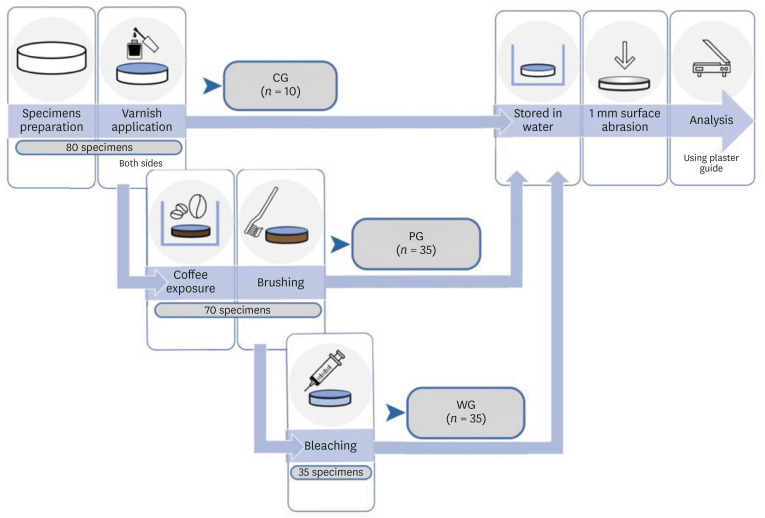
Specimen preparation
A cylindrical polypropylene mold with a thickness of 2.0 mm and an internal diameter of 8.4 mm was used to make the specimens. Composite resin was inserted inside the mold until it was filled. Subsequently, the mold was enclosed between 2 glass panes (1.0 mm thick) in order to complete the polymerization of the material surface, avoiding the possible inhibition by oxygen, and obtaining smooth and circular surfaces for each specimen. The resin inserted into the mold was cured for 20 seconds, for each of the 2 sides, through direct contact between the tip of the light-curing unit (Bluephase 10, High Power Program 1,200 mW/cm
2, Ivoclar Vivadent, Schaan, Liechtenstein). Once the curing process was completed, the glass tops were removed, and the resin specimen was extracted from the polypropylene mold. Any irregularities at the edges were eliminated by using abrasive discs (Sof-Lex Pop On, grit 1982 F and SF, 3M ESPE). The use of discs also allowed us to obtain a smooth surface, and therefore non-retentive for chromogenic substances. A layer of transparent nail polish (Smart Nail Lacquer, KIKO, Bergamo, Italy) was applied to both flat surfaces of each specimen of composite resin, taking care to leave only the lateral surface free. This allowed us to isolate the upper and lower part of the disc, ensuring that the pigment agent, and then the bleaching gel, only penetrated from the lateral surface. The protocol of a previous
in vitro test was followed for the production of the composite disks [
24]. Ten specimens for the CG were immersed in a physiological solution and stored at 37°C for 15 days. The remaining 70 specimens of composite resin were subjected to the pigment treatment with coffee solution. For the preparation of the solution, 1.6 g of coffee (Nescafè Classic, Nestlè, Vevey, Switzerland) was dissolved in 100 mL of boiling water. The specimens were stored in this solution at 37°C for 15 days. At the end of the treatment, they were extracted from the solution and washed under tap water for 1 minute, then the lateral surfaces of the specimens were cleaned with an electric toothbrush (Oral B Pro 600, Procter and Gamble, Cincinnati, OH, USA) and toothpaste (Az Pro Expert, Procter and Gamble) for about 30 seconds.
Subsequently, 35 of the 70 specimens were immersed in a physiological solution and stored at 37°C for 15 days (PG). The remaining 35 (WG), were subjected to the whitening treatment with Opalescence PF 10% Gel (Ultradent, South Jordan, UT, USA) for a duration of 10 days for 8 hours every day, following the manufacturer's instruction. During the period of the whitening treatment, the specimens were completely covered with the whitening gel and kept in an oven at 37°C with 100% humidity. After 8 hours of treatment, the specimens were rinsed under running water and placed in an oven at 37°C in a physiological solution.
Specimen analysis
After the pigmentation and whitening process, the specimens were analyzed using a flatbed computerized scanner (Epson Perfection V850Pro, Epson, Suwa, Japan) with 1,200 DPI resolution, following the same process used previously [
24].
