Abstract
-
Objectives
This study aimed to investigate the color stability, solubility, and surface characteristics of 3 calcium silicate-based cements (CSCs) after immersion in different solutions.
-
Materials and Methods
ProRoot white mineral trioxide aggregate (MTA), Biodentine, and Endosequence Root Repair Material (ERRM) were placed in cylindrical molds and stored at 37°C for 24 hours. Each specimen was immersed in distilled water, 5% sodium hypochlorite (NaOCl), 2% chlorhexidine, or 0.1% octenidine hydrochloride (OCT) for 24 hours. Color changes were measured with a spectrophotometer. Solubility was determined using an analytical balance with 10−5 g accuracy. The surface characteristics were analyzed using scanning electron microscopy and energy-dispersive spectroscopy. Data were analyzed using 2-way analysis of variance, the Tukey test, and the paired t-test.
-
Results
MTA exhibited significant discoloration in contact with NaOCl (p < 0.05). White precipitation occurred on the surfaces of Biodentine and ERRM after contact with the solutions, and none of the materials presented dark brown discoloration. All materials showed significant solubility after immersion in the solutions (p < 0.05), irrespective of the solution type (p > 0.05). The surface topography and elemental composition of the samples showed different patterns of crystal formation and precipitation depending on the solution type.
-
Conclusions
All materials presented some amount of solubility and showed crystal precipitation after contact with the solutions. Biodentine and ERRM are suitable alternatives to ProRoot MTA as they do not exhibit discoloration. The use of OCT can be considered safe for CSCs.
-
Keywords: Bioceramic; Bismuth oxide; Irrigation; Physical property; Staining
INTRODUCTION
Mineral trioxide aggregate (MTA) is a calcium silicate-based cement (CSC) commonly used for several endodontic procedures, including perforation repair, vital pulp therapies, and regenerative procedures, owing to its biocompatibility, bioactivity, and good sealing ability [
1]. Despite its benefits, MTA has some drawbacks such as difficult handling characteristics, long setting time, and discoloration potential [
2]. There are 2 types of MTA, including gray and white-colored forms. Gray MTA is composed of tricalcium silicate, dicalcium silicate, tetracalcium aluminoferrite, tricalcium aluminate, calcium sulfate, and bismuth oxide [
3]. White MTA, which lacks tetracalcium aluminoferrite, was developed due to aesthetic concerns [
3].
More recently, Biodentine (Septodont, Saint-Maur-des-Fosses, France) and Endosequence Root Repair Material (ERRM; Brasseler USA, Savannah, GA, USA) have been developed for similar indications to those of MTA. The powder of Biodentine is composed of tricalcium silicate, dicalcium silicate, calcium carbonate, and zirconium oxide. Its water-based liquid contains calcium chloride as the setting accelerator and a water-reducing agent [
3]. Biodentine has been reported to have a short setting time and high mechanical properties [
4]. ERRM is a premixed cement that contains calcium silicate, calcium phosphate, zirconium oxide, and tantalum oxide [
3]. ERRM is a bioactive and biocompatible material with similar mechanical and antimicrobial properties to those of MTA [
4].
CSCs can come into contact with root canal irrigants, especially when they are used in the treatment of perforation or resorption. Sodium hypochlorite (NaOCl) is a widely used root canal irrigant due to its organic tissue dissolution capacity and antimicrobial activity [
5]. However, NaOCl may cause severe damage if extruded to the periapical area and negatively affect the mechanical properties of dentin and bonding of materials [
5]. Chlorhexidine (CHX) has been recommended as an alternative irrigant to NaOCl due to its substantial antimicrobial effect [
5]. However, CHX has no tissue-dissolving properties and forms an orange-colored toxic precipitate when used with NaOCl [
6]. Octenisept (Schülke & Mayr, Nordersdedt, Germany) is a broad-spectrum antimicrobial solution containing octenidine hydrochloride (OCT) and phenoxyethanol [
7]. OCT is a cationic bispyridine that is effective on both gram-positive and gram-negative bacteria, fungi, and several viruses and has been widely used for wound disinfection [
8,
9]. Phenoxyethanol, an ethanol derivate, serves as a preservative component in OCT that improves the antimicrobial efficacy of the solution synergistically [
7]. In previous studies, OCT presented higher antimicrobial activity against
Enterococcus faecalis biofilms than CHX and produced better intratubular disinfection than NaOCl and CHX [
10,
11]. Unfortunately, OCT does not have tissue-dissolving properties, similarly to CHX. However, it can be used with NaOCl, since a colorless and non-harmful product is formed [
12]. In recent research, OCT has been found to have lower cytotoxicity than CHX and NaOCl and to reduce the cytotoxicity of NaOCl without altering the free chlorine level when they are used together [
13,
14]. OCT can also be used with ethylenediaminetetraacetic acid (EDTA) for smear layer removal [
15]. Its high antimicrobial properties, capacity to disinfect and clean dentin tubules and low toxicity profile make OCT a promising root canal irrigant [
10,
11,
14,
15].
