Abstract
-
Objectives
This study aimed to investigate the anti-erosive/abrasive effect of resin infiltration of previous deproteinized dentin.
-
Materials and Methods
Dentin slabs were randomly assigned to 3 groups (n = 15): Control (no deproteinization; no resin infiltrant applied), RI (no deproteinization; resin infiltrant applied), and DRI (deproteinization; resin infiltrant applied). After undergoing the assigned treatment, all slabs were subjected to an in vitro cycling model for 5 days. The specimens were immersed in citric acid (0.05 M, pH = 3.75; 60 seconds; 3 times/day) and brushed (150 strokes). Between the challenges, the specimens were exposed to a remineralizing solution (60 minutes). The morphological alterations were analyzed by mechanical profilometry (µm) and scanning electron microscopy (SEM). Data were submitted to one-way analysis of variance (ANOVA) and Tukey tests (p < 0.05).
-
Results
Control and RI groups presented mineral wear and did not significantly differ from each other (p = 0.063). DRI maintained a protective layer preserving the dentin (p < 0.001). After erosive/abrasive cycles, it was observed that in group RI, only 25% of the slabs partially evidenced the presence of the infiltrating, while, in the DRI group, 80% of the slabs presented the treated surface entirely covered by a resin-component layer protecting the dentin surface as observed in SEM images.
-
Conclusions
The removal of the organic content allows the resin infiltrant to efficiently protect the dentin surface against erosive/abrasive lesions.
-
Keywords: Dentin; Icon infiltrant; Sodium hypochlorite; Tooth erosion; Tooth abrasion
INTRODUCTION
Erosive tooth wear (ETW) has increased in recent decades, affecting about 30% of adults [
1]. The high consumption of acidic food, as is seen in modern diets [
2,
3], may be associated with this higher erosive wear and could compromise the enamel surface. Consequently, it could generate dentin exposure and lead to dentin sensitivity in the presence of external stimuli, a common complaint among patients with ETW [
4,
5,
6,
7].
Chemical-mechanical processes by acid demineralization and mechanical wear by attrition and abrasion work synergistically on the progress of erosive tooth wear. Preventive measures are studied continuously in order to prevent the advance of dental structure loss [
8,
9,
10,
11,
12]. Most of the preventive erosion products do not form a permanent physical barrier over the target tissue. Often, the current strategies rely on patient compliance and require frequent and ideal concentrations to impart preventive effects.
Successful innovations in the management of dental erosion have remained limited [
13]. The standard of care has remained mostly unchanged over the past decades, suggesting the need for new strategies. Recently, efforts have been devoted to identifying a protective coating that can be applied to dentin surfaces in an attempt to avoid erosive and abrasive progression. Polymeric methacrylate-based dental materials intended as sealing agents have been tested to prevent the progression of tooth erosion and abrasion with promising outcomes [
13,
14,
15].
Based on the recent encouraging results on the arresting of early stages of caries progression [
16], low-viscosity methacrylate resin infiltrants have been investigated for sealing eroded surfaces [
17,
18]. Resin infiltrants act by conditioning high porosity and subsequentially infiltration of a monomeric component with enhanced penetration ability after applying ethanol. The cured material presents mechanical resistance and no toxicity or alteration of tooth aesthetics [
19].
The current dental literature shows knowledge gaps and areas of controversy on resin infiltrants’ application as a preventive strategy for dental erosion [
15,
17,
18]. Some studies were able to show positive trends in its applicability [
17,
18], but retention of the material has been reported as major importance for the acid-protective effect. For resin materials’ retention, the micromechanical interlocking of tiny polymerized resin tags within the acid-etched enamel surface’s porosities provides the primary achievable bond mechanism for the dental substrates.
An approach that was discovered to facilitate the penetration of resin infiltrants and improve the bonding to dentin was the pre-treatment of dentin with deproteinizing agents, such as sodium hypochlorite. This deproteinization dissolves the smear layer’s organic components, leaving the inorganic crystals to act as a filling of the hybrid layer, but maintaining the collagen intact and healthy [
20,
21,
22].
The present in vitro study aimed to investigate the anti-erosive/abrasive effect of resin infiltration of previous deproteinized dentin. The null hypothesis tested was that there is no difference between the surface treatments in preventing erosive and abrasive wear.
