Abstract
-
Objective
This study investigated the effects of various concentrations of sodium hypochlorite (NaOCl) on human whole-blood clotting kinetics, the structure of the blood clots formed, and transforming growth factor (TGF)-β1 release.
-
Materials and Methods
Human whole blood was collected from 5 healthy volunteers and divided into 4 groups: CG (control, 0.5 mL of blood), BN0.5 (0.5 mL of blood with 0.5 mL of 0.5% NaOCl), BN3 (0.5 mL of blood with 0.5 mL of 3% NaOCl), and BN5.25 (0.5 mL of blood with 0.5 mL of 5.25% NaOCl). The effects of NaOCl on clotting kinetics, structure of fibrin and cells, and release of TGF-β1 were assessed using thromboelastography (TEG), scanning electron microscopy (SEM), and enzyme-linked immunosobent assay, respectively. Statistical analysis was conducted using the Kruskal Wallis and Mann-Whitney U tests, followed by the post hoc Dunn test. A p value < 0.05 indicated statistical significance.
-
Results
The blood samples in BN0.5 and BN3 did not clot, whereas the TEG of BN5.25 showed altered clot formation. Samples from the CG and BN3 groups could only be processed with SEM, which showed that the latter lacked fibrin formation and branching of fibers, as well as clumping of red blood cells with surface roughening and distortion. TGF-β1 release was significantly highest in BN3 when all groups were compared to CG (p < 0.05).
-
Conclusions
Each concentration of NaOCl affected the release of TGF-β1 from blood clots and altered the clotting mechanism of blood by affecting clotting kinetics and cell structure.
-
Keywords: Blood clot; Fibrin; Sodium hypochlorite; TGF-β1; Vital pulp therapy
INTRODUCTION
Wound healing is a complex mechanism involving blood coagulation, inflammatory and immune reactions, angiogenesis, cell migration and proliferation [
1]. Platelets are usually the first cells to migrate and initiate platelet plug formation and release platelet-derived growth factor, transforming growth factor-beta 1 (TGF-β1), epidermal growth factor, and fibroblast growth factor. Fibrin acts as a temporary support and plays a pivotal role in the binding of cells and proteins, which is important to the wound healing process. High- and low-molecular-weight fibrinogen regulates the recruitment of cells for cell adhesion, migration, proliferation, and tubule formation, which are beneficial for wound repair [
2]. The cell seeding and nutrient transport properties of blood clots, as well as their physical and mechanical strength, make them ideal scaffolds for wound healing [
3].
Vital pulp therapy is aimed at preserving and maintaining healthy pulp tissue that has been compromised by trauma, caries, or restorative procedures [
4]. The European Society of Endodontology position statement on the management of deep caries and exposed pulp suggests the use of 0.5%–5% sodium hypochlorite (NaOCl) as a hemostatic and disinfectant agent during pulpotomy procedures [
5].
Hypochlorous acid, a by-product of NaOCl, modifies clotting factors by inhibiting platelet aggregation [
6]. Chlorine, a chaotropic agent, interacts in a similar way with blood proteins and causes significantly unfavorable effects on fibrin polymerization by preventing the growth of thicker, stiffer, and straighter fibers [
7,
8]. Furthermore, NaOCl is a strong base and dissolves pulp due to its high pH (> 11) [
9].
Pashley
et al. [
10] reported complete hemolysis of red blood cells (RBCs) after exposure to 5.25% NaOCl, even at dilutions of 1:1,000. It has also been proven that NaOCl affects the protein component of blood and the protein supernatants, which tended to decompose with an increase in NaOCl concentration [
11].
To the best of the authors’ knowledge, no evidence-based literature exists on the effect of various NaOCl concentrations on blood clotting kinetics, fibrin structure, and growth factor release. The objective of this study was to evaluate the effect of various concentrations (0.5%, 3%, and 5.25%) of NaOCl on human whole-blood clotting kinetics, blood clot structure, and TGF-β1 release.
MATERIALS AND METHODS
The study protocol was reviewed and approved by the Institutional Review Board (MADC/IRB-XXV/2018/385), and informed consent was obtained from the 5 volunteers prior to the phlebotomy procedure. The manuscript has been prepared based on the Preferred Reporting Items for Laboratory Studies in Endodontology 2021 guidelines (
Supplementary Figure 1) [
12].
