Abstract
-
Objectives
This study compared the clinical and radiological outcomes of regenerative endodontic procedures (REPs) using blood clots (BCs), platelet-rich plasma (PRP), and platelet-rich fibrin (PRF) through intraoral periapical radiography (IOPAR) and cone-beam computed tomography (CBCT).
-
Materials and Methods
Forty-five single-rooted necrotic teeth with periapical pathology were randomly allocated to receive BC, PRP, or PRF as an individual scaffold. Outcomes were evaluated in 35 teeth in 23 patients with a follow-up period of 12–24 months through qualitative IOPAR scoring and quantitative CBCT measurements. Healing of periapical lesions and in immature teeth, changes in the apical foramen diameter (AFD), root wall thickness (RWT), and root length (RL) were assessed. A p value less than 0.05 was considered to indicate statistical significance.
-
Results
All teeth were asymptomatic except 1 in the PRP group. Periapical lesion healing was seen in all except 2 teeth in the BC group and 3 in the PRP group. Both IOPAR and CBCT revealed no significant differences in bone healing or changes in AFD, RWT, and RL among the 3 groups. A positive pulp sensibility response to the cold test was seen in 2 teeth in the BC group, but none to the electric pulp test. Intracanal calcification (ICC) was evident in more teeth in the BC group than in the PRP and PRF groups, and was also significantly higher in immature teeth.
-
Conclusions
Our results revealed that BC, PRP, and PRF have similar potential as scaffolds in REPs, and ICC may be a concern for long-term outcomes.
-
Keywords: Blood clot; Platelet rich plasma; Platelet rich fibrin; Regenerative endodontic procedure
INTRODUCTION
Biologically based regenerative endodontic procedures (REPs) are gaining popularity in the field of endodontics. The success of REPs depends on effectively implementing the triad of tissue engineering, adequate disinfection, and a coronal seal for maintaining the disinfected environment. The 3 key elements for tissue engineering are stem cells, the scaffold, and growth factors. Most reported
in vivo studies were based on the concept of cell homing by recruitment of the patient’s endogenous stem cells located around the periapical region [
1,
2]. These include stem cells from the apical papilla, inflammatory periapical progenitor cells, periodontal ligament stem cells, bone marrow stem cells, and dental pulp stem cells [
3]. Growth factors act as signaling molecules and induce cellular migration, adhesion, proliferation, and differentiation [
4]. Cell homing instead of cell transplantation for dentin-pulp tissue regeneration relies mainly on the delivery of growth factors into the canal space. In REPs, there are 2 common sources of growth factors: internal (i.e., dentin) and the scaffold. Growth factors are found embedded in the extracellular matrix of dentin and can be utilized by conditioning the dentin surface [
5].
A scaffold is a 3-dimensional (3D) structure that supports cell organization and vascularization. The principal objective of scaffold design is that it should mimic the physical and biochemical microenvironment of the root canal. Furthermore, it should be able to simulate the native extracellular matrix until cells seeded within the scaffold and/or derived from the host tissue can synthesize a new, natural matrix [
6]. The scaffold not only acts as a template, but can also provide growth factors [
6]. The scaffold can be obtained from natural sources. These scaffolds contain signaling molecules and can be obtained either from the same host or from other natural sources, although the use of the latter is limited due to the risk of pathogen transmission and a foreign body response. However, natural scaffolds may possess inconsistent mechanical properties compared to artificial scaffolds [
6]. Host-derived natural scaffolds include blood clots (BCs) and autologous platelet concentrates, of which platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) are commonly used. In addition to the advantages of being autologous, they are inexpensive, making them the most common choice of scaffolds for REPs. Artificial or synthetic scaffolds such as poly-L-lactic acid, polyglycolic acid, and poly-D,L-lactic-co-glycolic acid, which are manufactured synthetically through standardized production processes and thus ensure consistent properties. However, these materials lack the intrinsic signaling abilities of naturally-derived scaffolds and have high costs resulting from their complex production processes [
6]. The American Association of Endodontists (AAE) protocol for REPs, which is based on the cell homing technique, if strictly followed, makes it possible to achieve adequate disinfection of the root canal system, a coronal seal, and release of growth factors from dentin [
7]. However, the use of different scaffolds not only provides a different environment for the developing tissues, but also supplies a variable amount of growth factors; therefore, it might significantly affect the process of tissue regeneration.
The effects of different scaffolds on the outcome of REP have been widely researched in the past, but few studies have compared these 3 host-derived natural scaffolds (i.e., BC, PRP, and PRF) [
1,
8,
9,
10]. Some studies compared BC with PRF, BC with PRP, and PRP and PRF, but all the above studies used 2D intraoral periapical radiography (IOPAR) for outcome assessment [
11,
12,
13,
14,
15,
16,
17]. Due to the possibility of inconsistent images during follow-up and inaccuracy in IOPAR interpretation, the use of 3D cone-beam computed tomography (CBCT) would provide a more accurate evaluation of the outcomes. Only 2 studies have reported quantitative CBCT evaluations of REP outcomes with a comparison between BC and PRP [
2,
18]. However, none of the studies evaluated all 3 of these scaffolds using CBCT. Therefore, the aim of the present study was to perform a comparative evaluation of the outcomes of REP in human non-vital permanent teeth with periapical pathology using BC, PRP, and PRF as individual scaffolds, as assessed by both IOPAR and CBCT. The null hypothesis was that there would be no significant difference in the clinical and radiographical outcomes of REPs using BC, PRP, or PRF as a scaffold.
MATERIALS AND METHODS
This prospective randomized single-blinded clinical trial was designed according to the CONSORT guidelines and was conducted from January 2019 to June 2021. The study protocol was approved by the institutional ethics committee of Dr. R. Ahmed Dental College and Hospital, Kolkata, West Bengal, India (RADCH/IEC/15-01-2019).
Inclusion and exclusion criteria
Single-rooted mature or immature permanent teeth with necrotic pulp and periapical pathology were selected from systemically healthy patients in the age group of 15–36 years. Medically compromised patients, teeth with root fractures or non-restorable crowns, and periodontally compromised teeth were excluded.
Sample size determination
In order to detect an appropriate effect with 80% power with 3 groups and an alpha level of 0.05, G*Power (G*Power for Windows, version 3.1.9.7, University of Düsseldorf, Düsseldorf, Germany) suggested that 11 participants would be required in each group (n = 33). However, 60 teeth were included in the study to cover possible dropouts.
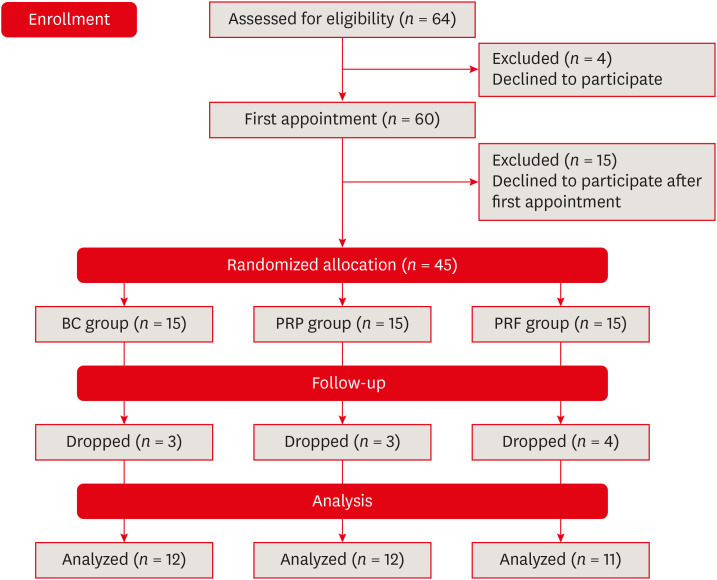
Sixty teeth in 42 patients (22 male and 21 female) were selected over a period of 6 months from the outpatient department of the hospital (
Figure 1). The following treatment options were given to the patients: root canal treatment in case of mature teeth, apical barrier formation in cases of immature teeth, or REP. After explaining the benefits and risks of all options, only those patients who agreed to undergo REP were included in this study, and written consent was obtained from all these patients.
Figure 1
Flow chart of the study.
BC, blood clot; PRP, platelet-rich plasma; PRF, platelet-rich fibrin.
Preoperative evaluation
A preoperative clinical examination was done to record the presence of pain, swelling, sinus tract, tenderness to percussion, mobility, and a probing depth of more than 3 mm. Pulp sensibility tests with cold (Endofrost, Coltene Whaledent, Langenau, Germany) and an electric pulp test (Confident Dental, Bangalore, India) were done for all teeth. Preoperative IOPAR was taken using a Rinn film holder, and CBCT scans were also performed (Skyview 3D Panoramic Imager, My-Ray Dental Imaging, Imola, Italy). The CBCT images were obtained with settings of 90 kVp and 10 mA, with a 0.14 mm × 0.14 mm × 0.14 mm voxel size, exposure time of 15 seconds, and field of view of 7 cm.