MATERIALS AND METHODS
Study design
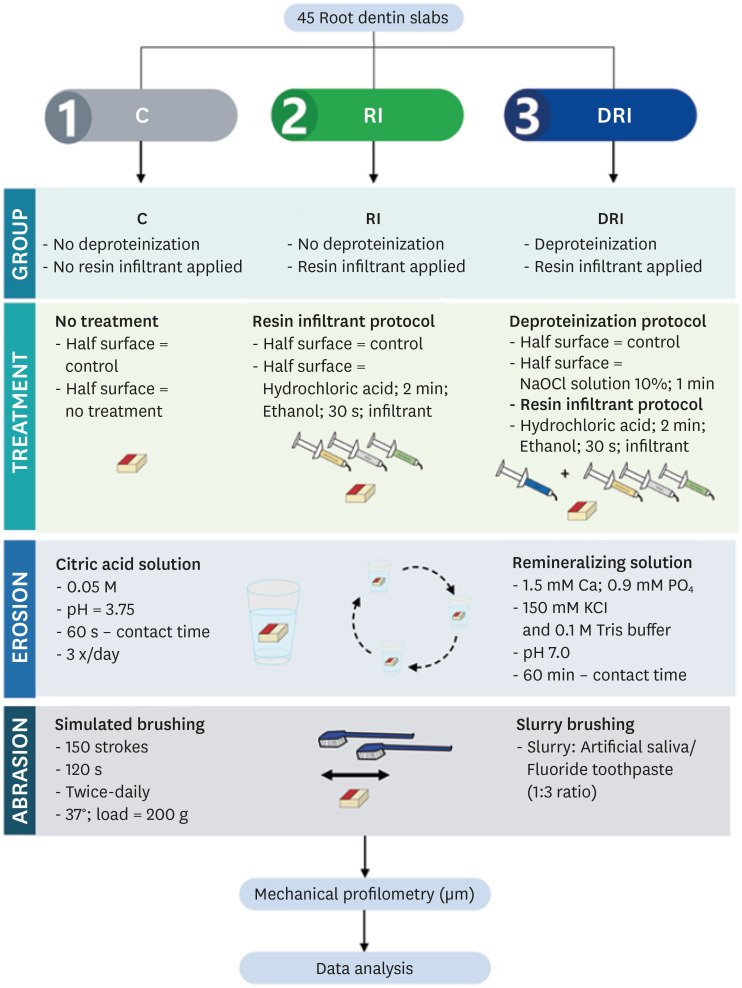
This research is an in vitro study that utilizes a quantitative and qualitative approach. The specimens were randomly distributed into 3 groups (n = 15), as follows: C (control; no deproteinization performed and no resin infiltrant applied), RI (no deproteinization performed and resin infiltrant applied), and DRI (deproteinization performed and resin infiltrant applied).
Preparation of slabs
Human third molars were collected after obtaining the patients’ informed consent form, under a protocol approved by a local Ethics Committee (protocol # 2.216.818). Teeth with stains, fractures, cracks, and any developmental failure were not included.
Root dentin slabs (4 mm width × 4 mm length × 2 mm thickness) were prepared. For such, a cutting machine (IsoMet 1000; Buehler, Lake Bluff, IL, USA) with a double-sided diamond disk (Buehler) was used with distilled water as an irrigant. Afterward, the dentin slabs were attached in acrylic resin disks with wax (Kota Industria E Comercio Ltda, São Paulo, SP, Brazil). Then, specimens were sequentially ground flat and polished with 320-, 600- and 1,200-grit paper, followed by felt paper and a 1 μm diamond suspension spray (Buehler). Between each flattening and the polishing steps, the dentin slabs were cleaned in an ultrasonic device with a frequency of 40 kHz for 2 minutes with distilled water.
For the standardization of samples, 5 surface microhardness measures were performed in each specimen. A Knoop diamond was used (FM 100; Future Techs, Tokyo, Japan), applying a 10 gf load for 5 seconds. Thus, 45 slabs of dentin presenting mean values of microhardness 20% above or 20% below the mean value of all fragments were included.
An adhesive tape (2.0 × 4.0 mm;
Figure 1) was deployed to delimit each dentin slab’s reference area for post cycling wear measure.