It has been reported that contact with root canal irrigants may affect several properties of CSCs, such as bond strength and microhardness [
16,
17]. Although the effect of NaOCl on the color of MTA has been studied, the literature regarding the effects of other irrigants, including CHX and OCT, on the behavior of CSCs is limited [
18,
19]. Therefore, the purpose of this study was to evaluate the color stability, solubility, and surface properties of different CSCs after contact with different irrigation solutions.
MATERIALS AND METHODS
Sample preparation
Three materials were used in this study, with each group containing 40 samples: ProRoot white MTA (Dentsply Tulsa Dental, Johnson City, TN, USA), Biodentine (Septodont, Saint-Maur-des-Fosses, France) and ERRM (Brasseler USA, Savannah, GA, USA). The materials were prepared according to the manufacturers’ instructions and placed in cylindrical molds that were 10 mm in diameter and 2 mm high. The specimens were stored at 37°C and 100% humidity for 24 hours. Then, each sample was separated from the mold and immersed in 1 of the following 4 solutions for 24 hours (n = 10):
1. Distilled water (DW) (pH = 7.4)
2. 5% NaOCl (Werax, İzmir, Turkey) (pH = 12)
3. 2% CHX (Drogsan, Ankara, Turkey) (pH = 6.5)
4. 0.1% OCT + 2% phenoxyethanol (Octenisept, Schülke & Mayr) (pH = 6.5)
Solubility analysis
The specimens were weighed 3 times before and after immersion in the irrigation solutions using an analytical balance with an accuracy of 10−5 g (Radwag Balances and Scales, Torunska, Poland), and the mean value of the readings was recorded. The mass loss was expressed as a percentage of the original mass.
Color analysis
The color of the specimens was assessed using a spectrophotometer (SpectroShade Micro, MHT Optic Research AG, Niederhasli, Switzerland). Standardization (in terms of angulation and distance to the light output) of the color measurements was ensured by the help of a custom-made silicone key that surrounded the spectrophotometer’s mouthpiece. Moreover, the silicone key served to block the exterior light during the measurements. A single operator who was blinded to the groups performed the color evaluations. The spectrophotometer was calibrated according to the manufacturer’s recommendations before each measurement. Each measurement was repeated thrice.
The Commission Internationale de l’Eclairage (CIE) system was used to calculate the color difference of the specimens before and after immersion in different solutions according to the following formula:
The values of L*, a*, and b* represent lightness and the chromaticity coordinates in the red-green axis and the yellow-blue axis, respectively. Images of the specimens were also captured before and after contact with the solutions using a digital camera (Cybershot DSCW510, Sony, Tokyo, Japan).
Additionally, the same evaluator descriptively analyzed each specimen before and after immersion in the solutions to record any local or generalized color changes (darker or lighter) and to detect the presence of precipitation on the surface.
Surface analysis
Three specimens from each group were selected for the analysis of microstructural surface morphology and elemental composition. The specimens were dried and gold-coated for analysis using scanning electron microscopy (SEM) (JEOL 6400, JEOL Corp., Tokyo, Japan) and energy dispersive spectroscopy (EDS) (EMAX-7000 Type S, Horiba Ltd., Kyoto, Japan). The central beam of the SEM was directed to the center of the specimen under ×10 magnification, which was gradually increased to ×1,000 to standardize the examined area of each specimen.
Statistical analysis
The statistical analysis was performed using SPSS version 20.0 (IBM Corp., Armonk, NY, USA). According to the Shapiro-Wilk test, the data showed a normal distribution. Data were analyzed using 2-way analysis of variance, the Tukey honest significant difference test, and the paired t-test. The level of significance was set at p < 0.05.
RESULTS
The results of the solubility analysis are presented in
Table 1. All materials showed significant solubility after immersion in the solutions (
p < 0.05). The solution type had no effect on the solubility of the materials (
p > 0.05).