Clinical procedure
The clinical procedure was performed according to the 2018 AAE guidelines [
7]. All procedural steps were performed by the same operator. During the first appointment, the access cavity was prepared after local anesthesia (2% lidocaine with 1:80,000 epinephrine) and rubber dam application. The working length (WL) was determined radiographically and recorded. A gentle and limited amount of filing was done in immature teeth (teeth with a minimum apical foramen diameter [AFD] of 1 mm were considered as immature) to preserve thin dentinal walls while removing the necrotic tissue and biofilm from the root canal. In mature teeth, preparation up to a size 30 K-file was done if the initial apical foramen was smaller than its tip diameter. Copious, gentle irrigation with 20 mL of 1.5% sodium hypochlorite (Prime Dental Products, Mumbai, India) over the course of 5 minutes using a side-vented needle (CanalClean, BioDent, Paju, Korea), keeping 2 mm short of the WL, was done. This was followed by irrigation with 20 mL of saline for 5 minutes. The canal was dried with paper points. A calcium hydroxide paste (Ultracal, Ultradent Products, Inc., South Jordan, UT, USA) was introduced into the canal (using a 29-gauge Navi tip) as an intracanal medicament for disinfection. The canal was sealed with 3–4 mm of temporary restorative material (Cavit, 3M ESPE, St Paul, MN, USA). The patients were recalled after 4 weeks.
In the second appointment, after a rubber dam was applied and the teeth were re-accessed, copious and gentle irrigation was done with saline to remove the intracanal calcium hydroxide. Then, irrigation was done with 20 mL of 17% ethylenediaminetetraacetic acid (Desmear, Ahmedabad, India) for 5 minutes, followed by saline [
19]. The canal was dried with paper points. If there were signs or symptoms of persistent infection, or if the canal was not dry, calcium hydroxide medication was repeated; otherwise, the scaffolds were then created according to the allocated groups.
Out of the 42 patients (60 teeth), 11 patients (15 teeth) did not return after the first appointment, so the remaining 31 patients (45 teeth) were allocated to 3 treatment groups. The randomization sequence was prepared using
www.random.org. For the randomized allocation, a dentist blinded to the study took an opaque sealed envelope from a bag containing the group codes. Blinding of the patient and operator was not possible due to the use of different protocols in each group.
In this group, after administration of local anesthesia without vasoconstrictor (2% lidocaine), an apically precurved size 20 K-file was rotated keeping it 2 mm past the apical foramen to induce bleeding up to the level of the cementoenamel junction (CEJ). A sterile moist cotton pellet was placed in the canal, 3–4 mm apical to the CEJ, and blood was allowed to clot. Blood clotting was confirmed by a sterile saline-soaked cotton pellet. A gelatin sponge (Gelatamp; Coltene Whaledent) was placed over the BC inside the canal.
PRF group
First, 10 mL of whole venous blood taken from the antecubital vein of the patient’s forearm was collected in a sterile tube without an anticoagulant and was immediately centrifuged at 2,110 rpm (400 ×
g; radius of centrifuge, 8 cm) for 10 minutes using a Remi R 8C Centrifuge (Remi Laboratories, Mumbai, India) [
20]. After centrifugation, PRF in the middle was removed with a sterile tweezer from the acellular plasma at the top and the red blood cells (RBCs) at the bottom of the test tube. It was then squeezed and cut into fragments, which were transferred with the help of a tweezer into the canal space and pushed inside the space using sterile hand pluggers, allowing for a space of 2–3 mm below the CEJ.
Whole venous blood (5 mL) was taken similarly as in the PRF group and collected in a glass test tube containing 1 mL of 3.8% sodium citrate (Universal Chemicals, Kolkata, India) as an anticoagulant. With the help of the same centrifuge machine, the first spin was done at 1,831 rpm (300 ×
g) for 5 minutes [
21]. This resulted in segregation of acellular plasma at the top, a buffy coat in the middle, and RBCs at the bottom. Then, the upper 2 layers were transferred to another glass test tube and subjected to a second round of centrifugation at 700 ×
g (2,797 rpm) for 17 minutes [
21]; thus, the residual RBCs settled at the bottom with platelet-poor plasma (PPP) (80% of the total volume) at the top and PRP in between the 2 layers. The outermost layer of PPP was removed with a syringe and discarded, and the remaining PRP was mixed with 10% calcium chloride (Universal Chemicals) to trigger platelet activation and fibrin polymerization. The mixture was shaken well. This solution was then injected into the canal below the level of the CEJ using an insulin syringe and allowed to clot for 10 minutes. Gelatamp (Coltene Whaledent) was placed over the scaffold inside the canal.
In all groups, 2–3 mm of Biodentine (Septodont, Saint-Maur-des-Faussés, France) as a capping material was placed inside the canal below the CEJ, directly over the scaffold in the PRF group and over Gelatamp in the BC and PRP groups. After waiting for 12 minutes as its setting time, coronal restoration was done with composite resin. IOPAR was then immediately taken.
Follow-up appointments
Postoperatively, the teeth were assessed at 3 months and then at 6-month intervals until 2 years. Patients were examined for clinical signs and symptoms and radiographically through IOPAR. CBCT was done at a 12-month appointment or thereafter. The radiographic technique was the same as presented for the preoperative evaluation.
IOPAR evaluation
Bone healing (BH) of the periapical lesion in relation to all teeth and changes in the AFD—more specifically, apical closure (AC), root wall thickness (RWT), and root length (RL) in immature teeth—were evaluated according to the following IOPAR scoring criteria: none (no obvious change), score 0; fair (minimal changes), score 1; good (between fair and excellent), score 2; excellent (significant changes), score 3 [
8]. The evaluation was performed at 2 different times by 2 different observers, who were blinded to the study groups.
Both preoperative and follow-up CBCT scans were viewed in 3 planes (sagittal, coronal, and axial). The sagittal plane was parallel to the long axis of the tooth and passed across the maximum dimensions of the pulp in the bucco-lingual plane. The coronal plane was perpendicular to the sagittal plane and passed across the maximum dimension of the pulp in the mesio-distal plane. The axial plane was perpendicular to both of the above planes and passed through the end of the root apex for periapical lesion area (PLA) and gray value (GV) measurements and through the CEJ for root measurements [
2]. To assess periapical lesions, all scanned samples were imported into iRYS viewer software version 6.2 (MyRay CBCT, Imola, Italy). PLA was measured using the freehand selection tool to trace out the border of the lesion, and the values obtained were automatically converted to square millimeters (mm
2) and indicators of bone density (i.e., the mean GV of the involved area expressed in Hounsfield units [HUs]) were also obtained in the scan simultaneously. These measurements were made in the 3 planes, the average of which was taken as the final value [
22] (
Supplementary Figure 1A-C). The percentage reduction in PLA [(Preoperative − Postoperative)/Preoperative × 100] and gain in GV were calculated.
To assess root development in immature teeth, all scanned samples were imported into the RadiAnt DICOM image viewer software version 2020.1.1 (Medixant, Poznan, Poland). AFD was measured as a line measurement from the mesial and distal root ends (
Supplementary Figure 1D and E). RWT was measured by subtracting the pulp space from the whole root thickness at the apical two-thirds RL (
Supplementary Figure 1F and G). RL was measured along the long axis of each tooth from the most apical point of the root perpendicular to the line connecting the mesial and distal CEJs (
Supplementary Figure 1H and I). These parameters were all measured in the sagittal and coronal planes. The average of these 2 readings was taken as the final value. The decrease in AFD and the increase in RWT and RL were then calculated. All measurements were made at 2 different time points by 2 observers, who were blinded to the study groups. The recorded data obtained from 2 observers at 2 different time intervals were subjected to (i) the Cohen kappa test to measure intra- and inter-rater reliability for IOPAR scores, for which nearly perfect agreement was obtained; and (ii) intra-class correlation and inter-rater correlation coefficients for quantitative CBCT values, which were found to be excellent.
The statistical analysis was carried out using SPSS version 26.0 (IBM Corp., Armonk, NY, USA). Both the Kolmogorov-Smirnov test and the Shapiro-Wilk test showed that the data were not normally distributed. Descriptive statistics were used to report the values of central tendency (median) and measures of dispersion (interquartile range [IQR]). Non-parametric tests were carried out for inferential statistics. Categorical variables (IOPAR scores) were compared across the groups using the Fisher exact test. Continuous variables (CBCT quantitative measurements) were compared across the groups using the Kruskal-Wallis test. The χ2 test was used to test the significance of differences between 2 proportions. A p value of 0.05 was considered as the level of significance.
RESULTS
In the present study, REPs were performed on 45 teeth in 31 patients. Outcomes were evaluated for teeth with a minimum of 12 months of follow-up, which included 35 teeth in 23 patients. The follow-up period ranged from 12 to 24 months. However, among them, BC and PRP were used as scaffolds in 12 teeth each, whereas PRF was used in 11 teeth. Out of the 23 patients, 3 female patients had bilateral involvement of teeth, and 2 different scaffolds (PRP and PRF) were used on either side of the same patient. The demographic data and preoperative findings, along with the IOPAR observations at the respective follow-up periods of the patients, are described in
Table 1.