Figure 1
Experimental design.
C, no deproteinization performed and no resin infiltrant applied (control); RI, no deproteinization performed and resin infiltrant applied; DRI, deproteinization performed and resin infiltrant applied.
Resin infiltration
In the DRI group, the slabs’ deproteinization was previously performed by applying sodium hypochlorite [
23] (NaOCl 10% - Dynamics; Indaiatuba, SP, Brazil) for 1 minute and then washed. Afterward, the infiltrant application protocol was carried out.
The resin infiltrant (Icon; DMG, Hamburg, Germany) was applied for groups RI and DRI following the manufacturer’s instructions. First, the Icon-Etch was applied for 2 minutes; then, the specimens were rinsed with distilled water for 30 seconds and air-dried. Next, Icon-Dry was applied for 30 seconds, air-dried, and then a slurry Icon-Infiltrant was applied, left to be processed for 3 minutes, and photopolymerized (Led Lux II; Ortus, Campo Mourão, PR, Brazil) for 40 seconds. Finally, the Icon-Infiltrant was applied again, left to be processed for 1 minute, and light-cured for 40 seconds.
Erosive/abrasive cycling model
The erosive/abrasive cycle (
Figure 1) was performed for 5 days, 3 times a day, as follows:
- Immersion in citric acid (0.05 M C6H8O7, pH 3.75) for 60 seconds [10].
- Rinsed with distilled water for 5 seconds and immersed in artificial saliva (1.5 mM Ca, 0.9 mM PO4, 150 mM KCl, and 0.1 M Tris buffer, pH 7.0) for 60 minutes [10].
- After each exposure to acids and artificial saliva, the slabs were abraded through a brushing machine (MSEt—1,500 W; Marcelo Nucci ME, São Carlos, SP, Brazil) with 150 back-and-forth movements (37°C) using a 200 g load [10]. During this procedure, the slabs were bathed in a dentifrice/artificial saliva suspension (1:3 by weight), using an 1,100 ppm Sodium Fluoride dentifrice (Oral-B Anti Cáries; Procter & Gamble, Rio de Janeiro, RJ, Brazil).
All procedures were performed under agitation (100 rpm) and at 37°C (TE-141; Tecnal Scientific Equipment, Piracicaba, SP, Brazil).
Specimens were visually inspected using a stereomicroscope with 80× magnification (Olympus, Tokyo, Japan) to identify the presence of resin infiltrant after the erosive/abrasive cycle. The specimens were classified into three categories: surface fully covered by the infiltrant, partially covered and fully uncovered.
Measurement of dental wear
After the experimental cycle, the adhesive tape was carefully removed. The height difference between the reference and treated surface was measured using a mechanical profilometer Hommel Etamic W10 (Hommelwerke GmbH, Germany). The program calculates this difference (Rz) using the highest peak-mean line-height (Rp) and the lowest valley-mean line-height (Rv), as follows:
For each specimen, at 100-μm intervals, three analyses were performed on an extension of 1.5 mm. Then, each sample had an average of the three measurements obtained.
Assessment by scanning electron microscopy
Two samples from each group were dehydrated for 24 hours and mounted onto stubs with double-sided carbon tape. Following that, the slabs were coated with gold/palladium alloy and observed in a scanning electron microscope (Quanta FEG 450; FEI Company, Hillsboro, OR, USA) at a voltage acceleration of 20 kV and magnification of 2,000×. On the treated surface, superficial morphological changes, presence of infiltrant and exposure of dentinal tubules were analyzed.
Statistical analysis
The Kolmogorov–Smirnov test was applied to assess the homogeneity of the data. Thus, since the values presented normal distribution for all groups, one-way analysis of variance (ANOVA) and Tukey tests were used to compare them. The level of significance was set at 5%. All statistical procedures were performed using Statistical Package for the Social Sciences 16.0 for Windows (SPSS Inc., Chicago, IL, USA).