Five healthy individuals aged 20–30 years, with no underlying systemic disorders, not on chronic medications, and classified as American Society of Anesthesiologists class I consented to participate in this study. Based on World Health Organization guidelines, 6 mL of blood was collected from the antecubital fossa of each volunteer (based on hematocrit values) by a qualified phlebotomist [
13].
Based on literature [
11], the estimation of sample size was performed for an alpha error of 5% at 80% statistical power with an expected mean ± standard deviation bilirubin level (hemolysis) of 0.925 ± 0.278 µg/mL when exposed to 2.5% NaOCl and of 0.355 ± 0.110 µg/mL when exposed to 5% NaOCl. A sample size of 16 was estimated (
n = 4 per group) using OpenEpi (
www.OpenEpi.org) [
14]. Thus, blood was collected from 5 healthy volunteers and divided into the 4 study groups.
Clotting kinetics were assessed using the TEG 5000 Hemostasis Analyzer System (Haemoscope Corporation, Niles, IL, USA). Vials coated with 1% kaolin (Haemoscope Corporation, Niles, IL, USA) were loaded with various concentrations of NaOCl (Sigma-Aldrich, St. Louis, MO, USA). A 2 mL sample of blood was drawn from each volunteer by the venipuncture syringe method with 21-gauge needles (Hindustan Syringes and Medical Devices Ltd., Faridabad, India).
The experimental groups, based on NaOCl concentration, were divided into the CG group (control, 0.5 mL blood), BN
0.5 group (0.5 mL blood mixed with 0.5 mL 0.5% NaOCl), BN
3 group (0.5 mL blood mixed with 0.5 mL 3% NaOCl), and BN
5.25 group (0.5 mL blood mixed with 0.5 mL 5.25% NaOCl). After inverting the vials 6 times to ensure adequate mixing, 340 μL was transferred to the TEG cup using a micropipette (Sartorius AG, Lower Saxony, Germany). Five major blood clotting kinetic parameters were assessed: reaction time (R), kinetics (K), alpha angle (α), maximum amplitude (MA), and coagulation index (CI) (
Table 1).
Table 1Intergroup and intragroup comparison of mean and standard deviation values of various thromboelastography parameters and concentration of TGF-β1 release (pg/mL)
|
Variables |
Mean ± SD |
Dunn’s pairwise comparison |
|
CG |
BN0.5
|
BN3
|
BN5.25
|
CG vs. BN0.5
|
CG vs. BN3
|
CG vs. BN5.25
|
BN0.5 vs. BN3
|
BN0.5 vs. BN5.25
|
BN3 vs. BN5.25
|
|
Thromboelastography parameters |
|
|
|
|
|
|
|
|
|
|
|
Interpretation (Ioscovich et al. 2016) |
Normal values (Ioscovich et al. 2016) |
|
|
|
|
|
|
|
|
|
|
|
|
R |
Activation phase of intrinsic coagulation pathway and initiation of fibrin deposition. |
4–8 min |
5.25 ± 0.918 |
57.4 ± 13.446 |
36.72 ± 6.45 |
26.94 ± 23.239 |
0.0003*
|
0.0137*
|
0.0270*
|
0.0772 |
0.0443*
|
0.3835 |
|
|
K |
Amplification of the fibrin deposition and cross-linking of fibrin threads. |
0–4 min |
2.36 ± 0.702 |
- |
- |
6.6 ± 2.253 |
- |
- |
0.0357*
|
- |
- |
- |
|
|
α |
Propagation phase of blood clot formation with the maximum speed of thrombin generation, fibrin deposition, and continued cross-linking of fibrin threads. |
47–74° |
56.96 ± 13.465 |
- |
- |
30.133 ± 8.181 |
- |
- |
0.0357*
|
- |
- |
- |
|
|
MA |
Maximal mechanical strength of the clot formed in the termination phase. |
54–72 mm |
64.9 ± 7.677 |
- |
- |
83.36 ± 7.90 |
- |
- |
0.0357*
|
- |
- |
- |
|
|
CI |
Coagulability state of the samples derived from the R, K, MA, and A values. Values greater than 3 indicate a hypercoagulability state, whereas values less than three indicate a hypocoagulability state. |
−3 to 3 |
0.82 ± 2.42 |
- |
- |
−4.53 ± 3.744 |
- |
- |
0.1429 |
- |
- |
- |
|
Concentration of TGF-β1 release (pg/mL) |