Table 1 Patients’ demographics, preoperative clinical findings, follow-up period, and intraoral periapical radiography (IOPAR) observations
|
No. |
Sex |
Age |
Tooth No. |
Tooth status |
PD |
Scaffold |
FU |
IOPAR score |
ICC |
|
BH |
AC |
RWT |
RL |
|
1 |
F |
29 |
22∥
|
IM |
AAA |
BC |
15 |
3 |
3 |
3 |
1 |
|
|
2 |
M |
16 |
21 |
IM |
AAP |
17 |
3 |
3 |
3 |
1 |
Y |
|
3 |
F |
23 |
45 |
IM |
AAA |
13 |
2 |
1 |
2 |
1 |
Y |
|
4 |
F |
19 |
21 |
IM |
AAP |
18 |
3 |
3 |
3 |
2 |
Y |
|
5 |
M |
17 |
11 |
M |
AAP |
16 |
2 |
|
|
|
|
|
6 |
F |
18 |
11 |
IM |
CAA |
24 |
1 |
1 |
1 |
0 |
|
|
7 |
F |
15 |
21 |
IM |
AAP |
15 |
3 |
3 |
3 |
2 |
Y |
|
8 |
M |
15 |
12∥
|
M |
AAP |
16 |
2 |
|
|
|
Y |
|
9 |
M |
30 |
21 |
M |
AAA |
12 |
3 |
|
|
|
Y |
|
10 |
22 |
M |
AAA |
12 |
3 |
|
|
|
Y |
|
11 |
M |
28 |
21 |
M |
AAP |
12 |
0 |
|
|
|
Y |
|
12 |
22 |
M |
AAP |
12 |
0 |
|
|
|
Y |
|
1 |
F†
|
15 |
21 |
IM |
CAA |
PRP |
24 |
3 |
3 |
3 |
3 |
Y |
|
2 |
22 |
M |
AAP |
24 |
3 |
|
|
|
|
|
3 |
F |
23 |
11 |
IM |
AAP |
14 |
2 |
2 |
3 |
2 |
Y |
|
4 |
M |
19 |
11 |
M |
CAA |
21 |
2 |
|
|
|
Y |
|
5 |
12 |
M |
AAP |
21 |
2 |
|
|
|
|
|
6 |
M |
18 |
21 |
IM |
AAP |
24 |
3 |
2 |
2 |
1 |
Y |
|
7 |
F‡
|
22 |
11 |
IM |
AAA |
12 |
2 |
3 |
1 |
1 |
Y |
|
8*
|
F |
25 |
11 |
IM |
AAP |
14 |
0 |
0 |
0 |
0 |
|
|
9 |
F |
36 |
11 |
M |
AAA |
14 |
1 |
|
|
|
|
|
10 |
12 |
M |
AAA |
14 |
1 |
|
|
|
|
|
11*¶
|
M |
30 |
35 |
M |
CAA |
12 |
0 |
|
|
|
Y |
|
12*
|
F§
|
27 |
21 |
IM |
AAP |
13 |
0 |
0 |
0 |
0 |
Y |
|
1 |
F†
|
15 |
11 |
IM |
AAP |
PRF |
24 |
3 |
3 |
3 |
2 |
Y |
|
2 |
12 |
M |
CAA |
24 |
3 |
|
|
|
|
|
3 |
M |
18 |
11 |
IM |
AAP |
20 |
2 |
3 |
3 |
0 |
Y |
|
4 |
M |
27 |
11 |
M |
AAA |
18 |
2 |
|
|
|
|
|
5 |
12 |
M |
AAA |
18 |
2 |
|
|
|
|
|
6 |
13 |
M |
AAA |
18 |
3 |
|
|
|
|
|
7 |
F |
28 |
21 |
IM |
AAP |
15 |
2 |
3 |
2 |
1 |
Y |
|
8 |
F‡
|
22 |
21 |
IM |
AAP |
12 |
3 |
2 |
2 |
2 |
Y |
|
9 |
F |
28 |
21 |
IM |
AAP |
12 |
2 |
2 |
2 |
1 |
Y |
|
10 |
F§
|
27 |
11 |
IM |
AAP |
13 |
1 |
3 |
1 |
1 |
Y |
|
11 |
12 |
M |
AAP |
13 |
1 |
|
|
|
|
All the patients had an etiology of dental trauma for pulp necrosis, except 1 patient (No. 3) in the BC group, who had dens evaginatus. Clinically, 1 patient in the PRP group (No. 11) complained of pain and swelling at a 12-month appointment, while the remaining patients were clinically asymptomatic at their follow-up appointments. The statistical analysis revealed no significant difference in the sex distribution (
p = 0.63), the median age of the patients (
p = 0.65), and the median follow-up period (
p = 0.55) among the 3 groups, nullifying the influence of these potential confounding factors on the outcomes (
Table 2).
Table 2 Analysis of demographic data and the follow-up period
|
Variables |
BC (n = 12) |
PRP (n = 12) |
PRF (n = 11) |
p value |
|
Sex |
|
|
|
0.63 |
|
Male |
5 (50.00) |
3 (33.33) |
2 (28.57) |
|
Female |
5 (50.00) |
6 (66.67) |
5 (71.43) |
|
Total*
|
10 (100.00) |
9 (100.00) |
7 (100.00) |
|
Mean age (yr) |
|
|
|
0.65 |
|
Median |
18.50 |
23.00 |
27.00 |
|
IQR |
16.00–28.00 |
18.50–28.50 |
18.00–28.00 |
|
Mean follow-up period (mon) |
|
|
|
0.55 |
|
Median |
15.00 |
14.00 |
18.00 |
|
IQR |
12.00–16.50 |
13.50–22.50 |
13.00–20.00 |
IOPAR evaluation
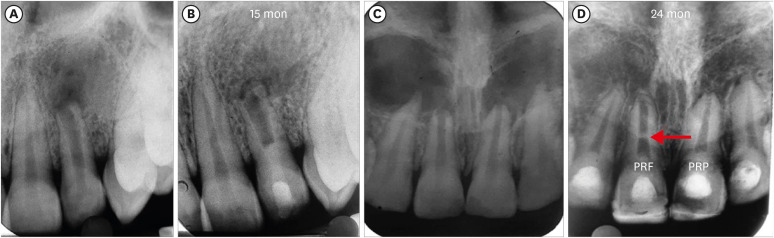
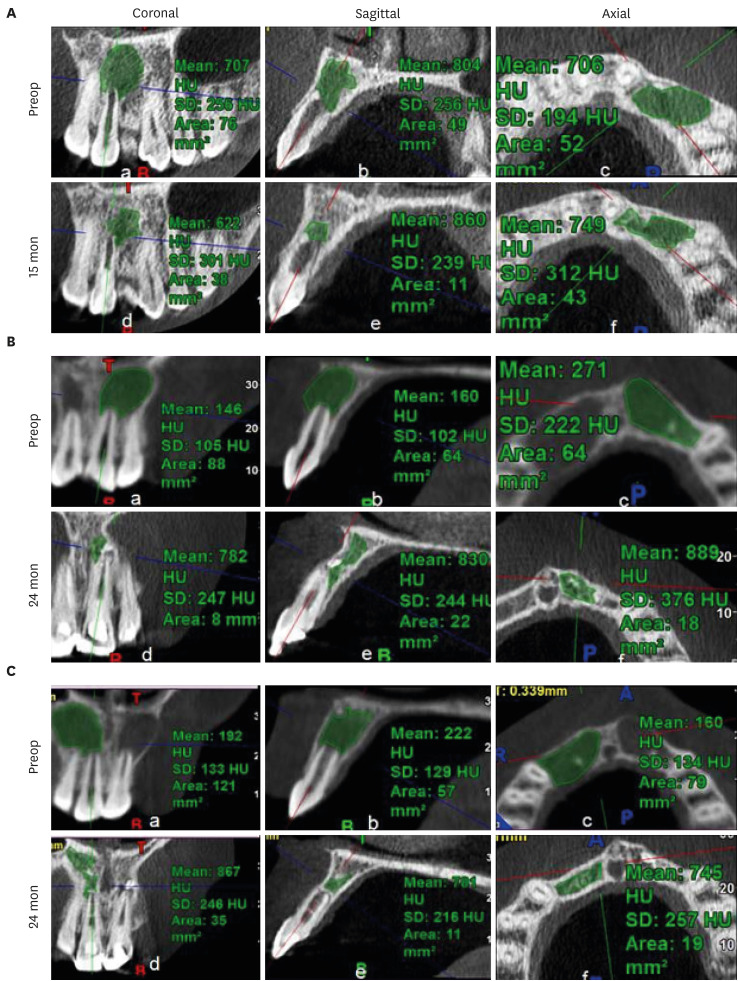
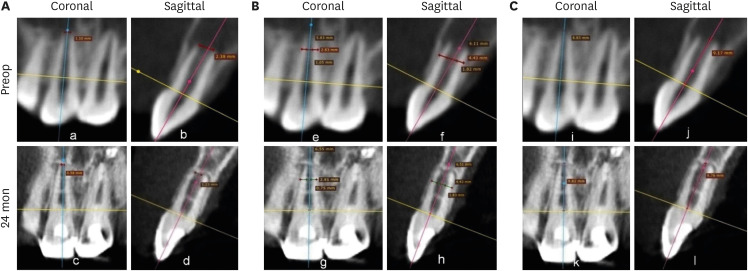
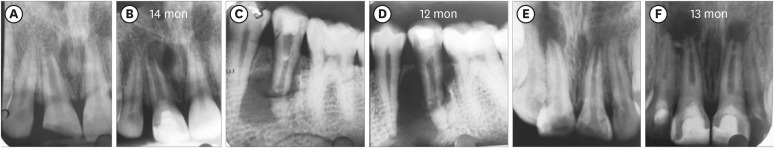
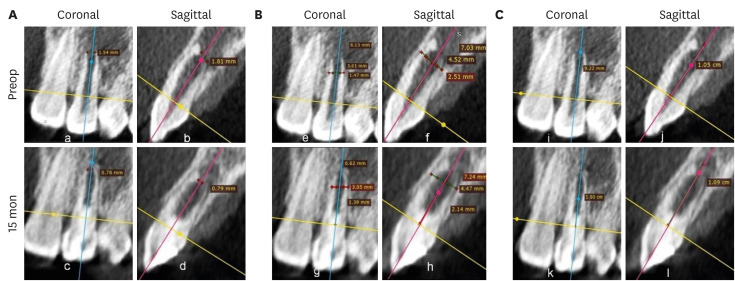
Figure 2 shows representative cases of each group BC, PRP, and PRF.