RESULTS
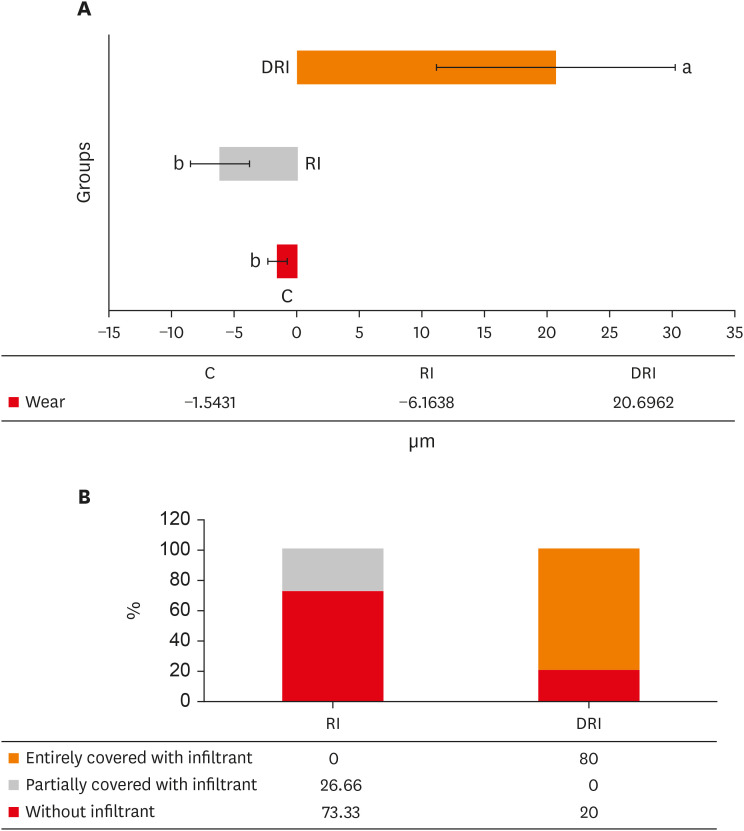
The mean and standard deviation of dentin wear or the formation of a protective barrier for all experimental groups are presented in
Figure 2A. There was wear for 2 groups (C and RI), and, for the DRI group, there was the formation of a protective barrier, which remained after 5 days of erosion/abrasion cycles. The wear observed in groups C and RI did not present a significant difference between each other (
p = 0.063). However, these groups differed from the DRI (
p < 0.001).
Figure 2
Wear and presence of infiltrant data on the dentin surface. (A) Mean and standard deviation of the wear/protective barrier obtained in the experimental groups. (B) Distribution of the presence of the infiltrant on the surface from groups that received the resinous infiltration.
C, no deproteinization performed and no resin infiltrant applied (control); RI, no deproteinization performed and resin infiltrant applied; DRI, deproteinization performed and resin infiltrant applied.
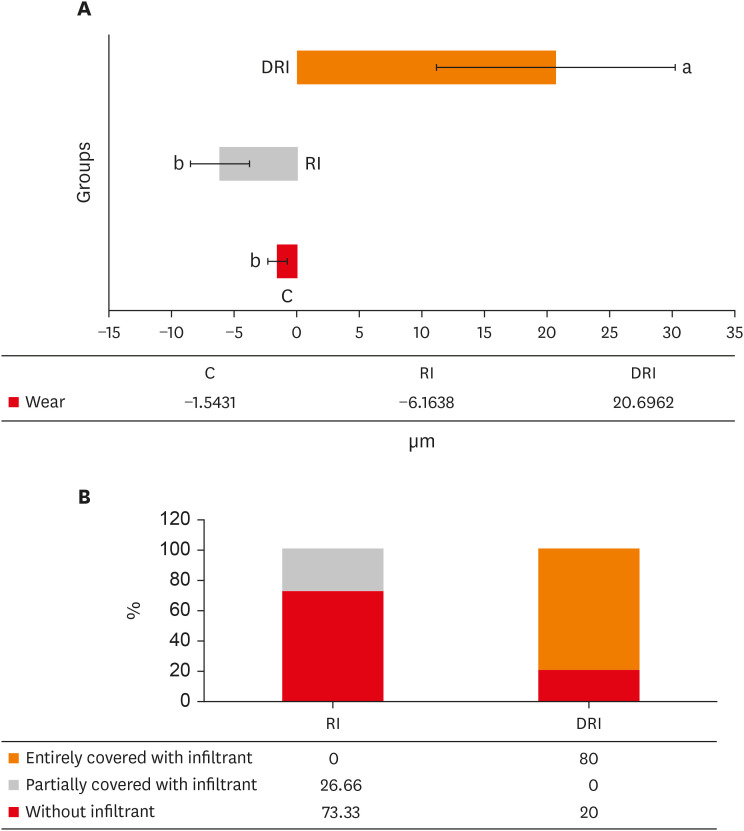
Concerning the presence of the Icon infiltrant barrier after the erosive/abrasive cycle, it was observed that only 25% of the slabs in group RI had the dentin partially covered, whereas, in the DRI group, 80% of the slabs presented the surface entirely covered (
Figure 2B).