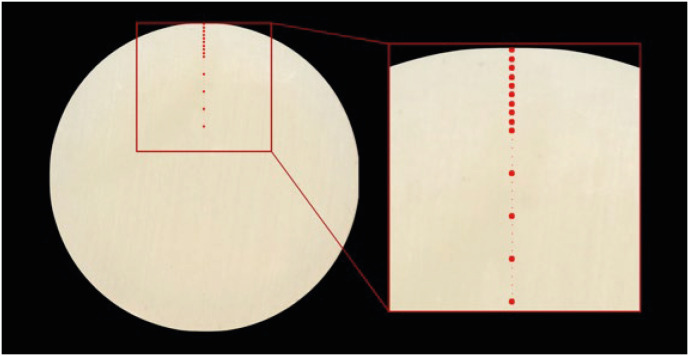
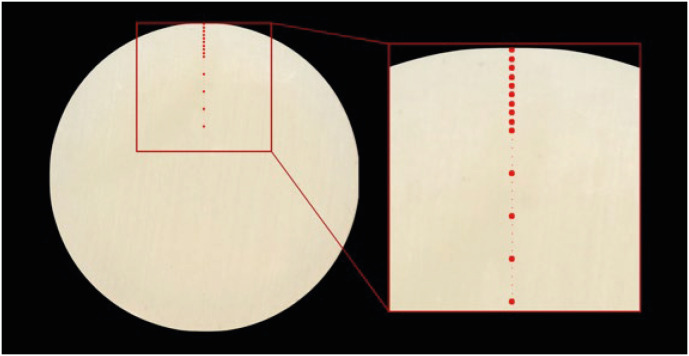
On each scanned image, the center of the circular surface was identified. By then, tracing a line passing through the center, 14 square-shaped points with a size of 25 pixels (5 × 5) were identified (
Figure 2). The first at 0.1 mm from the outer perimeter, the following at 0.2 mm, 0.3 mm, 0.4 mm, 0.5 mm, 0.6 mm, 0.7 mm, 0.8 mm, 0.9 mm, 1.0 mm, 1.5 mm, 2.0 mm, 2.5 mm, 3.0 mm from the outer perimeter.
Figure 2The picture shows how the points where the color detection was made were identified.
The color CIELab system (Commission Internationale de l'Eclairage) was used to quantify colors. Different color parameters were measured for each recorded area. In particular, the average values of L* (lightness), a* (redness) and b* (yellowness) were recorded, then the W (Whiteness index) was calculated. W is a value based on CIELab measurements [
31]. In particular, a nominal white point is used, defined as L* = 100, a* = 0 and b* = 0. The distance, of the color to be quantified, in the color space from this nominal white point was calculated by using the following formula:
For each group and for each measurement depth mean and standard deviation were calculated. Analysis of variance t-test and post hoc Tukey Kramer test were used to compare groups. For each statistical model, the residuals were investigated for the assumptions of normality and homoscedasticity.
RESULTS
Table 2 summarizes data obtained from the test and statistical analysis.