Figure 2Intraoral periapical radiograph evaluation. (A, B) Blood clot group: No. 1: bone healing (BH)-3, apical closure (AC)-3, root wall thickness (RWT)-3, root length (RL)-1. (C, D) Platelet-rich plasma group: No. 1 (tooth #21): BH-3, AC-3, RWT-3, RL-3, No. 2 (tooth #22: mature): BH-3. Platelet-rich fibrin group: No. 1 (tooth #11): BH-3, AC-3, RWT-3, RL-2, No. 2 (tooth #12: mature): BH-3. Arrow indicates intracanal calcification.
1. BH
Two teeth in the BC group (No. 11 and 12) did not show any sign of BH, and 3 teeth in the PRP group (No. 8, 11, and 12:
Figure 3) showed an increased size of the lesion radiographically (
Table 1) and were scored 0. In contrast, in the PRF group, all the 11 teeth showed evidence of BH, having a score of excellent (3) in 4, good (2) in 5, and fair (1) in 2 teeth. Among the 3 groups, excellent (3) scores were most often found (
n = 6) in the BC group (50%), while 3 teeth exhibited a good (2) score, and 1 tooth had a fair (1) score. In the PRP group, the number of teeth having excellent (3) BH was 3, while a good (2) score was found in 4 teeth, and a fair (1) score in 2 teeth. Considering the number of teeth with good and excellent scores in the 3 groups, it appeared that better BH took place in the teeth of the BC group, followed by the PRF and then the PRP groups, although no significant difference was observed (
p = 0.62) (
Table 3).
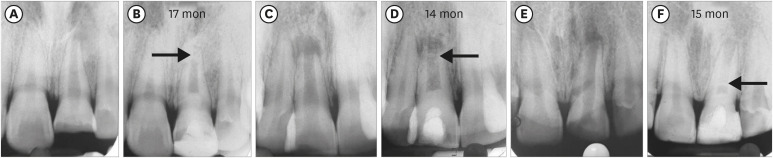
Figure 3Three cases: platelet-rich plasma group: increased lesion size postoperatively. (A, B) No. 8. (C, D) No. 11. (E, F) No. 12.
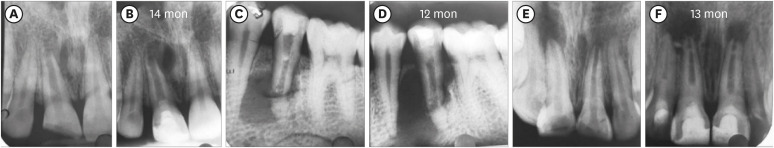
Table 3Analysis of intraoral periapical radiography evaluations
|
Parameter |
BC |
PRP |
PRF |
p value |
|
Mature and immature teeth |
(n = 12) |
(n = 12) |
(n = 11) |
|
|
BH |
|
|
|
0.62 |
|
|
0 |
2 (16.67) |
3 (25.00) |
0 (0.00) |
|
|
1 |
1 (8.33) |
2 (16.67) |
2 (18.18) |
|
|
2 |
3 (25.00) |
4 (33.33) |
5 (45.45) |
|
|
3 |
6 (50.00) |
3 (25.00) |
4 (36.36) |
|
Immature teeth |
(n = 6) |
(n = 6) |
(n = 6) |
|
|
AC |
|
|
|
0.10 |
|
|
0 |
0 (0.00) |
2 (33.33) |
0 (0.00) |
|
|
1 |
2 (33.33) |
0 (0.00) |
0 (0.00) |
|
|
2 |
0 (0.00) |
2 (33.33) |
2 (33.33) |
|
|
3 |
4 (66.67) |
2 (33.33) |
4 (66.67) |
|
RWT |
|
|
|
0.63 |
|
|
0 |
0 (0.00) |
2 (33.33) |
0 (0.00) |
|
|
1 |
1 (16.67) |
1 (16.67) |
1 (16.67) |
|
|
2 |
1 (16.67) |
1 (16.67) |
3 (50.00) |
|
|
3 |
4 (66.67) |
2 (33.33) |
2 (33.33) |
|
RL |
|
|
|
0.79 |
|
|
0 |
1 (16.67) |
2 (33.33) |
1 (16.67) |
|
|
1 |
3 (50.00) |
2 (33.33) |
3 (50.00) |
|
|
2 |
2 (33.33) |
1 (16.67) |
2 (33.33) |
|
|
3 |
0 (0.00) |
1 (16.67) |
0 (0.00) |
|
Mature and immature teeth |
|
|
|
|
|
ICC |
|
|
|
0.68 |
|
|
No |
3 (25.00) |
5 (41.67) |
5 (45.45) |
|
|
Yes |
9 (75.00) |
7 (58.33) |
6 (54.55) |
2. AC
Incidentally, the number of immature teeth was 6 in each group. The BC and PRF groups had the maximum number of teeth with excellent (3) scores (4; 66.67% each) in regard to AC, whereas the remaining 2 teeth had fair (1) scores in the former group and good (2) scores in the latter group. In the PRP group, excellent (3) and good (2) scores of AC were seen in 2 teeth each, and in the remaining 2 teeth, no sign (0) of AC was observed. Based on these results, more evidence of AC was seen in the PRF group than in the BC and PRP groups, but no significant difference (
p = 0.10) was identified (
Table 3).
3. RWT
The BC group had the maximum number (4; 66.67%) of teeth with an excellent (3) score for RWT. One tooth each scored fair (1) and good (2) in this group, whereas 2 teeth in each of the PRP and PRF groups exhibited an excellent (3) score and 50% of teeth in the latter group showed a good (2) score for RWT, while the remaining tooth had a fair (1) score. However, in the former group, 2 teeth showed no sign (0) of RWT, with scores of fair (1) and good (2) for the remaining 2 teeth. Therefore, RWT was evidenced more in the BC group, followed in descending order by the PRF and PRP groups, although there were no significant differences among them (
p = 0.63) (
Table 3).
4. RL
Fifty percent of the immature teeth in BC and PRF group showed a fair (1) score. Two teeth exhibited a good (2) score in both groups, and the remaining 1 tooth showed no sign (0) of RL in each group. Excellent (3) RL was seen in 1 tooth in the PRP group only. In another tooth, the score was good (2) and in the remaining 4 teeth, it was equally distributed between fair (1) and no sign (0). Considering the distribution of good and excellent scores among the 3 groups, it appears that better RL was seen in the PRP group, followed by BC and PRF groups, but with no significant difference among them (
p = 0.79) (
Table 3).