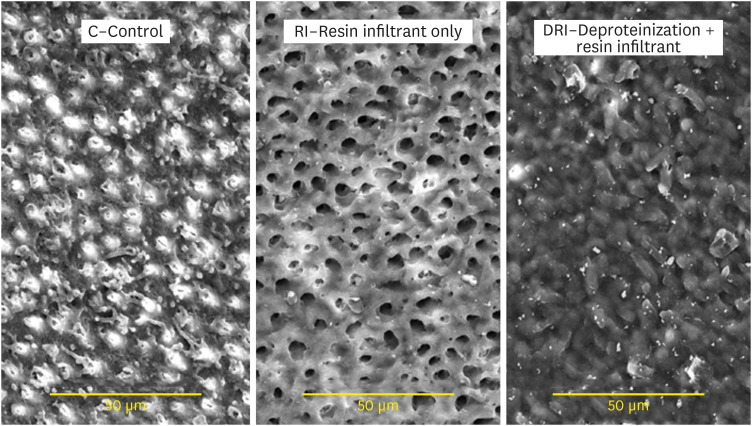
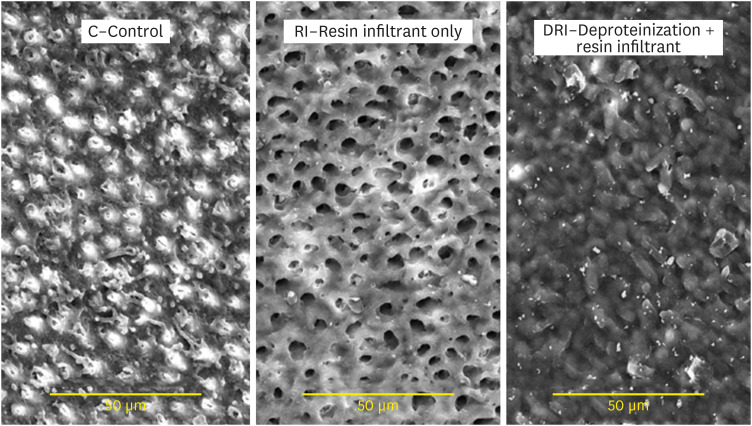
Figure 3 presents images of the dentin surface for each group tested. In
Figure 3A, the C group presents a dentin surface with exposed and relatively open dentinal tubules, demonstrating similarity with eroded dentin. In
Figure 3B, the group RI presented a dentin pattern with wide-open tubules aperture, thus demonstrating, after the erosion and abrasion processes, the absence of any resinous product previously applied. In
Figure 3C, the DRI group still presented a resin-component layer protecting the dentin surface, present even after erosion/abrasion cycles.
Figure 3 Scanning electron microscopy images of the dentin surface after different surface treatments. (×2,000).
DISCUSSION
Considering that several studies demonstrate that resin infiltration has an anti-erosive and abrasive effect in enamel [
17,
18], the question arises whether its use on dentin would promote the same outcome. After all, the patients affected by ETW quickly develop dentin exposure with the chemical-mechanical processes involved [
4,
5,
6,
7]. So, the present study aimed to investigate the anti-erosive/abrasive effect of resin infiltration of previous deproteinized dentin.