Table 1 Solubility of calcium silicate-based cements in irrigation solutions
|
Groups |
Initial weight (mg) |
Final weight (mg) |
Mean percentage change (%) |
|
MTA-DW |
116.13 ± 13.79a
|
114.38 ± 14.02b
|
1.51 |
|
MTA-NaOCl |
113.07 ± 11.43a
|
111.46 ± 11.50b
|
1.42 |
|
MTA-CHX |
112.47 ± 11.59a
|
111.08 ± 11.65b
|
1.24 |
|
MTA-OCT |
114.14 ± 8.54a
|
112.17 ± 8.60b
|
1.73 |
|
Biodentine-DW |
116.69 ± 13.69a
|
114.99 ± 12.94b
|
1.46 |
|
Biodentine-NaOCl |
118.29 ± 10.84a
|
116.85 ± 10.55b
|
1.22 |
|
Biodentine-CHX |
111.52 ± 13.02a
|
109.46 ± 13.29b
|
1.85 |
|
Biodentine-OCT |
110.64 ± 10.88a
|
109.08 ± 10.92b
|
1.41 |
|
ERRM-DW |
111.48 ± 15.38a
|
109.93 ± 14.87b
|
1.39 |
|
ERRM-NaOCl |
112.27 ± 12.07a
|
110.96 ± 12.86b
|
1.17 |
|
ERRM-CHX |
116.46 ± 10.04a
|
115.16 ± 10.83b
|
1.12 |
|
ERRM-OCT |
117.80 ± 10.41a
|
115.49 ± 10.31b
|
1.96 |
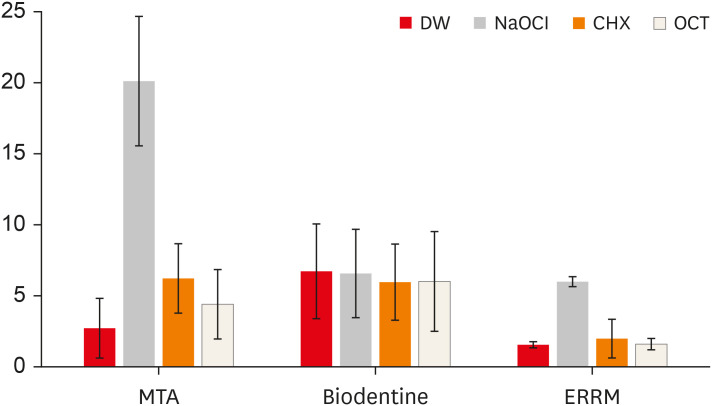
Figure 1 presents digital images of the CSCs before and after contact with different solutions. The mean ΔE values of the groups are shown in
Figure 2. NaOCl significantly affected the color of ProRoot MTA (darker) and ERRM (lighter) compared with the other irrigants (
p < 0.05). The color change of ProRoot MTA was greater than that of the other materials after immersion in NaOCl (
p < 0.05). ProRoot MTA presented dark brown discoloration, whereas a white precipitate was observed on the surface of ERRM after contact with NaOCl. Biodentine also exhibited a white precipitate on its surface after immersion in the solutions, and all solutions similarly affected its color (lighter) (
p > 0.05). Biodentine showed a greater color change when immersed in DW than the other materials (
p < 0.05). The color change of ProRoot MTA and Biodentine was significantly greater than that of ERRM after immersion in CHX (lighter) (
p < 0.05). Biodentine presented a greater color change than ERRM after immersion in OCT (lighter) (
p < 0.05).
Figure 1
Images of calcium silicate-based cements before and after contact with different solutions.
MTA, mineral trioxide aggregate; ERRM, Endosequence Root Repair Material; CHX, chlorhexidine; NaOCl, sodium hypochlorite; OCT, octenidine hydrochloride.