485.1 ± 39.35 |
516.8 ± 73.33 |
695.5 ± 148.03 |
519.833 ± 148.26 |
0.1842 |
0.0001*
|
0.3848 |
0.0021*
|
0.1166 |
0.0000*
|
Structural analysis of the blood clot under scanning electron microscopy (SEM)
Blood samples were collected as they were for TEG analysis. The samples were left undisturbed at room temperature for 1 hour to allow complete polymerization of the fibrin [
15]. The clot/clot-like specimens obtained were first washed in 0.05 M sodium cacodylate (pH 7.4) (Sisco Research Laboratories, Mumbai, India) followed by 0.10 M sodium chloride (Sisco Research Laboratories) for 30 minutes. At room temperature, the clots were then fixed in 2.5% glutaraldehyde in phosphate-buffered saline (Sigma-Aldrich) for 1 hour. The specimens were then washed and dehydrated in grades of ethanol dilutions (Sigma-Aldrich) ranging from 30%–100%. Blood clot specimens were later dried and treated with hexamethyldisilazane (Sigma-Aldrich) under a steel fume hood (Bionics Scientific Technologies Pvt. Ltd., Delhi, India) for 10 minutes. The samples were then sputter coated with gold and visualized up to ×12,000 magnification under the SEM FEI Quanta FEG 200 (FEI Company, Hillsboro, OR, USA). Each specimen was imaged at 3 locations along the surface.
Blood samples were collected and divided into the same experimental groups as they were for the TEG analysis. The samples were left undisturbed for 3 hours and centrifuged (Thermo Fisher Scientific Inc., Waltham, MA, USA) at 1,000 × g for 10 minutes (according to the manufacturer’s instructions for ELISA testing). The serum supernatant collected from the samples was frozen at −80°C. Sample dilutions were done for both high and low target protein concentrates with dilutions of 1:100 and 1:2, respectively, using the Human TGF-β1 PicoKine ELISA Kit (EK0513) (Boster Biological Technology, Pleasanton, CA, USA). Triplicates of the samples were coated onto a 96-well plate and incubated 3 times. Tetramethylbenzidine stop solution (Boster Biological Technology) was then put into each well, and the optical density absorbance per microplate reader (BioTek Instruments Inc., Winooski, VT, USA) was measured at 450 nm immediately after adding the stop solution.
Statistical analysis
Stata 16.1 software (Stata Corp., College Station, TX, USA) was used to perform the statistical analysis. The normality of the distribution was analyzed using the Shapiro-Wilk test followed by skewness and kurtosis tests, and was found to be not normal. After a descriptive analysis, intergroup comparisons for reaction time and concentration of TGF-β1 were conducted using the Kruskal Wallis test, followed by the post hoc Dunn test. The intergroup comparisons for K, MA, α, and CI were analyzed using the Mann-Whitney U test followed by the post hoc Dunn test. A statistically significant difference was indicated by p < 0.05.
RESULTS
TEG
Thromboelastographic results were autogenerated by the Thromboelastograph 5000 Hemostasis Analyzer and were correlated with normal values, then reviewed by an experienced hematologist. A graphical representation of the clotting kinetics of the CG and BN
5.25 samples is shown in
Figure 1. The mean and standard deviation of the R, K, α, MA, and CI values are tabulated in
Table 1. The intergroup comparisons of R values showed a significant difference between CG and BN
0.5, CG and BN
3, CG and BN
5, and BN
0.5 and BN
5 (
p < 0.05). The intergroup comparisons between CG and BN
5 showed a significant difference in relation to the K, MA, and α values (
p < 0.05) (
Table 1).