Surface treatments influenced the performance of the resinous infiltrant after the cycling. The deproteinized dentin maintained the resin infiltrant’s presence that prevented the progression of the demineralization. Therefore, discrepancies between the erosive protection ability of the groups were observed. Besides, dentin deproteinization enhanced the efficacy of resin infiltration. Considering that, the null hypothesis was rejected. In the erosive/abrasive cycle conducted, the brushing was performed with 150 strokes three times a day, similar to a prior study [
10]. The
in vitro simulated brushing was set to extrapolate daily habits and simulate the people most exposed to erosive and abrasive wear. Furthermore, the resin infiltrant was applied over a sturdy surface, simulating its application on dentin exposed to the oral environment. The citric acid mimics juice or soft drinks often ingested by the population, such as orange juice, with a pH close to 3.75 [
12,
24].
Considering the visual inspection, a slight loss of resin infiltrant was observed in the specimens deproteinized with sodium hypochlorite. They demonstrated the formation of a protective barrier as a mechanism of action, as verified in
Figure 3C. This barrier avoids contact of abrasive and erosive impacts with dentin, preventing demineralization. The effectiveness of Icon was noticed in 80% of specimens from the DRI group. This efficacy probably was achieved due to deproteinization with sodium hypochlorite, which enabled better mechanical interpenetration of the resin infiltrant, resulting in enhanced surface protection of the dentin against erosive/abrasive challenges. Deproteinization was a technique developed as an alternative to increase dentin porosity and thus improve the interpenetration of resin restorative materials and the sealing of dentinal tubules [
23].
Sodium hypochlorite is a robust solution that eliminates the organic elements. The degree of penetration of an adhesive resin in caries lesions in the enamel, using NaOCl as the deproteinization agent per 1 minute, increases adhesive materials’ retention [
25]. A similar behavior could be observed in dentin, where NaOCl promotes disinfection and increases the hybridization between resin and dentin [
20].
For the samples not previously deproteinized, only 25% of them presented the remaining resin infiltrant at the end of the experiment, exhibiting wear similar to the negative control group. This finding agrees with a prior study, which observed that the application of Icon with and without acid etching was not effective in preventing erosive and abrasive enamel wear [
15]. The presence of collagen fibers in the RI group probably impaired the penetration of the resin infiltrant, reducing the bond strength of the infiltrant with the dentin surface. After all, the resin infiltrant does not present a significant dentin penetration since it is composed of triethylene glycol dimethacrylate (TEGDMA), camphorquinone, and ethyl-4-dimethylaminobenzoate. Furthermore, the erosive challenge may have affected the resin infiltrant’s resistance through resin matrix decomposition, exhibiting cracks and fractures [
26].
A protective effect of Icon was observed in an
in situ erosive and abrasive challenge for 20 days on etched enamel [
18], demonstrating that resin infiltrant has resistance as a coating material over time. To achieve this effect with resin infiltration, it is indispensable the presence of wide pores on the surface to the infiltration of resin into a subsurface enamel lesion [
27]. However, the mechanism in dentin is complex and less predictable. Hence, in dentin, only acid etching may not be sufficient to accomplish a satisfactory infiltration, requiring the surface's deproteinization.
Some previous studies have shown a more significant reduction in erosive and abrasive wear with conventional resin composites and sealants when compared to the resinous infiltrant [
15,
28]. This outcome was observed because they have better resistance against daily erosive and abrasive challenges, such as acidic beverages and toothbrushing [
15]. However, despite this lower resin infiltration effect, the calcium dissolution was significantly lower than in control, not treated with the infiltrating agent Icon [
28]. Therefore, resin infiltration is an encouraging technique to be used as an additional treatment to inhibit erosion progression.
Since there are feasible approaches to assess ETW quantitatively in clinical situations, the
in vitro simulating models allow controlled studies that simulate oral environment conditions. Furthermore,
in vitro studies allow accurate quantitative measurements, such as profilometry, which quantifies dentin loss compared to a non-treated reference area [
29]. The profilometry results were complemented by scanning electron microscopy micrographs, allowing the verification of the resin infiltrant’s wear and presence on the surface. However, even when a controlled laboratory environment similar to the oral cavity is achieved, some limitations still are noticed. Therefore, further investigations are warranted to reduce these limitations, using HOCl for deproteinization, stannous fluoride as a positive control, human saliva in the cycle, and simulation of different designs, such as
in situ.
CONCLUSIONS
Resin infiltration was effective in preventing erosive tooth wear on deproteinized dentin. Further investigations assessing the long-term effects and clinical conditions are warranted to provide more evidence in the prevention and progression of erosive and abrasive tooth wear of Icon.