5. Intracanal calcification (ICC)
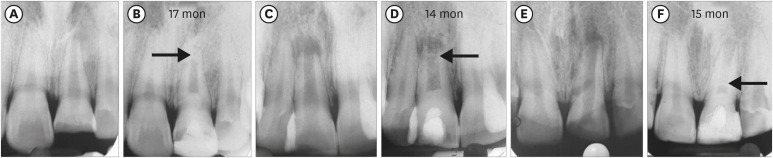
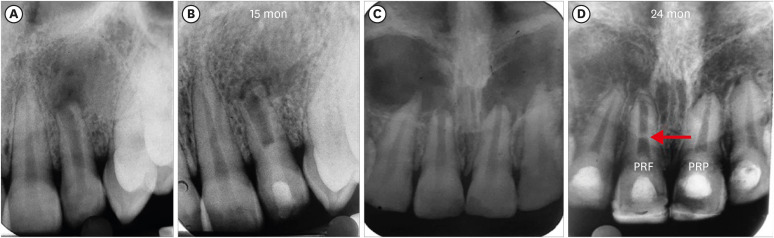
ICC occurring in the root canal space anywhere between capping material and apical foramen was evident in a total of 22 teeth. It was seen more frequently in the BC group (9 teeth: 75%) than in the PRP and PRF groups, in which the number of teeth involved was 7 and 6, respectively (
Figure 4). Calcification was seen in the apical third in 13 teeth, in the middle third in 7 teeth, and in the coronal third of the canal in 2 teeth. It took the form of diffuse deposits in 15 teeth; however, 5 teeth showed calcific bridge-like deposits running from the mesial to the distal canal wall. Nonetheless, the statistical analysis revealed no significant difference in the occurrence of ICC among the 3 groups (
p = 0.68) (
Table 3).
Figure 4Intracanal calcification. (A, B) No. 2 of the blood clot group: apical one-third. (C, D) No. 3 of the platelet-rich plasma group: apical one-third. (E, F) No. 7 of the platelet-rich fibrin group: coronal one-third close to Biodentine.
CBCT evaluation
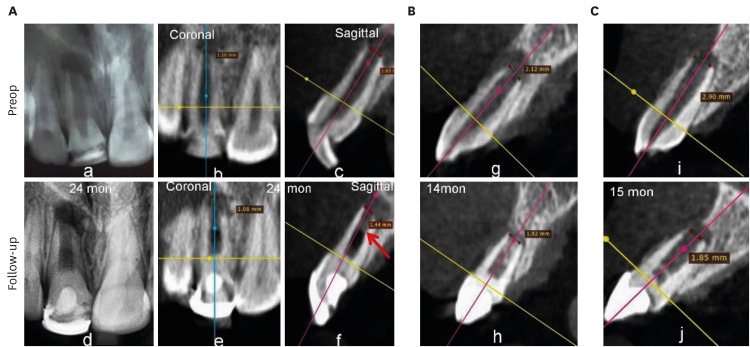
1. PLA
Reduction of PLA was observed in all cases except in the same 3 teeth of the PRP group (No. 8 and 11 [
Figure 5], and 12), in which the lesions increased in size (
Supplementary Table 1), as was seen in the IOPAR observations. The median percentage reduction in PLA in the PRF group (69.57%; IQR: 23.85%–71.24%) was higher than in the BC group (44.02%; IQR: 25.58%–84.48%) and the PRP group (23.08%; IQR: 31.03%–78.17%). However, this difference was not statistically significant (
p = 0.42) (
Table 4,
Figure 6).
Figure 5
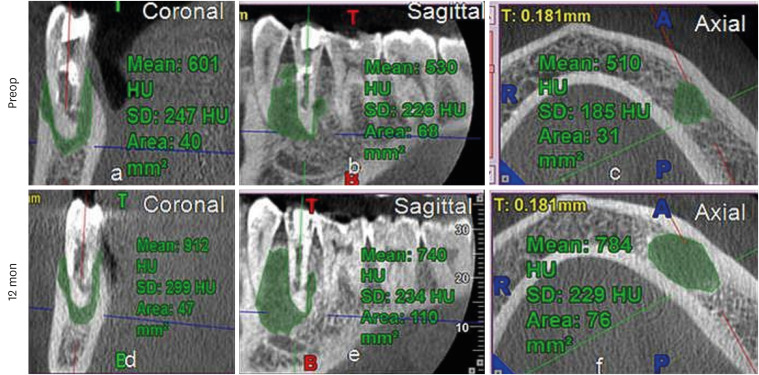
Increased lesion size and gray values in No. 11 of the platelet-rich plasma group.
HU, Hounsfield unit; SD, standard deviation.
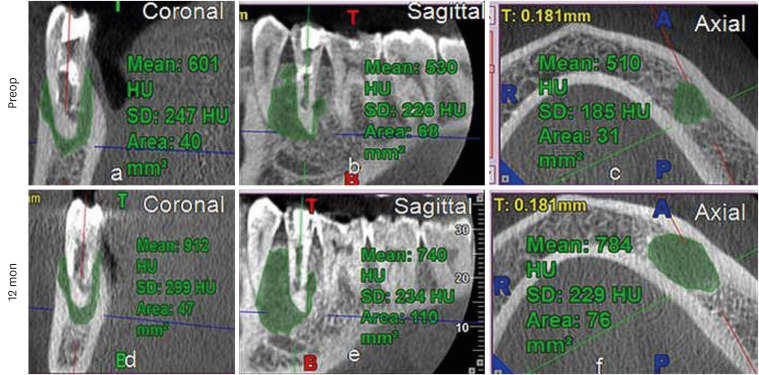
Table 4Analysis of quantitative cone-beam computed tomography measurements
|
Parameter |
BC |
PRP |
PRF |
p value |
|
PLA (mm2) |
|
|
|
|
|
Preop |
|
|
|
0.93 |
|
|
Median |
26.67 |
30.33 |
38.00 |
|
|
IQR |
11.33–74.66 |
13.17–63.50 |
5.33–84.25 |
|
Postop |
|
|
|
0.47 |
|
|
Median |
10.33 |
19.00 |
9.00 |
|
|
IQR |
6.67–30.67 |
10.33–51.71 |
5.00–26.75 |
|
% reduction
|
|
|
|
0.42
|
|
|
Median
|
44.02
|
23.08
|
69.57
|
|
|
IQR
|
25.58–84.48
|
31.03–78.17
|
23.85–71.24
|
|
GV (HU) |
|
|
|
|
|
Preop |
|
|
|
0.46 |
|
|
Median |
591.00 |
489.00 |
460.67 |
|
|
IQR |
374.00–636.00 |
327.50–656.67 |
191.25–572.00 |
|
Postop |
|
|
|
0.29 |
|
|
Median |
761.34 |
737.33 |
938.80 |
|
|
IQR |
673.00–1,017.70 |
583.90–859.90 |
688.00–1,010.00 |
|
Difference
|
|
|
|
0.14
|
|
|
Median
|
261.13
|
174.00
|
438.00
|
|
|
IQR
|
45.60–462.00
|
130.34–382.50
|
276.00–600.75
|
|
AFD (mm) |
|
|
|
|
|
Preop |
|
|
|
0.65 |
|
|
Median |
1.55 |
1.56 |
1.81 |
|
|
IQR |
1.31–1.68 |
1.22–1.83 |
1.34–2.55 |
|
Postop |
|
|
|
0.74 |
|
|
Median |
0.93 |
1.19 |
1.24 |
|
|
IQR |
0.38–1.26 |
0.89–1.64 |
0.74–1.57 |
|
Decrease
|
|
|
|
0.22
|
|
|
Median
|
0.89
|
0.34
|
0.91
|
|
|
IQR
|
0.23–1.16
|
0.13–0.68
|
0.32–1.32
|
|
RWT (mm) |
|
|
|
|
|
Preop |
|
|
|
0.95 |
|
|
Median |
2.20 |
2.27 |
2.31 |
|
|
IQR |
1.94–2.46 |
2.10–2.35 |
2.20–2.38 |
|
Postop |
|
|
|
0.99 |
|
|
Median |
2.45 |
2.58 |
2.61 |
|
|
IQR |
2.15–2.90 |
2.29–2.81 |
2.33–2.86 |
|
Increase
|
|
|
|
0.91
|
|
|
Median
|
0.24
|
0.23
|
0.24
|
|
|
IQR
|
0.19–0.46
|
0.08–0.50
|
0.13–0.42
|
|
RL (mm) |
|
|
|
|
|
Preop |
|
|
|
0.77 |
|
|
Median |
10.28 |
10.70 |
10.95 |
|
|
IQR |
9.07–11.40 |
10.45–11.40 |
9.94–12.25 |
|
Postop |
|
|
|
0.89 |
|
|
Median |
11.23 |
11.55 |
11.15 |
|
|
IQR |
9.66–12.25 |
10.55–11.75 |
10.75–12.80 |
|
Increase
|
|
|
|
0.62
|
|
|
Median
|
0.59
|
0.32
|
0.55
|
|
|
IQR
|
0.08–0.96
|
0.08–0.76
|
0.30–0.80
|
|
ICC |
|
|
|
0.14 |
|
No |
1 (8.33) |
4 (33.33) |
5 (45.45) |
|
Yes |
11 (91.67) |
8 (66.67) |
6 (54.55) |
|
Total |
12 (100.00) |
12 (100.00) |
11 (100.00) |
|
Figure 6
Decrease in periapical lesion area and gain in gray values (Hounsfield unit [HU]). (A) No. 1 of the blood clot group. (B) No. 1 of the platelet-rich plasma group. (C) No. 1 of the platelet-rich fibrin group.
SD, standard deviation.