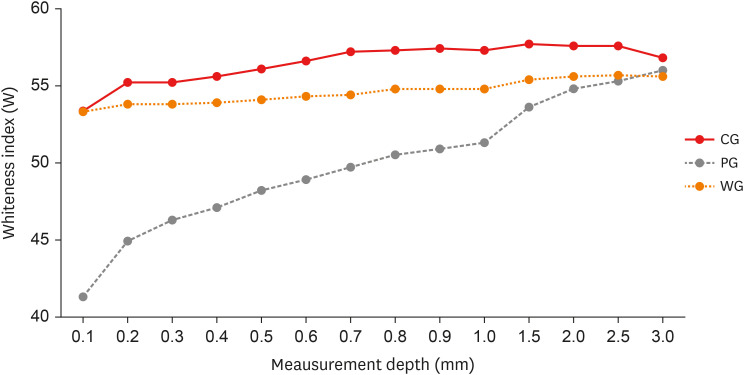
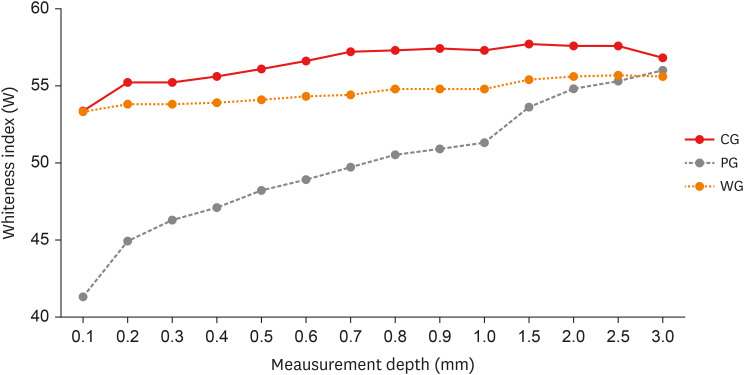
Figure 3 shows the trend of W, relative to the 3 groups analyzed, at the different depth levels. The mean and standard deviation of the W value were calculated for each group and for each individual measurement level. The PG shows statistically significant differences with the CG up to 2.5 mm in depth. The group treated with the WG shows similar values to the CG up to 0.5 mm. Between 0.6 mm and 1.5 mm, WG shows significant differences compared to CG and PG.
Table 2 The table shows the mean and standard deviation of Whiteness index in the different groups
|
Measurement depth (mm) |
CG (n = 10) |
PG (n = 35) |
WG (n = 35) |
p value |
|
0.1 |
53.4 (1.7)a
|
41.3 (0.9)c
|
53.3 (0.9)a
|
< 0.0001 |
|
0.2 |
55.2 (1.4)a
|
44.9 (0.7)c
|
53.8 (0.7)a
|
< 0.0001 |
|
0.3 |
55.2 (1.1)a
|
46.3 (0.6)c
|
53.8 (0.6)a
|
< 0.0001 |
|
0.4 |
55.6 (0.9)a
|
47.1 (0.5)c
|
53.9 (0.5)a
|
< 0.0001 |
|
0.5 |
56.1 (0.9)a
|
48.2 (0.5)c
|
54.1 (0.5)a
|
< 0.0001 |
|
0.6 |
56.6 (0.8)a
|
48.9 (0.4)c
|
54.3 (0.4)b
|
< 0.0001 |
|
0.7 |
57.2 (0.8)a
|
49.7 (0.4)c
|
54.4 (0.4)b
|
< 0.0001 |
|
0.8 |
57.3 (0.8)a
|
50.5 (0.4)c
|
54.8 (0.4)b
|
< 0.0001 |
|
0.9 |
57.4 (0.7)a
|
50.9 (0.4)c
|
54.8 (0.4)b
|
< 0.0001 |
|
1.0 |
57.3 (0.6)a
|
51.3 (0.3)c
|
54.8 (0.3)b
|
< 0.0001 |
|
1.5 |
57.7 (0.6)a
|
53.6 (0.3)c
|
55.4 (0.3)b
|
< 0.0001 |
|
2.0 |
57.6 (0.5)a
|
54.8 (0.3)c
|
55.6 (0.3)c
|
< 0.0001 |
|
2.5 |
57.6 (0.6)a
|
55.3 (0.3)c
|
55.7 (0.3)c
|
0.0042 |
|
3.0 |
56.8 (0.6)a
|
56.0 (0.3)a
|
55.6 (0.3)a
|
0.1797 |
Figure 3 Trend of Whiteness index (W), relative to the 3 groups analyzed, at the different depth levels. The trends of the Control Group (CG) and Whitening Group (WG) are similar. In the Pigmentation Group (PG), the stains have marked values on the surface that gradually decrease.
DISCUSSION
The hypothesis of this study, which predicted statistically significant differences in color, among 3 tested groups, was confirmed. In fact, significant differences were registered between the W value in PG, WG and CG. The specimens stored in coffee showed a greater discoloration than the control. Pigmentation was maintained down to a depth level of 2.5 mm. This therefore highlights the pigmented power of the coffee, to act not only on the surface but also in the deeper layers of the specimens [
24]. Later, the second part of the test again showed a significant difference between the CG and the group of specimens subjected to bleaching (WG). The results showed the ability of the bleaching gel to restore the color of the resin almost to its original one, down to a depth of 0.5 mm. The whitening effect was still detected up to 1.5 mm in depth, albeit with different values compared to the CG. Observing
Figure 3, a slight deviation between the W trend of the WG and CG groups could be observed, a deviation also present in the surface layers, although this difference is not statistically significant up to 0.5 mm.