ACKNOWLEDGEMENTS
The authors would like to thank the Central Analítica-UFC/CT-INFRA/MCTI-SISANO/Pró-Equipamentos CAPES for the support.
-
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
-
Author Contributions:
Conceptualization: de Albuquerque ATQ.
Data curation: de Albuquerque ATQ.
Formal analysis: de Albuquerque ATQ, Leal IC.
Funding acquisition: de Albuquerque ATQ, de Moraes MDR.
Investigation: de Albuquerque ATQ, Bezerra BO, Leal IC.
Methodology: Passos VP, de Moraes MDR.
Project administration: Passos VP, de Moraes MDR.
Resources: de Albuquerque ATQ, de Moraes MDR.
Software: Passos VP.
Supervision: Passos VP, de Moraes MDR.
Validation: Passos VP, de Moraes MDR.
Visualization: Passos VP, de Moraes MDR.
Writing - original draft: Leal IC, de Albuquerque ATQ.
Writing - review & editing: Passos VP, de Moraes MDR, S. Melo MA.
REFERENCES
- 1. Bartlett DW, Lussi A, West NX, Bouchard P, Sanz M, Bourgeois D. Prevalence of tooth wear on buccal and lingual surfaces and possible risk factors in young European adults. J Dent 2013;41:1007-1013.ArticlePubMed
- 2. Grippo JO, Simring M, Coleman TA. Abfraction, abrasion, biocorrosion, and the enigma of noncarious cervical lesions: a 20-year perspective. J Esthet Restor Dent 2012;24:10-23.ArticlePubMed
- 3. Lussi A, Carvalho TS. Erosive tooth wear: a multifactorial condition of growing concern and increasing knowledge. Monogr Oral Sci 2014;25:1-15.ArticlePubMed
- 4. Ganss C. Definition of erosion and links to tooth wear. Monogr Oral Sci 2006;20:9-16.ArticlePubMed
- 5. Ganss C, Lussi A. Diagnosis of erosive tooth wear. Monogr Oral Sci 2006;20:32-43.ArticlePubMed
- 6. O’Toole S, Bartlett D. The relationship between dentine hypersensitivity, dietary acid intake and erosive tooth wear. J Dent 2017;67:84-87.ArticlePubMed
- 7. Peumans M, Politano G, Van Meerbeek B. Treatment of noncarious cervical lesions: when, why, and how. Int J Esthet Dent 2020;15:16-42.PubMed
- 8. de Moraes MDR, Carneiro JR, Passos VF, Santiago SL. Effect of green tea as a protective measure against dental erosion in coronary dentine. Braz Oral Res 2016;30:1-6.
- 9. Machado AC, Bezerra SJC, João-Souza SH, Caetano TM, Russo LC, Carvalho TS, Scaramucci T. Using fluoride mouthrinses before or after toothbrushing: effect on erosive tooth wear. Arch Oral Biol 2019;108:104520.ArticlePubMed
- 10. Passos VF, de Vasconcellos AA, Pequeno JH, Rodrigues LK, Santiago SL. Effect of commercial fluoride dentifrices against hydrochloric acid in an erosion-abrasion model. Clin Oral Investig 2015;19:71-76.ArticlePubMedPDF
- 11. Passos VF, Melo MAS, Lima JPM, Marçal FF, Costa CAGA, Rodrigues LKA, Santiago SL. Active compounds and derivatives of camellia sinensis responding to erosive attacks on dentin. Braz Oral Res 2018;32:e40.PubMed
- 12. Passos VF, Rodrigues LKA, Santiago SL. The effect of magnesium hydroxide-containing dentifrice using an extrinsic and intrinsic erosion cycling model. Arch Oral Biol 2018;86:46-50.ArticlePubMed
- 13. Wegehaupt FJ, Tauböck TT, Sener B, Attin T. Long-term protective effect of surface sealants against erosive wear by intrinsic and extrinsic acids. J Dent 2012;40:416-422.ArticlePubMed
- 14. Wegehaupt FJ, Tauböck TT, Attin T. Durability of the anti-erosive effect of surfaces sealants under erosive abrasive conditions. Acta Odontol Scand 2013;71:1188-1194.ArticlePubMed
- 15. Zhao X, Pan J, Zhang S, Malmstrom HS, Ren YF. Effectiveness of resin-based materials against erosive and abrasive enamel wear. Clin Oral Investig 2017;21:463-468.ArticlePubMedPDF
- 16. Paris S, Bitter K, Krois J, Meyer-Lueckel H. Seven-year-efficacy of proximal caries infiltration - randomized clinical trial. J Dent 2020;93:103277.ArticlePubMed
- 17. Oliveira GC, Boteon AP, Ionta FQ, Moretto MJ, Honório HM, Wang L, Rios D.