2. GV
All cases showed increased GV, except 2 teeth in the PRP group (No. 8 and 12), which showed a decrease in GV. However, interestingly, an increase by 265 HU was observed in No. 11 in PRP group, with an increase in lesion size (
Figure 5,
Supplementary Table 1). The lowest increase in GV was seen in the PRP group (174 HU; IQR: 130.34–382.5 HU). The maximum value was in the PRF group (438.00 HU; IQR: 276–600.75 HU), followed by the BC group (261.13 HU; IQR: 45.60–462 HU). This difference, however, was not statistically significant (
p = 0.14) (
Table 4,
Figure 6).
3. AFD, RWT, and RL
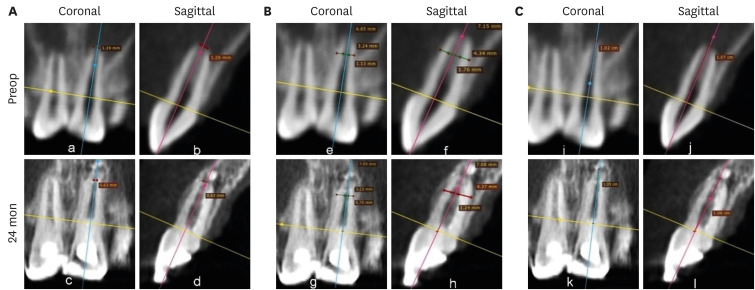
Figures 7,
8,
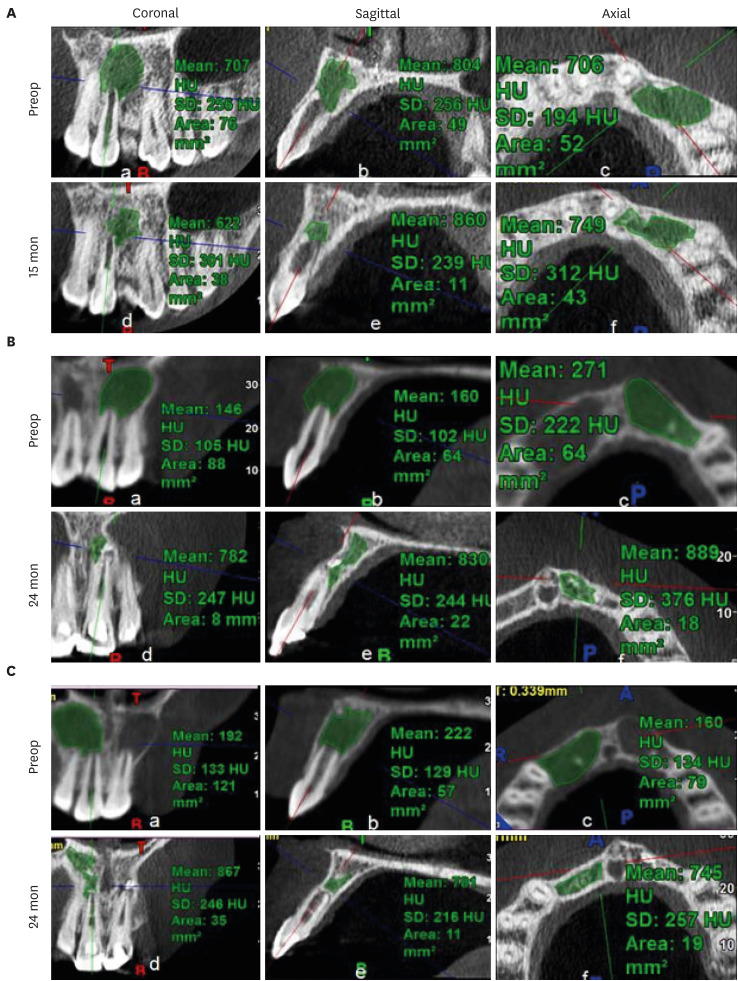
9 shows the measurement of AFD, RWT, and RL in one representative case from each group (BC, PRP, and PRF, respectively). In immature teeth, postoperative measurements of the roots revealed that a decrease in AFD and some amount of increase in RWT and RL took place in all 3 groups (
Supplementary Table 1). The median decrease in AFD was highest in the PRF group (0.91 mm; IQR: 0.32–1.32 mm), followed by the BC group (0.89 mm; IQR: 0.23–1.16 mm) and the PRP group (0.34 mm; IQR: 0.13–0.68 mm). The median increase in RWT was approximately the same in all the 3 groups; the median values were 0.24 mm (IQR: 0.19–0.46 mm) in the BC group, 0.23 mm (IQR: 0.08–0.5 mm) in the PRP group, and 0.24 mm (IQR: 0.13–0.42 mm) in the PRF group. The median increase in RL differed within a millimeter, but was highest in the BC group (0.59 mm; IQR: 0.08–0.96). The statistical analysis showed that there was no significant difference in root maturation in the 3 groups, as revealed by quantitative measurements of the decreased AFD (
p = 0.22), increased RWT (
p = 0.91), and increased RL (
p = 0.62) (
Table 4).
Figure 7No. 1 of the blood clot group. (A) Decrease in the apical foramen diameter, (B) increase in root wall thickness, and (C) increase in root length.
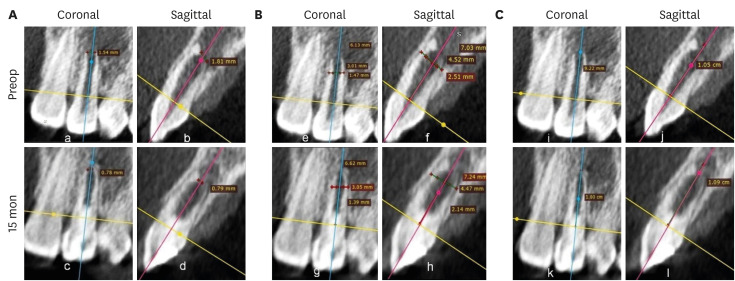
Figure 8No. 1 of the platelet-rich plasma group. (A) Decrease in apical foramen diameter, (B) increase in root wall thickness, and (C) increase in root length.
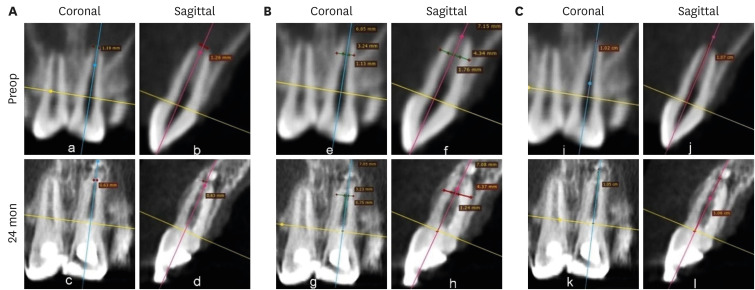
Figure 9No. 1 of the platelet-rich fibrin group. (A) Decrease in apical foramen diameter, (B) increase in root wall thickness, (C) increase in root length.
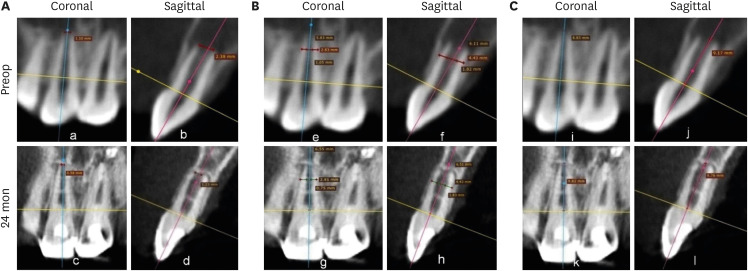
4. ICC
Figure 10 represents occurrence of ICC in each group. ICC was seen in 25 teeth, with the highest frequency in the BC group (11: 91.67%), followed by the PRP group (8: 66.667%) and the PRF group (6: 54.55%) (
Supplementary Table 1,
Table 4). The presence of ICC was also significantly greater in immature than in mature teeth. This observation was seen in both IOPAR (
p = 0.015) and CBCT (
p = 0.003) observations (
Table 5).
Figure 10Intracanal calcification. (A) No. 6 in the blood clot group: discernible on cone-beam computed tomography only, (B) No. 3: platelet-rich plasma group: diffuse in nature, (C) No. 7: platelet-rich fibrin group: calcific bridge apical to Biodentine.