Figure 1
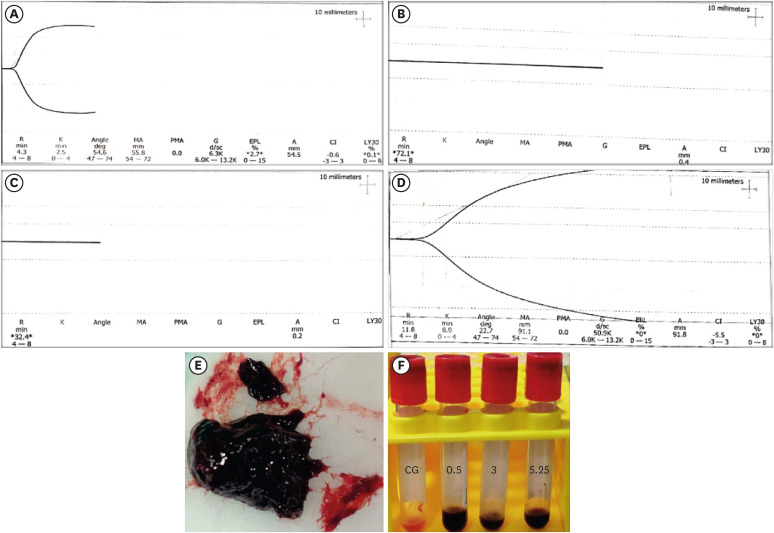
Graphical representation of thromboelastography parameters from 1 sample of each group. (A) CG group, (B) BN0.5 group, (C) BN3 group, (D) and BN5.25 group. (E) A weak, fragile, and black agglomerated mass formed when blood was mixed with 5.25% NaOCl. (F) Serum collected from sample groups (BN0.5, BN3, and BN5.25) showing black discoloration in comparison to the CG group.
CG, control (0.5 mL of blood); BN0.5, 0.5 mL of blood with 0.5 mL of 0.5% NaOCl; BN3, 0.5 mL of blood with 0.5 mL of 3% NaOCl; BN5.25, 0.5 mL of blood with 0.5 mL of 5.25% NaOCl; R, reaction time; K, kinetics; MA, maximum amplitude; CI, coagulation index; PMA, projected maximum amplitude; G, shear elastic modulus strength; EPL, estimated percent lysis at 30 minutes; A, amplitude; LY30, percent lysis at 30 minutes.
SEM
SEM results were assessed by an expert technician in the Sophisticated Analytical Instrument Facility of our institution. The CG samples demonstrated healthy biconcave RBCs intermeshed with thick fibers of fibrin. Platelets with pseudopodia were interspersed within the structure. Superficially, thick fibers of fibrin with branches were observed (
Figure 2A-E).
Figure 2
Pictorial representation of scanning electron microscopic images. (A, B) Healthy biconcave RBCs from the control group (at ×3,000 and ×12,000). (C, D) Fibrin mesh with thick fibrin fibers visualized in the control group (at ×5,000 and ×10,000). (E) Platelet (red arrow) with pseudopodia interspersed with RBCs in the control group (at ×6,000). (F) Roughened RBCs with surface distortions in response to 3% NaOCl (at ×12,000). (G) Clumping of RBCs and lysis of cells with 3% NaOCl (at ×2,500). (H) NaOCl crystals (yellow arrow) observed to be interspersed within the aggregated mass of cells (at ×5,000).
RBCs, red blood cells; NaOCl, sodium hypochlorite.

The BN0.5 samples did not clot, even after 1 hour, and thus could not be processed for visualization under the SEM.
The BN
3 samples demonstrated a clot-like structure at the end of 1 hour. On SEM analysis, neither fibrin formation nor branching of fibers (either superficially or in the deeper layers) was observed. The surface of the RBCs was observed to be roughened, even at 12,000× magnification. This distortion, as well as clumping of RBCs, was seen throughout the samples. Evidence of NaOCl crystals was also interspersed within the aggregated mass of cells (
Figure 2F-H).
The BN
5.25 samples formed a clot-like black mass immediately, but the structure was weak and fragile and could not withstand the fixation procedures necessary for visualization under SEM (
Figure 1E).
The serum supernatant obtained from the BN
0.5, BN
3, and BN
5.25 samples showed black discoloration (
Figure 1F). The concentration of TGF-β1 release (pg/mL) for the different study groups is tabulated in
Table 1. The TGF-β1 release was seen to be significantly higher in BN
3 than in the other study groups (BN
3 vs. BN
5.25, BN
3 vs. CG, and BN
3 vs. BN
0.5;
p < 0.05). However, the marginal increase in TGF-β1 release in BN
0.5 and BN
5.25 compared to CG was not statistically significant (BN
0.5 vs. BN
5.25, BN
0.5 vs. CG, BN
5.25 vs. CG;
p > 0.05).
DISCUSSION
TEG is a non-invasive, rapid, and robust tool for the detection and quantification of blood clotting kinetics and viscoelastic changes. It also helps measure the functional ability of blood to make a hemostatic plug [
16]. Hence, clotting kinetics and strength in this study were assessed using TEG.