Other studies were set up to evaluate the effectiveness of whitening systems on composites, but the analysis was limited to the surface of the material [
2,
3,
4,
5]. Whitening procedures are generally effective in reducing the effect of pigmentation, the entity of color change depends on the staining solution and the material nature [
5]. Some authors hypothesized that a superficial cleansing effect causes the color change of the composite surface after the application of the bleaching products, not an intrinsic whitening effect [
29,
30]. The results of the present study do not agree with this statement, since the whitening effect can also be observed in the subsurface layers of the composite. The teeth whitening mechanism assumes that the hydrogen peroxide molecules, released by the whitening gels, penetrate the dental tissues to interact with the organic compounds responsible for pigmentation (chromophores). The interprismatic spaces and dentinal tubules allow for the flow of fluids into the enamel and dentin, respectively. Precisely for this reason, dental hard tissues are highly permeable to fluids. Thanks to this characteristic, the enamel and the dentin allow the hydrogen peroxide to carry out its action in the dental tissues [
26]. Less is known about whether whitening products may also interact with the 3-dimensional polymer network of dental composite materials. Durner
et al. [
32] speculate that whitening products may lead to oxidative cleavage of the 3-dimensional polymer network. This could contribute to the diffusion of bleaching molecules into the structure of the material, and this hypothesis could explain the results of this study.
The present study analyzed 80 specimens of a nano-filled composite resin (Filtek Supreme XTE, 3M ESPE) and subjected 70 of these to a pigmentation protocol in a coffee solution, following the materials and methods of a previous
in vitro test [
24]. The composite disks were stored in the pigmentation solution for 15 days at 37°C. If 24 hours of water storage correspond to approximately one month of oral aging, it could be assumed that specimens have undergone aging comparable to more than one year of clinical service [
14].
Subsequently, 35 of the pigmented specimens underwent bleaching treatment with 10% carbamide peroxide gel for 10 days, following the times and methods of application suggested for clinical use. This kind of bleaching treatment was chosen as it seemed to be more effective on composite resins than a treatment that involves high concentrations of the whitening agent [
33]. Carbamide peroxide (CH
6N
2O
3) is a crystalline white solid that, when in contact with H
2O, releases oxygen. When used for whitening procedures, its concentration ranges from 10% to 35%. Precisely, a 10% carbamide peroxide solution breaks down into 3.35% hydrogen peroxide and 6.65% urea. The urea further breaks down into ammonia and carbon dioxide. The high pH of ammonia facilitates bleaching procedures [
27,
28]. Commercially available carbamide peroxide products usually contain in their formulations also a glycerin or a carbopol base. The carbopol base makes the whitening product operative longer as it slows down the release of hydrogen peroxide [
26].
Conventionally, “chromophore theory” is used to explain tooth whitening mechanism. Organic chromophores are colored molecules that consist of organic compounds containing a large number of single and double bonds. Chromophores are found in the tooth structure and are able to interact with hydrogen peroxide [
26,
27].
Hydrogen peroxide decomposes in reactive oxygen species (ROS) that are highly oxidative. It performs its action by reaching the adsorbed stains, and the ROS breaks the double bonds of the staining molecules. It results in the breakdown and dispersion of stained molecules, or a size reduction that makes them appear lighter thanks to lower absorption of light, chemical oxidation reactions play a key role [
26,
32,
33,
34,
35].
Coffee was selected as a staining agent because of its capability to pigment composite resin materials. The roasting process thermally degrades monosaccharides into a brownish substance that reacts with chlorogenic acids and produces brown-black pigments. The pigments become dispersed, when brewed, either as water-soluble or colloidally dispersed compounds [
1]. Filtek Supreme XTE is a widely used nano-hybrid resin composite. Its formulation contains larger filler particles, made up of nano-particles clusters. Silanization is not performed on individual particles of the cluster [
36]. It can be viewed that the absence of a complete silanization of the matrix-filler interface can lead to the possibility of water infiltration, and therefore of pigment infiltration [
37]. This is the reason why it was chosen to utilize this type of composite.