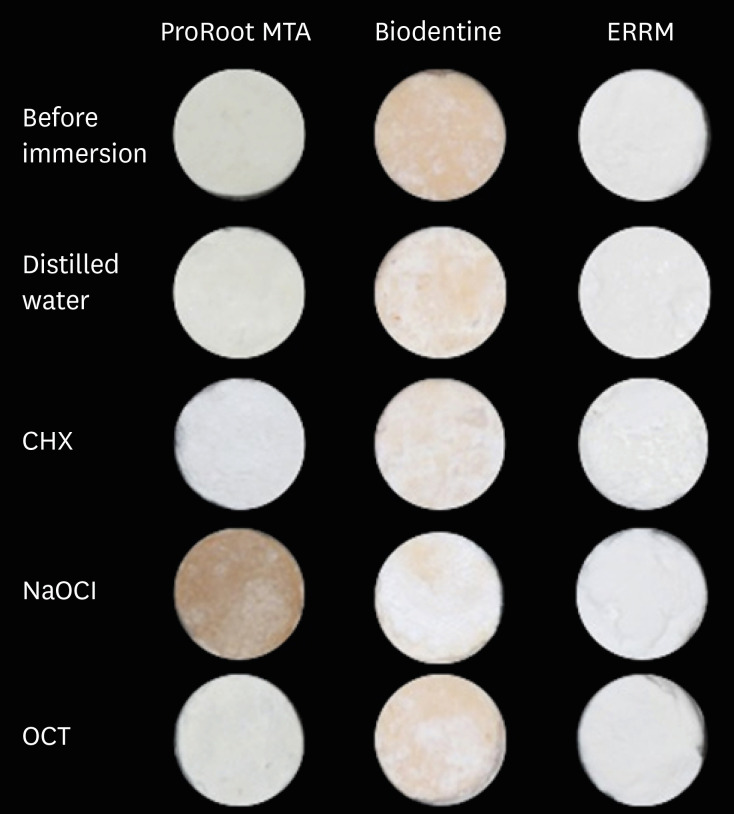
Figure 2
Mean ΔE values of the groups.
DW, distilled water; NaOCl, sodium hypochlorite; CHX, chlorhexidine; OCT, octenidine hydrochloride; MTA, mineral trioxide aggregate; ERRM, Endosequence Root Repair Material.
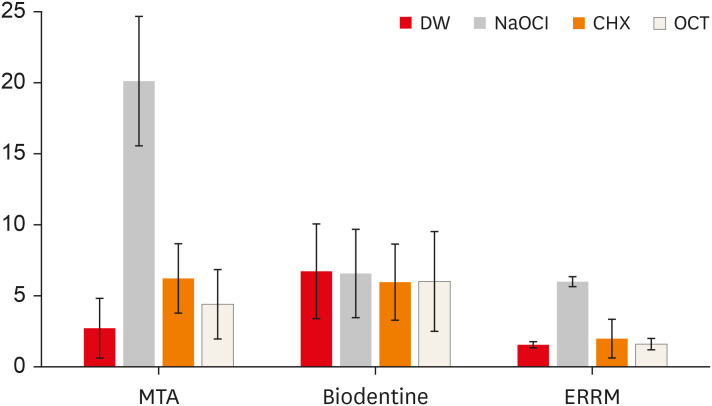
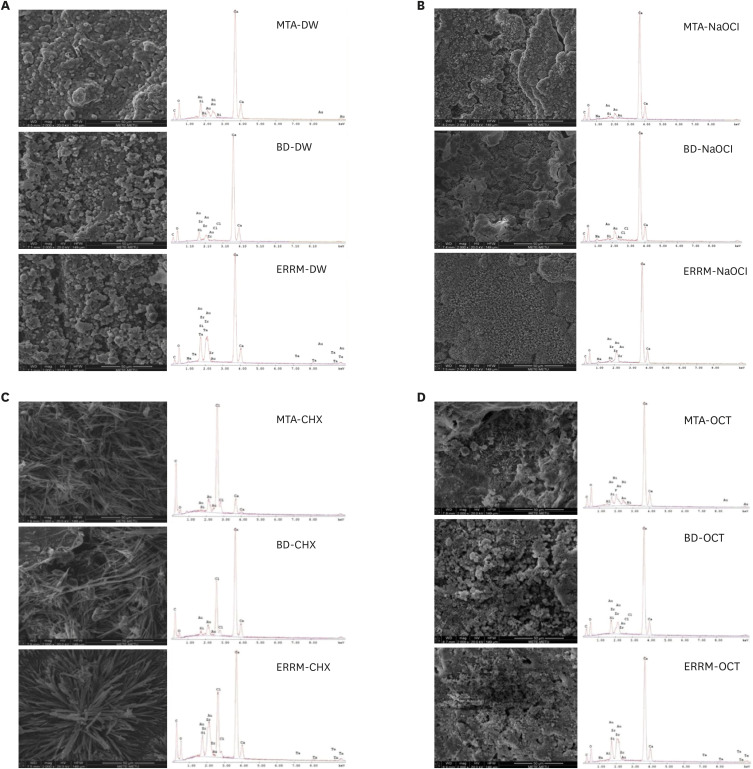
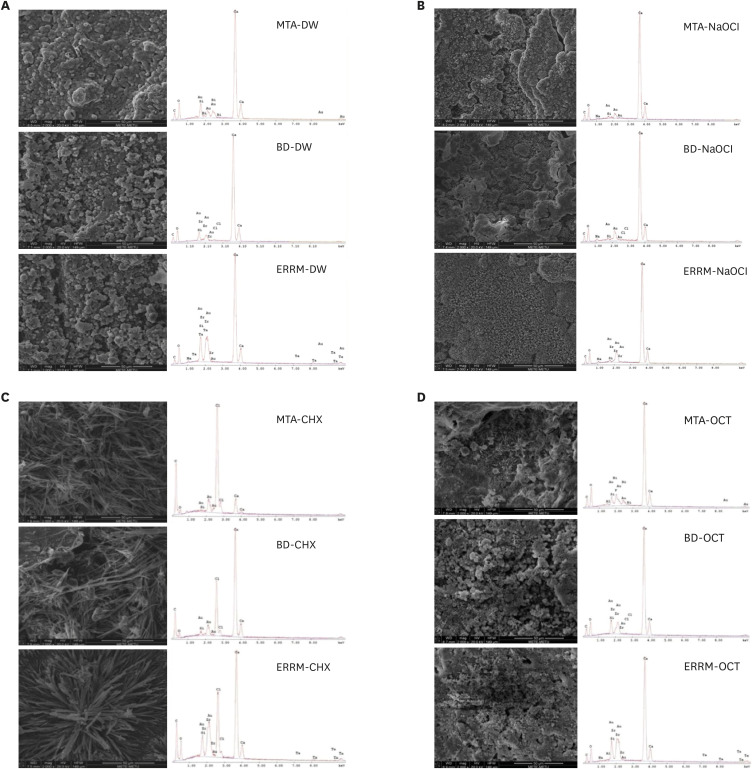
Figure 3 presents representative SEM images (×2,000 magnification) and EDS graphs of each group. The typical surface appearance of CSCs, with round, cubic, and cylindrical crystals, was observed on the surface of materials after immersion in DW (
Figure 3A). After exposure to NaOCl, cracks, a few plate-like crystals, and crystal clusters were seen on the surfaces (
Figure 3B). Marked morphological changes were observed on the surface of all materials placed in CHX, and needle-shaped crystals were mainly seen on the surfaces after contact with CHX (
Figure 3C). After exposure to OCT, the surfaces were full of crystal clusters, which mainly showed petal-like formations (
Figure 3D). EDS analysis displayed calcium and silicon peaks for all materials. Bismuth and zirconium peaks were observed on the surfaces of MTA and Biodentine, respectively. The ERRM samples presented zirconium and tantalum peaks. Sodium and chloride peaks were present on the surfaces of all materials after immersion in NaOCl and CHX, respectively.
Figure 3
Scanning electron microscopy images and energy dispersive spectroscopy graphs of calcium silicate-based cements after immersion in (A) DW, (B) NaOCl, (C) CHX, and (D) OCT.
DW, distilled water; NaOCl, sodium hypochlorite; CHX, chlorhexidine; OCT, octenidine hydrochloride; MTA, mineral trioxide aggregate; BD, Biodentine; ERRM, Endosequence Root Repair Material.
DISCUSSION
Root canal procedures performed after repair material placement may lead to physicochemical alterations of cement [
16,
17,
18,
19]. In the present study, the effects of different irrigation solutions on commonly used CSCs were analyzed. For this purpose, the materials were stored in a humid environment for 24 hours to enable complete setting and to achieve the optimum physical characteristics before exposure to the solutions. Then, the entire specimen was placed in the solutions for 24 hours, as in previous studies [
18,
19]. It should be mentioned that the prolonged time of exposure to the irrigants and the number of contact surfaces may not precisely reflect the clinical situation. Furthermore, in routine clinical procedures, the materials immediately interact with the solutions once they are placed. However, in specific conditions such as cases of resorption or perforation, it is appropriate to wait for complete setting of the repair material and to perform the root canal procedure on the second visit [
20].