In vitro effects of resin infiltration on enamel erosion inhibition. Oper Dent 2015;40:492-502.PubMed
- 18. Rios D, Oliveira GC, Zampieri CR, Jordão MC, Dionisio EJ, Buzalaf M, Wang L, Honório HM. Resin-based materials protect against erosion/abrasion-a prolonged in situ study. Oper Dent 2019;44:302-311.PubMed
- 19. Skucha-Nowak M, Machorowska-Pieniążek A, Tanasiewicz M. Assessing the penetrating abilities of experimental preparation with dental infiltrant features using optical microscope: preliminary study. Adv Clin Exp Med 2016;25:961-969.ArticlePubMed
- 20. Alshaikh KH, Hamama HHH, Mahmoud SH. Effect of smear layer deproteinization on bonding of self-etch adhesives to dentin: a systematic review and meta-analysis. Restor Dent Endod 2018;43:e14.ArticlePubMedPMCPDF
- 21. Thanatvarakorn O, Prasansuttiporn T, Thittaweerat S, Foxton RM, Ichinose S, Tagami J, Hosaka K, Nakajima M. Smear layer-deproteinizing improves bonding of one-step self-etch adhesives to dentin. Dent Mater 2018;34:434-441.ArticlePubMed
- 22. Augusto MG, Torres C, Pucci CR, Schlueter N, Borges AB. Bond stability of a universal adhesive system to eroded/abraded dentin after deproteinization. Oper Dent 2018;43:291-300.ArticlePubMedPDF
- 23. Esteves SR, Huhtala MF, Gomes AP, Ye Q, Spencer P, De Paiva Gonçalves SE. Longitudinal effect of surface treatments modified by NaOCl-induced deproteinization and Nd:YAG laser on dentin permeability. Photomed Laser Surg 2016;34:68-75.ArticlePubMed
- 24. Reddy A, Norris DF, Momeni SS, Waldo B, Ruby JD. The pH of beverages in the United States. J Am Dent Assoc 2016;147:255-263.ArticlePubMed
- 25. Abdelmegid FY. Effect of deproteinization before and after acid etching on the surface roughness of immature permanent enamel. Niger J Clin Pract 2018;21:591-596.ArticlePubMed
- 26. Soares LE, Soares AL, De Oliveira R, Nahórny S. The effects of acid erosion and remineralization on enamel and three different dental materials: FT-Raman spectroscopy and scanning electron microscopy analysis. Microsc Res Tech 2016;79:646-656.ArticlePubMed
- 27. Arnold WH, Gaengler P. Light- and electronmicroscopic study of infiltration of resin into initial caries lesions--a new methodological approach. J Microsc 2012;245:26-33.ArticlePubMed
- 28. Yetkiner E, Wegehaupt FJ, Attin R, Wiegand A, Attin T. Stability of two resin combinations used as sealants against toothbrush abrasion and acid challenge in vitro
. Acta Odontol Scand 2014;72:825-830.PubMed
- 29. Schlueter N, Hara A, Shellis RP, Ganss C. Methods for the measurement and characterization of erosion in enamel and dentine. Caries Res 2011;45(Supplement 1):13-23.ArticlePDF
 , Bruna Oliveira Bezerra1
, Bruna Oliveira Bezerra1 , Isabelly de Carvalho Leal2
, Isabelly de Carvalho Leal2 , Maria Denise Rodrigues de Moraes1
, Maria Denise Rodrigues de Moraes1 , Mary Anne S. Melo3,4
, Mary Anne S. Melo3,4 , Vanara Florêncio Passos2
, Vanara Florêncio Passos2





 KACD
KACD
 ePub Link
ePub Link Cite
Cite