The composite disks measured 2.0 mm in thickness and 8.4 mm in diameter so as to ensure a complete and homogeneous polymerization. The chosen thickness (2.0 mm) is the maximum thickness to ensure uniform polymerization of the whole material [
38,
39,
40]. Adequate polymerization is the basis of the mechanical and esthetic properties of the resin material. On the contrary, the absorption of water and the reception of exogenous substances, which may be responsible for a progressive deterioration of the material, could be caused by an incomplete polymerization [
14]. Finally, the disk diameter measure corresponds to the size of the curing light tip, this allowed us to obtain a polymerization in a single phase.
To eliminate any extrinsic pigmentations adhering to the surface, the lateral surfaces of the composite discs were brushed with toothpaste and an electronic toothbrush [
41]. In fact, residual pigmentations could influence the color recording. By using a flatbed scanner (Epson Perfection V850Pro) a computerized scan was started. In this way, using photo-graphic software (ADOBE Photoshop CC 2021, Adobe, San Jose, CA, USA), the scan of the sectional surface of the specimens was acquired. During the scanning process, the specimens were placed in a specially constructed white plaster mold. This allowed us to avoid ambient light and the light emitted by the scanner itself from creating shadow areas that could have affected the measurement.
A possible limitation of this study is that of having discarded the ΔE value for the color analysis, which is well known and diffused parameter. On the contrary, the W was chosen to calculate the extent of pigmentation. This is because, W is an “absolute” index, in fact, it compares the detected color with an “a priori” defined value. In the literature, there are many indices that attest to the changes in color or the white index itself. Luo
et al. [
42] state that all indices (W, WIO and WIC), b* and ΔE values showed a similar trend; they are equally reliable in attesting the color change.
Ultimately, from the results obtained, it emerged that the nano-filled composite used is not inert to the action of the 10% carbamide peroxide-based gel. Contrary to what was hypothesized in other studies, the effectiveness of whitening seems to have its effect even within the structure of the material [
29,
30]. This opens the way to further studies, where it will be possible to examine the behavior of different types of composite resins and the action of different whitening systems.
CONCLUSIONS
Within the limitations of this current study, it was concluded that previously pigmented nano-filled composite resin can be effectively treated with a whitening agent. The action of the whitening gel is effective in restoring the white value close to the initial one, in the more superficial layers of the sample. The effect of the whitening procedure is noticeable at an even greater depth, but it is less effective.
-
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
-
Author Contributions:
Conceptualization: Giachetti L.
Data curation: Scaminaci Russo D, Cinelli F, Nieri M.
Formal analysis: Scaminaci Russo D, Cinelli F.
Investigation: Scaminaci Russo D, Cinelli F.
Methodology: Giachetti L, Scaminaci Russo D.
Supervision: Giachetti L.
Writing - original draft: Cinelli F.
Writing - review & editing: Giachetti L, Scaminaci Russo D.