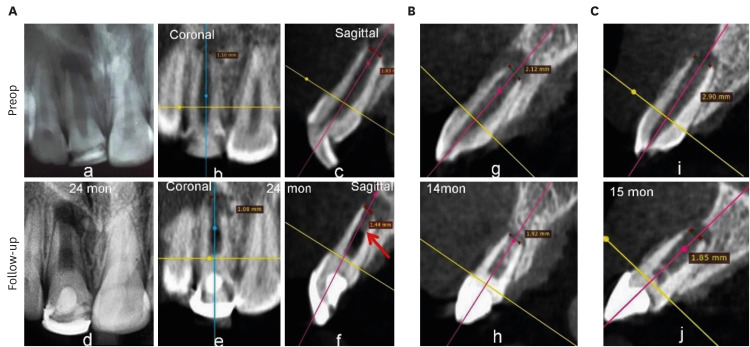
Table 5Comparison of intracanal calcification (ICC) in mature and immature teeth
|
ICC |
Immature (n = 18) |
Mature (n = 17) |
Total (n = 35) |
p value |
|
IOPAR |
|
|
|
0.015 |
|
No |
3 (16.67) |
10 (58.82) |
13 (37.14) |
|
Yes |
15 (83.33) |
7 (41.18) |
22 (62.86) |
|
CBCT |
|
|
|
0.003 |
|
No |
1 (5.56) |
9 (52.94) |
10 (28.57) |
|
Yes |
17 (94.44) |
8 (47.60) |
25 (71.43) |
Pulp sensibility test
Two teeth in the BC group responded positively to the cold test in the present study at 15- and 16-month follow-up visits. However, none of the teeth responded positively to the electric pulp test (
Table 1).
DISCUSSION
There has been an ongoing search for the most suitable scaffold for REPs, as well as for the most appropriate evaluation method to assess the outcomes. The present study incorporated 3 commonly used scaffolds (i.e., BC, PRP, and PRF) and analyzed them through both IOPAR and CBCT using various parameters. Qualitative IOPAR scoring and quantitative CBCT measurements were analyzed, as it is known that CBCT quantitative measurements are not significantly different from the actual measurements of teeth [
23]. In the present study, IOPAR was used instead of radiovisiography (RVG), as IOPAR is a conventional method. Although RVG offers less radiation exposure, a study reported that there were no significant differences in bone loss measurements made using IOPAR and RVG [
24].
The present study revealed that the primary outcome (i.e., the absence of clinical signs/symptoms and evidence of BH) was achieved in 85.71% of teeth (30/35), and the secondary outcome (i.e., continued root development of immature tooth) was seen in 88.88% (16/18). The tertiary outcome (i.e., a positive response to the pulp sensibility test) was seen in 5.71% of teeth (2/35) (
Table 1).
There was no significant difference in the extent of BH of periapical lesions among the 3 groups, evaluated through IOPAR, which is consistent with the studies of Ulusoy
et al. [
1] and Murray [
10]. A similar result was also seen in several other studies involving a comparison of either 2 of these 3 scaffolds [
2,
11,
12,
16,
17]. Some studies have also expressed conflicting opinions, stating that PRP was better than BC or PRF, or identifying PRP as superior when these 3 scaffolds were compared [
8,
9,
15].
No study was found in the review of the literature regarding the quantitative evaluation of BH among 3 host-derived scaffolds using CBCT. However, 2 reported studies with BC and PRP concluded that there was no significant difference in the reduction of the lesion size and the increase in bone density at a 12-month follow-up period [
2,
18]. In contrast, the present study investigated BC, PRP, and PRF, and no significant differences were found in the quantitative analysis of these 2 parameters for the evaluation of BH (i.e., reduction of lesion size and increase in bone density) within the median follow-up period of the respective groups, which was longer than in the previous 2 studies [
2,
18].
RL, RWT, and AC are important parameters for assessing REPs in immature teeth. In the IOPAR evaluation, there were no significant differences in the above 3 parameters among the 3 groups, which is similar to the study of Ulusoy
et al. [
1]. No significant difference was found in RL and RWT by 2 other studies and in AC by another study when 3 groups were compared [
8,
9,
10]. In comparisons of 2 groups, such as BC and PRF, BC and PRP, and PRP and PRF, similar results were obtained regarding these 3 parameters [
2,
12,
16]. However, AC was more evident in autologous platelet concentrates than in BC in the studies of Murray (a meta-analysis) [
10], Turky
et al. [
14], and Rizk
et al. [
15]. However, a significantly greater increase in RL was seen in the PRP group in the latter 2 studies and in RWT in the last study when BC and PRP were compared [
14,
15]. RWT and RL were seen more frequently in the PRF group in the study of Narang
et al. [
8], which compared 3 groups.
In the present study, the quantitative measurements of these 3 parameters did not differ significantly among the 3 groups. As with BH, a search of the literature failed to find a single study that conducted a comparative evaluation of these 3 host-derived scaffolds in terms of root maturation through CBCT. CBCT studies by Alagl
et al. [
18] and ElSheshtawy
et al. [
2] compared BC with PRP, and a significant increase in RL was shown in the PRP group in the former study, but all 3 parameters did not significantly differ in the latter. The minimum increase in RL in all 3 groups was supported by the study of Kahler
et al. [
25], with an 8-year follow-up period, and by Lin
et al. [
26], who reported that the etiology had an impact on REP outcomes; specifically, dens evaginatus cases had better increases in RL and width than teeth in which trauma was the etiology of necrosis. In the present study, the etiology was dental trauma in all cases except 1 tooth.
A positive response to the pulp sensibility test after REPs, as reported in previous studies, was also seen in 2 teeth in the present study (
Table 1) [
2,
9,
17]. However, the negative findings in the pulp sensibility test could have been due to the thickness of mineral trioxide aggregate [
27]. Moreover, dentin sensitivity in the natural pulp-dentin complex is related to the hydrodynamic activity of dentinal tubules in association with A-β sensory fibers, whereas a histopathological study revealed that newly regenerated, mineralized, root canal tissue did not appear to have well-organized dentinal tubules and thus may not exhibit the same sensitivity as natural tissue [
28]. In the present study, even cases with significant hard tissue deposition showed a negative response to the pulp sensibility test (
Figure 4B and D).
In the present study, CBCT detected ICC in more teeth than IOAPR, as both sagittal and coronal sections could be analyzed for any opacities within the root canal space indicating ICC, and its incidence was higher in immature teeth. The literature on trauma also supports this finding [
29]. The higher incidence of ICC in the present study may be due to the compound effect of the use of calcium hydroxide as an intracanal medicament, along with the other contributing factors, as revascularization-associated intracanal calcification (RAIC) was reported to be present in 76.9% in cases medicated with calcium hydroxide, constituting a higher percentage than was observed when antibiotic paste was used (46.2%) in the study of Song
et al. [
30]. ICC occurred more frequently in the BC group than in the groups using platelet concentrates, as inducing bleeding from the periapex may carry periodontal ligament stem cells and bone marrow stem cells from the alveolar bone, thereby recruiting cells with cementogenic and osteogenic differentiation capacities into the root canal space, leading to ectopic cementum and bone formation inside the lumen of the root canal [
31,
32]. As in the present study, Song
et al. [
30] also reported that RAIC was more prevalent when bleeding was induced in the canal (69.6%) than in cases without bleeding (33.4%). Ulusoy
et al. [
1] also reported a lower risk of ICC when platelet concentrates were used. However, ICC did also occur in cases where platelet concentrates were used, as all these scaffolds have the same source of growth factors (i.e., platelets). This could suggest the possibility that the current REP protocols and blood/blood-derived scaffolds recruit osteogenic and cementogenic cells into the root canal. Pulp hemorrhage after trauma can also become a nidus for calcification and lead to narrowing of the pulp canal space [
33]. Additionally, ICC may be related to the osteoinductive activity of calcium silicate materials [
34]. The incidence of the calcific bridge just below Biodentine was also seen in a few cases in the present study; however, these calcifications were seen in all groups. Thus, an analysis of the results of the present study confirmed the null hypothesis.
This study has some limitations, as follows. First, the sample size for the final evaluation was small due to the non-reporting of some patients due to the coronavirus disease 2019 (COVID-19) pandemic. Second, no volumetric measurements were done using CBCT; instead, only 2D measurements were performed. Third, the inability to blind the operator and patient might have introduced some procedural bias. Lastly, the follow-up period for all patients was variable, either because patients did not report for follow-up visits at the specified time or due to COVID-19-related restrictions.
CONCLUSIONS
REPs proved to be a viable treatment option for both non-vital immature and mature teeth with periapical pathology. Qualitative IOPAR evaluations and quantitative CBCT measurements revealed that BC, PRP, and PRF as scaffolds had similar potential for periapical lesion healing and AC, RWT, and RL in immature tooth. The effects on ICC may be discernable in subsequent long-term follow-up visits.
-
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
-
Author Contributions:
Conceptualization: Markandey S, Adhikari HD.
Data curation: Markandey S.
Formal analysis: Markandey S.
Funding acquisition: Markandey S.
Investigation: Markandey S.
Methodology: Markandey S.
Project administration: Adhikari HD.
Resources: Adhikari HD.
Software: Markandey S.
Supervision: Adhikari HD.
Validation: Adhikari HD.
Visualization: Adhikari HD.
Writing - original draft: Markandey S.
Writing - review & editing: Markandey S, Adhikari HD.