The solubility test was performed after materials were stored in solutions for 24 hours in accordance with ISO standard 6876:2012, similar to previous studies [
21,
22]. Although all CSCs presented significant solubility, the changes did not exceed 3% by mass, meeting the ISO solubility requirements. CSCs, also called hydraulic cements, form calcium silicate hydrate and calcium hydroxide (Ca(OH)
2) in contact with water [
23]. These cements create an alkaline environment, possess antimicrobial properties, and promote mineralized tissue formation with calcium and hydroxyl ion release [
24]. Therefore, some amount of dissolution is necessary for CSCs to exert these favorable effects. The solubility detected in the materials in the present study, in which all the materials presented similar solubility after storage in solutions for 24 hours, was probably related to the dissolution of calcium and hydroxyl ions from the cements. In line with the present findings, previous studies that also evaluated solubility after a 24-hour storage period found no significant difference between CSCs [
21,
22]. This could be explained by the similar bioactivity potential of the materials [
25]. A previous study found that an acidic environment reduced calcium release from CSCs [
25]. Despite being close to neutral, the slightly acidic pH of CHX and OCT can lead to inhibition of crystallization over the surface, negatively affecting solubility. In contrast, another study reported that an acidic environment did not affect the surface level and the roughness of CSCs over time [
26]. Based on the present findings, the solution type had no effect on the solubility of the tested CSCs. However, it is worth mentioning that CSCs exhibit a tendency for washout, which refers to disintegration of the material upon contact with fluids [
27,
28]. This washout can be minimized if the material sets quickly before it is exposed to fluids [
28]. In the present study, materials were exposed to irrigants after the completion of setting. Therefore, the washout tendency of the materials could not be evaluated. This should be considered when interpreting the present findings.
Color analysis using visual spectrophotometry according to the CIE system is accepted as a gold standard method in dentistry [
29]. Spectrophotometric analysis in the present study provided repeatability and objectivity for the detection of small changes in color due to the standardization of measurement parameters and calibration of the spectrophotometer before each measurement. According to the present study, the color of the tested CSCs was generally brighter due to white precipitate formation on their surfaces after immersion in the solutions, while NaOCl caused severe discoloration of MTA [
18,
19]. MTA contains bismuth oxide as a radiopacifier; this component is responsible for the dark brown discoloration of the material after contact with NaOCl [
19]. The mechanism of this change is probably related to the reduction of NaOCl to sodium chloride after contact with bismuth oxide [
19]. As a result, a dark brown or black precipitate is formed through the overoxidation of bismuth oxide [
30]. As oxygen becomes unstable, it reacts with carbon dioxide in the air, and forms bismuth carbonate, which leads to discoloration [
30]. It has previously been reported that other radiopacifiers, such as zirconium oxide and tantalum oxide, do not cause discoloration [
31]. As Biodentine and ERRM contain these components instead of bismuth oxide as radiopacifiers, no dark discoloration was observed with these materials after contact with NaOCl in the present study.
This is the first study to analyze the effect of OCT on the color stability of CSCs. According to the current results, color changes occurred due to white crystal precipitation on the surface, and no dark discoloration was observed in any materials after immersion in OCT. Although the mechanism of this action is not well-defined, a previous study reported discoloration of CSCs after immersion in CHX [
18]. On the contrary, in this study, the surfaces of all materials were covered with white crystal formation, and no dark discoloration was observed after contact with CHX. Similar to the present findings, a previous study reported formation of calcium silicate hydrate crystals and calcium phosphate on the surface of MTA after immersion in a solution containing CHX [
32]. Furthermore, the addition of CHX to MTA was found to increase its pH and calcium ion release [
33].
Biodentine exhibited greater color changes than the other materials due to white precipitate formation after immersion in DW. This result could be related to the higher biomineralization ability of the material [
34,
35]. Although Biodentine has been reported to be more appropriate than MTA for use in acidic conditions, the present results showed no significant differences between MTA and Biodentine in color stability or solubility after exposure to CHX and OCT [
36]. The EDS analysis showed that all materials presented high levels of calcium on the surface after immersion in NaOCl. Similar to a recent study, a CSC after contact with NaOCl had dense Ca(OH)
2 and calcium phosphate crystal formation [
32]. Previously, the microhardness of ERRM was found to be lower than that of MTA after exposure to low-pH conditions [
37]. A possible explanation for this finding might be inhibited crystallization in the hydration reaction of the material at a low pH. The lower amount of white crystal precipitation in low-pH conditions may explain the smaller color change of ERRM compared to the other materials after exposure to CHX and OCT.