TGF-β1 plays a major role in vital pulp therapy in the proliferation, collagen turnover, and differentiation of dental pulp cells, as well as increasing odontoblast secretory activity, thereby aiding in tertiary dentinogenesis [
17]. Therefore, the release of TGF-β1 was analyzed using ELISA.
In this study, all concentrations of NaOCl affected the formation of blood clots. NaOCl has antiaggregant properties that prevent the aggregation of human platelets by direct and indirect effects [
18]. The direct effects include the modification of fibrinogen receptors. The indirect effects are a result of oxidative stress and modification of the sulfur-containing groups present in the plasma membrane of platelets to sulfinic acid residues [
19].
An interesting finding in our study was that when blood was mixed with NaOCl, it immediately turned black (
Figure 1F). This was attributed to the oxidizing effect of NaOCl on blood components, as it is a strong oxidizing agent [
18]. The singlet oxygen produced during oxidative stress plays a complex role in the blood coagulation cascade by affecting coagulation factors and the aggregation of platelets [
20]. NaOCl causes oxidative modification of fibrinogen, leading to the formation of thinner fibers, with a decrease in fiber thickness (nm) and the average number of fiber strands (μm
2) [
18].
The TEG results confirmed this absence of clot formation when human whole blood was mixed with 0.5% and 3% NaOCl. A graphic representation of the clotting kinetics obtained with BN
5.25 shows that the quality parameters of the clot formed were not within normal ranges. The TEG R value signified the initiation of fibrin deposition [
21]. The R values of BN
0.5 and BN
3 were 7-11 times longer than the CG and showed no clot formation, even after 40 minutes. The graphical representation of BN
5.25 showed that it was 5 times longer than the CG, suggesting altered initiation of fibrin deposition. The K value indicates the time taken to reach a certain level of clot strength, the α indicates the rapidity of fiber build-up and cross-linking, and the MA indicates the direct function of the properties of fibrin and platelet aggregation [
21]. Although the K, MA, and α values were not recorded for BN
0.5 and BN
3, for BN
5.25 they were 2.8 times greater, 1.3 times greater, and 2 times less than CG, respectively. These findings correlate with the derangement of platelet aggregation and fibrin deposition and confirm altered clot formation.
The CI was derived from the above parameters and was −4 for BN
5.25, which represents an altered coagulability state. Blood forms a mass either by normal coagulation or by forming three-dimensional aggregates of cells [
20]. Despite the absence of clot formation with BN
5.25, a graphical representation was obtained. This could have been a result of the resistance measured between the metal pin of the TEG and the cell aggregate formation rather than the presence of fibrin structure.
The samples from BN
0.5 and BN
5.25 could not be assessed under SEM because the former did not clot, even after an extended period of 1 hour, and the latter produced an agglomerated structure that was weak and fragile and could not withstand the fixation procedures, leading to disintegration of the samples. The 3-dimensional aggregate of cells formed with BN
3 was more stable than that of BN
5.25 and thus allowed fixation processing for SEM analysis. Nevertheless, the samples from BN
3 demonstrated a roughening of surface RBCs along with distortion and clumping of cells and they lacked fibrin formation or branching of fibers. A study by Murina
et al. [
19] showed hemolysis of RBCs even when exposed to 0.01 mM of NaOCl. Nevertheless, the concentrations of NaOCl used in endodontics are much higher. The clot-like structure produced in BN
5.25 was fragile and degenerated during processing, which merits further discussion. The high concentration of NaOCl could have caused massive disruption of the cell membrane, making the three-dimensional aggregates unstable. This is in accordance with a previous study [
18], which proved that the fibrin fibers were markedly reduced by NaOCl mixtures and that oxidized fibrinogen results in the formation of small three-dimensional aggregates. These aggregates increase with an increase in oxidation, thus negatively affecting the blood micro-rheology by decreasing the intensity of platelet aggregation and suppressing the generation of thrombin, clot elasticity, and condensation [
20]. These aggregates are prone to destruction during shear forces [
20].
The concentrations of TGF-β1 released in the BN0.5 and BN3 groups were 1.06 and 1.4 times higher than in the CG, respectively. This finding could have been due to degranulation of the platelets when exposed to oxidized fibrinogen. Interestingly, the BN5.25 group did not show the highest concentrations, as projected, although it displayed higher TGF-β1 concentrations than the CG. A probable reason could be the aggregation of the RBCs due to the presence of oxidized fibrinogen, resulting in a clumping of cells that formed a thick mass, which could have hindered the release and migration of TGF-β1.