SUPPLEMENTARY MATERIALS
Supplementary Table 1
Quantitative evaluation of blood clot (BC), platelet-rich plasma (PRP), and platelet-rich fibrin (PRF) group through cone-beam computed tomography
rde-47-e41-s001.xls
Supplementary Figure 1
Cone-beam computed tomography scan for measurement of different parameters. (A-C) Periapical lesion area and gray values (Hounsfield unit [HU]) in 3 planes, (D, E) apical foramen diameter, (F, G) root wall thickness, and (H, I) root length.
rde-47-e41-s002.ppt
REFERENCES
- 1. Ulusoy AT, Turedi I, Cimen M, Cehreli ZC. Evaluation of blood clot, platelet-rich plasma, plateletrich fibrin, and platelet pellet as scaffolds in regenerative endodontic treatment: a prospective randomized trial. J Endod 2019;45:560-566.ArticlePubMed
- 2. ElSheshtawy AS, Nazzal H, El Shahawy OI, El Baz AA, Ismail SM, Kang J, Ezzat KM. The effect of platelet-rich plasma as a scaffold in regeneration/revitalization endodontics of immature permanent teeth assessed using 2-dimensional radiographs and cone beam computed tomography: a randomized controlled trial. Int Endod J 2020;53:905-921.ArticlePubMedPDF
- 3. Egusa H, Sonoyama W, Nishimura M, Atsuta I, Akiyama K. Stem cells in dentistry--part I: stem cell sources. J Prosthodont Res 2012;56:151-165.ArticlePubMed
- 4. Wingard JR, Demetri GD. Clinical applications of cytokines and growth factors. New York, NY: Springer; 1999.
- 5. Zhao S, Sloan AJ, Murray PE, Lumley PJ, Smith AJ. Ultrastructural localisation of TGF-β exposure in dentine by chemical treatment. Histochem J 2000;32:489-494.ArticlePubMedPDF
- 6. O’Brien FJ. Biomaterials & scaffolds for tissue engineering. Mater Today 2011;14:88-95.Article
- 7. American Association of Endodontists. Clinical considerations for a regenerative procedure. updated April 1, 2018]. cited June 9, 2021]. Available from: https://www.aae.org.
- 8. Narang I, Mittal N, Mishra N. A comparative evaluation of the blood clot, platelet-rich plasma, and platelet-rich fibrin in regeneration of necrotic immature permanent teeth: a clinical study. Contemp Clin Dent 2015;6:63-68.ArticlePubMedPMC
- 9. Shivashankar VY, Johns DA, Maroli RK, Sekar M, Chandrasekaran R, Karthikeyan S, Renganathan SK. Comparison of the effect of PRP, PRF and induced bleeding in the revascularization of teeth with necrotic pulp and open apex: a triple blind randomized clinical trial. J Clin Diagn Res 2017;11:ZC34-ZC39.Article
- 10. Murray PE. Platelet-rich plasma and platelet-rich fibrin can induce apical closure more frequently than blood-clot revascularization for the regeneration of immature permanent teeth: a meta-analysis of clinical efficacy. Front Bioeng Biotechnol 2018;6:139.ArticlePubMedPMC
- 11. Prabhakar AR, Rani NS, Yavagal C. Revascularization of immature necrotic teeth with platelet- rich fibrin and blood clot. Int J Oral Health Sci 2016;6:4-10.Article
- 12. Lv H, Chen Y, Cai Z, Lei L, Zhang M, Zhou R, Huang X. The efficacy of platelet-rich fibrin as a scaffold in regenerative endodontic treatment: a retrospective controlled cohort study. BMC Oral Health 2018;18:139.ArticlePubMedPMCPDF
- 13. Bezgin T, Yilmaz AD, Celik BN, Kolsuz ME, Sonmez H. Efficacy of platelet-rich plasma as a scaffold in regenerative endodontic treatment. J Endod 2015;41:36-44.ArticlePubMed
- 14. Turky M, Kataia MA, Ali MMM, Hassan RE. Revascularization induced maturogenesis of human non-vital immature teeth via platelets- rich plasma (PRP): radiographic study. J Dent Oral Health 2017;3:097.
- 15. Rizk HM, Al-Deen MS, Emam AA. Regenerative endodontic treatment of bilateral necrotic immature permanent maxillary central incisors with platelet-rich plasma versus blood clot: a split mouth double-blinded randomized controlled trial. Int J Clin Pediatr Dent 2019;12:332-339.ArticlePubMedPMC
- 16. Rizk HM, Salah Al-Deen MS, Emam AA. Comparative evaluation of platelet rich plasma (PRP) versus platelet rich fibrin (PRF) scaffolds in regenerative endodontic treatment of immature necrotic permanent maxillary central incisors: a double blinded randomized controlled trial. Saudi Dent J 2020;32:224-231.ArticlePubMed
- 17. Adhikari HD, Sujith R, Gupta A, Markandey S. Comparative evaluation of platelet rich plasma and platelet rich fibrin as a scaffold for regenerative endodontic procedure: a clinical study. IOSR J Dent Med Sci 2021;20:51-58.
- 18. Alagl A, Bedi S, Hassan K, AlHumaid J. Use of platelet-rich plasma for regeneration in non-vital immature permanent teeth: clinical and cone-beam computed tomography evaluation. J Int Med Res 2017;45:583-593.ArticlePubMedPMCPDF
- 19. Farhad Mollashahi N, Saberi E, Karkehabadi H. Evaluation of cytotoxic effects of various endodontic irrigation solutions on the survival of stem cell of human apical papilla. Iran Endod J 2016;11:293-297.PubMedPMC
- 20. Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, Gogly B. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006;101:e37-e44.ArticlePubMed
- 21. Amable PR, Carias RB, Teixeira MV, da Cruz Pacheco I, Corrêa do Amaral RJ, Granjeiro JM, Borojevic R. Platelet-rich plasma preparation for regenerative medicine: optimization and quantification of cytokines and growth factors. Stem Cell Res Ther 2013;4:67.ArticlePubMedPMCPDF
- 22. Tanomaru-FIlho M, Jorge ÉG, Guerreiro-Tanomaru JM, Reis JM, Spin-Neto R, Gonçalves M. Two- and tridimensional analysis of periapical repair after endodontic surgery. Clin Oral Investig 2015;19:17-25.ArticlePubMedPDF
- 23. Sherrard JF, Rossouw PE, Benson BW, Carrillo R, Buschang PH. Accuracy and reliability of tooth and root lengths measured on cone-beam computed tomographs. Am J Orthod Dentofacial Orthop 2010;137:S100-S108.ArticlePubMed
- 24. Ashwinirani SR, Suragimath G, Jaishankar HP, Kulkarni P, Bijjaragi SC, Sangle VA. Comparison of diagnostic accuracy of conventional intraoral periapical and direct digital radiographs in detecting interdental bone loss. J Clin Diagn Res 2015;9:ZC35-ZC38.
- 25. Kahler B, Kahler SL, Lin LM. Revascularization-associated Intracanal calcification: a case report with an 8-year review. J Endod 2018;44:1792-1795.ArticlePubMed
- 26. Lin J, Zeng Q, Wei X, Zhao W, Cui M, Gu J, Lu J, Yang M, Ling J. Regenerative endodontics versus apexification in immature permanent teeth with apical periodontitis: a prospective randomized controlled study. J Endod 2017;43:1821-1827.ArticlePubMed
- 27. Torabinejad M, Turman M. Revitalization of tooth with necrotic pulp and open apex by using platelet-rich plasma: a case report. J Endod 2011;37:265-268.ArticlePubMed
- 28. Huang GT. Dental pulp and dentin tissue engineering and regeneration: advancement and challenge. Front Biosci (Elite Ed) 2011;3:788-800.ArticlePubMedPMC
- 29. Andreasen FM, Kahler B. Pulpal response after acute dental injury in the permanent dentition: clinical implications-a review. J Endod 2015;41:299-308.ArticlePubMed
- 30. Song M, Cao Y, Shin SJ, Shon WJ, Chugal N, Kim RH, Kim E, Kang MK. Revascularization- associated intracanal calcification: assessment of prevalence and contributing factors. J Endod 2017;43:2025-2033.ArticlePubMed
- 31. Adhikari HD, Gupta A. Report of a case of platelet-rich fibrin-mediated revascularization of immature 12 with histopathological evaluation. J Conserv Dent 2018;21:691-695.ArticlePubMedPMC
- 32. Austah O, Joon R, Fath WM, Chrepa V, Diogenes A, Ezeldeen M, Couve E, Ruparel NB. Comprehensive characterization of 2 immature teeth treated with regenerative endodontic procedures. J Endod 2018;44:1802-1811.ArticlePubMed
- 33. Andreasen FM. Pulpal healing after luxation injuries and root fracture in the permanent dentition. Endod Dent Traumatol 1989;5:111-131.ArticlePubMed
- 34. Wang X, Thibodeau B, Trope M, Lin LM, Huang GT. Histologic characterization of regenerated tissues in canal space after the revitalization/revascularization procedure of immature dog teeth with apical periodontitis. J Endod 2010;36:56-63.ArticlePubMed
 , Haridas Das Adhikari
, Haridas Das Adhikari












 KACD
KACD
 ePub Link
ePub Link Cite
Cite