SEM images showed changes in the microstructural characteristics of materials depending on the solution type. Round, cubic, and cylindrical crystals or crystal clusters were observed on the surfaces after contact with DW, NaOCl, and OCT, reflecting the typical particles of CSCs. This crystalline deposition on the surfaces had a high calcium peak, which may indicate bioactivity [
35]. Interestingly, needle-shaped crystals were formed on the surfaces of CSCs after contact with CHX. Similarly, a previous study also demonstrated needle-shaped crystals in MTA after immersion in CHX [
38]. This microstructural change could be related to the interaction between CHX and CSCs. It is well known that CSCs release Ca(OH)
2 during hydration, leading to an alkaline environment [
4]. Previous studies have shown that CHX degrades immediately and completely after contact with Ca(OH)
2 resulting in the generation of
para-chloroaniline (pCA) [
39,
40]. This chemical reaction may explain the different surface morphology of CSCs after contact with CHX and the high level of chlorine detection with EDS analysis. pCA is known to have long-term toxicity, and its precipitation can adversely affect the biological properties of CSCs such as bioactivity and cell attachment to the surface [
39,
40]. Further studies are needed to confirm this possibility. Despite these possible effects of the solutions on the material properties, the solution type had no influence on the solubility of the tested CSCs according to the current findings. However, depending on the solution type, the change in surface characteristics appears to have affected precipitate formation and hence color stability.
CONCLUSIONS
All materials showed solubility after immersion in the solutions irrespective of the solution type, and precipitation of crystals was observed after contact with the solutions. ProRoot MTA presented dark brown discoloration after contact with NaOCl. Biodentine and ERRM can be suitable alternatives to ProRoot MTA, since they do not exhibit discoloration. The use of OCT can be considered safe for CSCs.
-
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
-
Author Contributions:
Conceptualization: Eren SK, Örs SA, Aksel H, Canay S, Karasan D.
Data curation: Eren SK, Karasan D.
Formal analysis: Eren SK, Karasan D.
Funding acquisition: Eren SK, Karasan D.
Investigation: Eren SK, Örs SA, Karasan D.
Methodology: Eren SK, Örs SA, Aksel H, Karasan D.
Project administration: Eren SK.
Resources: Eren SK, Örs SA, Aksel H, Canay S, Karasan D.
Software: Eren SK, Örs SA, Aksel H, Canay S, Karasan D.
Supervision: Eren SK, Canay S, Karasan D.
Validation: Eren SK, Karasan D.
Writing - original draft: Selen Küçükkaya Eren.
Writing - review & editing: Eren SK, Örs SA, Aksel H, Canay S, Karasan D.
REFERENCES
- 1. Torabinejad M, Chivian N. Clinical applications of mineral trioxide aggregate. J Endod 1999;25:197-205.ArticlePubMed
- 2. Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review--part III: clinical applications, drawbacks, and mechanism of action. J Endod 2010;36:400-413.ArticlePubMed
- 3. Parirokh M, Torabinejad M, Dummer PMH. Mineral trioxide aggregate and other bioactive endodontic cements: an updated overview - part I: vital pulp therapy. Int Endod J 2018;51:177-205.ArticlePubMedPDF
- 4. Dawood AE, Parashos P, Wong RHK, Reynolds EC, Manton DJ. Calcium silicate-based cements: composition, properties, and clinical applications. J Investig Clin Dent 2017;8:e12195.ArticlePDF
- 5. Zehnder M. Root canal irrigants. J Endod 2006;32:389-398.ArticlePubMed
- 6. Nocca G, Ahmed HMA, Martorana GE, Callà C, Gambarini G, Rengo S, Spagnuolo G. Chromographic analysis and cytotoxic effects of chlorhexidine and sodium hypochlorite reaction mixtures. J Endod 2017;43:1545-1552.ArticlePubMed
- 7. Tandjung L, Waltimo T, Hauser I, Heide P, Decker EM, Weiger R. Octenidine in root canal and dentine disinfection ex vivo
. Int Endod J 2007;40:845-851.PubMed
- 8. Sedlock DM, Bailey DM. Microbicidal activity of octenidine hydrochloride, a new alkanediylbis[pyridine] germicidal agent. Antimicrob Agents Chemother 1985;28:786-790.ArticlePubMedPMCPDF
- 9. Tirali RE, Turan Y, Akal N, Karahan ZC.
In vitro antimicrobial activity of several concentrations of NaOCl and Octenisept in elimination of endodontic pathogens. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009;108:e117-e120.