The strength of this study is that a wide range of NaOCl concentrations were used to assess the blood clot structure, clotting kinetics, and growth factor release. NaOCl at any concentration hampers clot formation and forms an unstable clot. In clinical scenarios, vital pulp therapy procedures are performed on pulp tissues that are already inflamed, and there may be high oxidative stress on the blood cells.
Short-term preoperative pulpal inflammation was shown not to affect treatment outcomes in an animal model study [
22]. However, an oxidizing agent like NaOCl can have a cumulative effect and might prolong bleeding from an amputated pulp or produce a fragile clot structure. Extended bleeding could alter treatment decisions, as the presence of profuse pulpal bleeding is indicative of severe inflammation and is deemed unsalvageable [
5].
Achieving hemostasis within 5 minutes during vital pulp therapy in mild to moderately inflamed pulp is considered crucial for creating an environment conducive to healing [
5]. Therefore, clinicians must avoid direct contact of NaOCl with pulp during vital pulp therapy since the depth of solution seepage into vital tissues is unknown.
A limitation of our study is that blood was drawn by venipuncture instead of from an inflamed pulp. Future studies are required to assess the role of biocompatible hemostatic agents in maintaining pulp vitality and to analyze the release of various other growth factors from the blood of inflamed pulp.
CONCLUSIONS
Within the limitations of our study, exposure of blood to NaOCl in concentrations ranging from 0.5% to 5.25% inhibited platelet aggregation, caused erythrocyte distortion, and oxidized fibrinogen formation resulting in a lack of fibrin fibers. Even low concentrations of 0.5% NaOCl inhibited the formation of blood clots, whereas the use of 3% and 5.25% NaOCl hampered the fibrin structure of the blood clot and TGF-β1 release.
ACKNOWLEDGEMENTS
The authors would like to acknowledge: the guidance of Dr. Ganesh Venkatraman, Professor and Ms. Vaishnavi Balasubramanian, Research Scholar, Department of Human Genetics, Sri Ramachandra Institute of Higher Education and Research in procuring and aiding in the analysis of TGF-β1 growth factor release using enzyme-linked immunosorbent assay; the Department of Biomedical Sciences, Apollo Hospitals, Greams Road, Chennai for permitting the use of the Thromboelastograph 5000 Hemostasis Analyzer; Prof. AK Mishra, Dean, IIT-Madras; Mr. Sundarraman and Ms. Kalpana, Sophisticated Analytical Instrument Facility, IIT-Madras; Dr. Thangavel, Vice Principal, Allied Health Sciences, Meenakshi Academy of higher Education and research; Dr. Lokeshwari, Department of Biotechnology, Sri Ramachandra Institute of Higher Education and Research for aiding in the analysis of samples under a scanning electron microscope.
-
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
-
Author Contributions:
Conceptualization: Suresh N.
Data curation: Anisha Mishra, Suresh N.
Formal analysis: Anisha Mishra, Suresh N.
Investigation: Anisha Mishra, Suresh N.
Methodology: Anisha Mishra, Suresh N.
Project administration: Natanasabapathy V, Suresh N.
Resources: Anisha Mishra, Natanasabapathy V, Suresh N.
Software: Anisha Mishra, Suresh N.
Supervision: Natanasabapathy V, Suresh N.
Validation: Natanasabapathy V, Suresh N.
Visualization: Anisha Mishra, Natanasabapathy V, Suresh N.
Writing - original draft: Anisha Mishra. Writing - review & editing.
SUPPLEMENTARY MATERIAL
REFERENCES
- 1. Monroe DM, Hoffman M. The clotting system - a major player in wound healing. Haemophilia 2012;18(Supplement 5):11-16.ArticlePubMed
- 2. Laurens N, Koolwijk P, de Maat MP. Fibrin structure and wound healing. J Thromb Haemost 2006;4:932-939.ArticlePubMed
- 3. Saber SE. Tissue engineering in endodontics. J Oral Sci 2009;51:495-507.ArticlePubMed
- 4. American Association of Endodontists. Glossary of endodontic terms. 10th ed. Chicago, IL: American Association of Endodontists; 2020.