REFERENCES
- 1. Reinhardt JW, Balbierz MM, Schultz CM, Simetich B, Beatty MW. Effect of tooth-whitening procedures on stained composite resins. Oper Dent 2019;44:65-75.ArticlePubMedPDF
- 2. Attin T, Hannig C, Wiegand A, Attin R. Effect of bleaching on restorative materials and restorations--a systematic review. Dent Mater 2004;20:852-861.ArticlePubMed
- 3. Abd Elhamid M, Mosallam R. Effect of bleaching versus repolishing on colour and surface topography of stained resin composite. Aust Dent J 2010;55:390-398.ArticlePubMed
- 4. Türkün LS, Türkün M. Effect of bleaching and repolishing procedures on coffee and tea stain removal from three anterior composite veneering materials. J Esthet Restor Dent 2004;16:290-301.ArticlePubMed
- 5. Alharbi A, Ardu S, Bortolotto T, Krejci I. In-office bleaching efficacy on stain removal from CAD/CAM and direct resin composite materials. J Esthet Restor Dent 2018;30:51-58.ArticlePubMedPDF
- 6. Mjör IA. The reasons for replacement and the age of failed restorations in general dental practice. Acta Odontol Scand 1997;55:58-63.ArticlePubMed
- 7. Malhotra N, Shenoy RP, Acharya S, Shenoy R, Mayya S. Effect of three indigenous food stains on resin-based, microhybrid-, and nanocomposites. J Esthet Restor Dent 2011;23:250-257.ArticlePubMed
- 8. Mutlu-Sagesen L, Ergün G, Özkan Y, Semiz M. Color stability of a dental composite after immersion in various media. Dent Mater J 2005;24:382-390.ArticlePubMed
- 9. Nasim I, Neelakantan P, Sujeer R, Subbarao CV. Color stability of microfilled, microhybrid and nanocomposite resins--an in vitro study. J Dent 2010;38(Supplement 2):e137-e142.PubMed
- 10. Topcu FT, Sahinkesen G, Yamanel K, Erdemir U, Oktay EA, Ersahan S. Influence of different drinks on the colour stability of dental resin composites. Eur J Dent 2009;3:50-56.ArticlePubMedPMC
- 11. Alharbi A, Ardu S, Bortolotto T, Krejci I. Stain susceptibility of composite and ceramic CAD/CAM blocks versus direct resin composites with different resinous matrices. Odontology 2017;105:162-169.ArticlePubMedPDF
- 12. Raptis CN, Powers JM, Fan PL, Yu R. Staining of composite resins by cigarette smoke. J Oral Rehabil 1982;9:367-371.ArticlePubMed
- 13. Gujjari AK, Bhatnagar VM, Basavaraju RM. Color stability and flexural strength of poly (methyl methacrylate) and bis-acrylic composite based provisional crown and bridge auto-polymerizing resins exposed to beverages and food dye: an in vitro study. Indian J Dent Res 2013;24:172-177.ArticlePubMed
- 14. Arocha MA, Mayoral JR, Lefever D, Mercade M, Basilio J, Roig M. Color stability of siloranes versus methacrylate-based composites after immersion in staining solutions. Clin Oral Investig 2013;17:1481-1487.ArticlePubMedPDF
- 15. Güler AU, Güler E, Yücel AC, Ertaş E. Effects of polishing procedures on color stability of composite resins. J Appl Oral Sci 2009;17:108-112.ArticlePubMedPMC
- 16. Asmussen E, Hansen EK. Surface discoloration of restorative resins in relation to surface softening and oral hygiene. Scand J Dent Res 1986;94:174-177.ArticlePubMed
- 17. Asmussen E. Factors affecting the color stability of restorative resins. Acta Odontol Scand 1983;41:11-18.ArticlePubMed
- 18. Ruyter IE. Composites--characterization of composite filling materials: reactor response. Adv Dent Res 1988;2:122-129.ArticlePubMedPDF
- 19. Garoushi S, Lassila L, Hatem M, Shembesh M, Baady L, Salim Z, et al. Influence of staining solutions and whitening procedures on discoloration of hybrid composite resins. Acta Odontol Scand 2013;71:144-150.ArticlePubMed
- 20. Ergücü Z, Türkün LS, Aladag A. Color stability of nanocomposites polished with one-step systems. Oper Dent 2008;33:413-420.ArticlePubMedPDF
- 21. Ikeda T, Nakanishi A, Yamamoto T, Sano H. Color differences and color changes in Vita shade tooth-colored restorative materials. Am J Dent 2003;16:381-384.PubMed
- 22. Ardu S, Braut V, Gutemberg D, Krejci I, Dietschi D, Feilzer AJ. A long-term laboratory test on staining susceptibility of esthetic composite resin materials. Quintessence Int 2010;41:695-702.PubMed
- 23. Ardu S, Duc O, Di Bella E, Krejci I. Color stability of recent composite resins. Odontology 2017;105:29-35.ArticlePubMedPDF
- 24. Cinelli F, Scaminaci Russo D, Nieri M, Giachetti L. Stain susceptibility of composite resins: pigment penetration analysis. Materials (Basel) 2022;15:4874.ArticlePubMedPMC
- 25. Davidi MP, Hadad A, Weiss EI, Domb A, Mizrahi B, Sterer N. The effect of a mild increase in temperature on tooth bleaching. Quintessence Int 2008;39:771-775.PubMed
- 26. Kwon SR, Wertz PW. Review of the mechanism of tooth whitening. J Esthet Restor Dent 2015;27:240-257.ArticlePubMedPDF
- 27. Wijetunga CL, Otsuki M, Hiraishi N, Luong MN, Tagami J. Effect of pH of bleaching agent on tooth bleaching action in vitro
. Dent Mater J 2021;40:566-572.ArticlePubMed
- 28. Torres CR, Crastechini E, Feitosa FA, Pucci CR, Borges AB. Influence of pH on the effectiveness of hydrogen peroxide whitening. Oper Dent 2014;39:E261-E268.ArticlePubMedPDF
- 29. Poggio C, Beltrami R, Scribante A, Colombo M, Chiesa M. Surface discoloration of composite resins: Effects of staining and bleaching. Dent Res J 2012;9:567-573.ArticlePubMedPMC
- 30. Villalta P, Lu H, Okte Z, Garcia-Godoy F, Powers JM. Effects of staining and bleaching on color change of dental composite resins. J Prosthet Dent 2006;95:137-142.ArticlePubMed
- 31. de Camargo EJ, Moreschi E, Baseggio W, Cury JA, Pascotto RC. Composite depth of cure using four polymerization techniques. J Appl Oral Sci 2009;17:446-450.ArticlePubMedPMC
- 32. Durner J, Stojanovic M, Urcan E, Spahl W, Haertel U, Hickel R, et al. Effect of hydrogen peroxide on the three-dimensional polymer network in composites. Dent Mater 2011;27:573-580.ArticlePubMed
- 33. Lago M, Mozzaquatro LR, Rodrigues C, Kaizer MR, Mallmann A, Jacques LB. Influence of bleaching agents on color and translucency of aged resin composites. J Esthet Restor Dent 2017;29:368-377.ArticlePubMedPDF
- 34. Thickett E, Cobourne MT. New developments in tooth whitening. The current status of external bleaching in orthodontics. J Orthod 2009;36:194-201.ArticlePubMed
- 35. Goldberg M, Grootveld M, Lynch E. Undesirable and adverse effects of tooth-whitening products: a review. Clin Oral Investig 2010;14:1-10.ArticlePubMedPDF
- 36. Curtis AR, Palin WM, Fleming GJ, Shortall AC, Marquis PM. The mechanical properties of nanofilled resin-based composites: the impact of dry and wet cyclic pre-loading on bi-axial flexure strength. Dent Mater 2009;25:188-197.ArticlePubMed
- 37. Cavalcante LM, Ferraz LG, Antunes KB, Garcia IM, Schneider LF, Collares FM. Silane content influences physicochemical properties in nanostructured model composites. Dent Mater 2021;37:e85-e93.ArticlePubMed
- 38. Bouschlicher MR, Rueggeberg FA, Boyer DB. Effect of stepped light intensity on polymerization force and conversion in a photoactivated composite. J Esthet Dent 2000;12:23-32.ArticlePubMed
- 39. Duc O, Di Bella E, Krejci I, Betrisey E, Abdelaziz M, Ardu S. Staining susceptibility of resin composite materials. Am J Dent 2019;32:39-42.PubMed
- 40. Rueggeberg FA, Caughman WF, Curtis JW Jr, Davis HC. Factors affecting cure at depths within light-activated resin composites. Am J Dent 1993;6:91-95.PubMed
- 41. Zanetti F, Zhao X, Pan J, Peitsch MC, Hoeng J, Ren Y. Effects of cigarette smoke and tobacco heating aerosol on color stability of dental enamel, dentin, and composite resin restorations. Quintessence Int 2019;50:156-166.PubMed
- 42. Luo W, Westland S, Brunton P, Ellwood R, Pretty IA, Mohan N. Comparison of the ability of different colour indices to assess changes in tooth whiteness. J Dent 2007;35:109-116.ArticlePubMed
 , Daniele Scaminaci Russo
, Daniele Scaminaci Russo , Michele Nieri
, Michele Nieri , Francesca Cinelli
, Francesca Cinelli




 KACD
KACD

 ePub Link
ePub Link Cite
Cite