- 5. European Society of Endodontology (ESE). Duncan HF, Galler KM, Tomson PL, Simon S, El-Karim I, Kundzina R, Krastl G, Dammaschke T, Fransson H, Markvart M, Zehnder M, Bjørndal L. European Society of Endodontology position statement: Management of deep caries and the exposed pulp. Int Endod J 2019;52:923-934.ArticlePubMedPDF
- 6. Sugiyama S, Kugiyama K, Aikawa M, Nakamura S, Ogawa H, Libby P. Hypochlorous acid, a macrophage product, induces endothelial apoptosis and tissue factor expression: involvement of myeloperoxidase-mediated oxidant in plaque erosion and thrombogenesis. Arterioscler Thromb Vasc Biol 2004;24:1309-1314.PubMed
- 7. Di Stasio E, Nagaswami C, Weisel JW, Di Cera E. Cl- regulates the structure of the fibrin clot. Biophys J 1998;75:1973-1979.ArticlePubMedPMC
- 8. Standeven KF, Ariëns RA, Grant PJ. The molecular physiology and pathology of fibrin structure/function. Blood Rev 2005;19:275-288.ArticlePubMed
- 9. Moorer WR, Wesselink PR. Factors promoting the tissue dissolving capability of sodium hypochlorite. Int Endod J 1982;15:187-196.ArticlePubMed
- 10. Pashley EL, Birdsong NL, Bowman K, Pashley DH. Cytotoxic effects of NaOCl on vital tissue. J Endod 1985;11:525-528.ArticlePubMed
- 11. Yamaguchi H, Hosoya N, Kobayashi K, Yokota T, Arai T, Nakamura J, Cox CF. The influence of two concentrations of sodium hypochlorite on human blood: changes in haemolysis, pH and protein. Int Endod J 2001;34:231-236.ArticlePubMedPDF
- 12. Nagendrababu V, Murray PE, Ordinola-Zapata R, Peters OA, Rôças IN, Siqueira JF Jr, Priya E, Jayaraman J, Pulikkotil SJ, Suresh N, Dummer PM. PRILE 2021 guidelines for reporting laboratory studies in Endodontology: explanation and elaboration. Int Endod J 2021;54:1491-1515.ArticlePubMedPDF
- 13. World Health Organization. WHO guidelines on drawing blood: best practices in phlebotomy. Geneva: World Health Organization; 2010.
- 14. Dean AG, Sullivan KM, Soe MM. OpenEpi: open source epidemiologic statistics for public health, version. updated April 6, 2013]. 2022]. Available from: www.OpenEpi.com.
- 15. Chernysh IN, Weisel JW. Dynamic imaging of fibrin network formation correlated with other measures of polymerization. Blood 2008;111:4854-4861.ArticlePubMedPMCPDF
- 16. Shaydakov ME, Sigmon DF, Blebea J. Thromboelastography [Internet]. Treasure Island, FL: StatPearls Publishing; 2022.
- 17. da Rosa WL, Piva E, da Silva AF. Disclosing the physiology of pulp tissue for vital pulp therapy. Int Endod J 2018;51:829-846.ArticlePubMedPDF
- 18. Štikarová J, Kotlín R, Riedel T, Suttnar J, Pimková K, Chrastinová L, Dyr JE. The effect of reagents mimicking oxidative stress on fibrinogen function. Sci World J 2013;2013:359621.
- 19. Murina MA, Roshchupkin DI, Kravchenko NN, Petrova AO, Sergienko VI. Antiaggregant effects of biogenic chloramines. Bull Exp Biol Med 2007;144:464-470.ArticlePubMedPDF
- 20. Azizova OA, Aseichev AV, Piryazev AP, Roitman EV, Shcheglovitova ON. Effects of oxidized fibrinogen on the functions of blood cells, blood clotting, and rheology. Bull Exp Biol Med 2007;144:397-407.ArticlePubMedPDF
- 21. Ioscovich A, Fadeev D, Kenet G, Naamad M, Schtrechman G, Zimran A, Elstein D. Thromboelastography as a surrogate marker of perisurgical hemostasis in gaucher disease. Clin Appl Thromb Hemost 2016;22:693-697.ArticlePubMedPDF
- 22. Santos JM, Marques JA, Diogo P, Messias A, Sousa V, Sequeira D, Palma PJ. Influence of preoperative pulp inflammation in the outcome of full pulpotomy using a dog model. J Endod 2021;47:1417-1426.ArticlePubMed
 , Velmurugan Natanasabapathy
, Velmurugan Natanasabapathy , Nandini Suresh
, Nandini Suresh





 KACD
KACD
 ePub Link
ePub Link Cite
Cite