- 10. Bukhary S, Balto H. Antibacterial efficacy of octenisept, alexidine, chlorhexidine, and sodium hypochlorite against Enterococcus faecalis biofilms. J Endod 2017;43:643-647.PubMed
- 11. Tirali RE, Bodur H, Sipahi B, Sungurtekin E. Evaluation of the antimicrobial activities of chlorhexidine gluconate, sodium hypochlorite and octenidine hydrochloride in vitro
. Aust Endod J 2013;39:15-18.PubMed
- 12. Thaha KA, Varma RL, Nair MG, Sam Joseph VG, Krishnan U. Interaction between octenidine-based solution and sodium hypochlorite: a mass spectroscopy, proton nuclear magnetic resonance, and scanning electron microscopy-based observational study. J Endod 2017;43:135-140.ArticlePubMed
- 13. Krishnan U, Saji S, Clarkson R, Lalloo R, Moule AJ. Free active chlorine in sodium hypochlorite solutions admixed with Octenidine, SmearOFF, Chlorhexidine, and EDTA. J Endod 2017;43:1354-1359.ArticlePubMed
- 14. Coaguila-Llerena H, Rodrigues EM, Santos CS, Ramos SG, Medeiros MC, Chavez-Andrade GM, Guerreiro-Tanomaru JM, Tanomaru-Filho M, Faria G. Effects of octenidine applied alone or mixed with sodium hypochlorite on eukaryotic cells. Int Endod J 2020;53:1264-1274.ArticlePubMedPDF
- 15. Coaguila-Llerena H, Stefanini da Silva V, Tanomaru-Filho M, Guerreiro Tanomaru JM, Faria G. Cleaning capacity of octenidine as root canal irrigant: a scanning electron microscopy study. Microsc Res Tech 2018;81:523-527.ArticlePubMedPDF
- 16. Nagas E, Cehreli ZC, Uyanik MO, Durmaz V, Vallittu PK, Lassila LV. Bond strength of mineral trioxide aggregate to root dentin after exposure to different irrigation solutions. Dent Traumatol 2014;30:246-249.ArticlePubMed
- 17. Chu JHR, Chia KY, Qui AL, Moule A, Ha WN. The effects of sodium hypochlorite and ethylenediaminetetraacetic acid on the microhardness of Mineral Trioxide Aggregate and TotalFill Bioceramic Putty. Aust Endod J 2020;46:33-39.ArticlePubMedPDF
- 18. Keskin C, Demiryurek EO, Ozyurek T. Color stabilities of calcium silicate-based materials in contact with different irrigation solutions. J Endod 2015;41:409-411.ArticlePubMed
- 19. Camilleri J. Color stability of white mineral trioxide aggregate in contact with hypochlorite solution. J Endod 2014;40:436-440.ArticlePubMed
- 20. Krupp C, Bargholz C, Brüsehaber M, Hülsmann M. Treatment outcome after repair of root perforations with mineral trioxide aggregate: a retrospective evaluation of 90 teeth. J Endod 2013;39:1364-1368.ArticlePubMed
- 21. Gandolfi MG, Siboni F, Botero T, Bossù M, Riccitiello F, Prati C. Calcium silicate and calcium hydroxide materials for pulp capping: biointeractivity, porosity, solubility and bioactivity of current formulations. J Appl Biomater Funct Mater 2015;13:43-60.ArticlePubMedPDF
- 22. Singh S, Podar R, Dadu S, Kulkarni G, Purba R. Solubility of a new calcium silicate-based root-end filling material. J Conserv Dent 2015;18:149-153.ArticlePubMedPMC
- 23. Camilleri J. Characterization and hydration kinetics of tricalcium silicate cement for use as a dental biomaterial. Dent Mater 2011;27:836-844.ArticlePubMed
- 24. Arias-Moliz MT, Farrugia C, Lung CYK, Wismayer PS, Camilleri J. Antimicrobial and biological activity of leachate from light curable pulp capping materials. J Dent 2017;64:45-51.ArticlePubMed
- 25. Natale LC, Rodrigues MC, Xavier TA, Simões A, de Souza DN, Braga RR. Ion release and mechanical properties of calcium silicate and calcium hydroxide materials used for pulp capping. Int Endod J 2015;48:89-94.ArticlePubMed
- 26. Aksel H, Küçükkaya Eren S, Askerbeyli Õrs S, Karaismailoğlu E. Surface and vertical dimensional changes of mineral trioxide aggregate and biodentine in different environmental conditions. J Appl Oral Sci 2018;27:e20180093.ArticlePubMedPMC
- 27. Grech L, Mallia B, Camilleri J. Investigation of the physical properties of tricalcium silicate cement-based root-end filling materials. Dent Mater 2013;29:e20-e28.ArticlePubMed
- 28. Choi Y, Park SJ, Lee SH, Hwang YC, Yu MK, Min KS. Biological effects and washout resistance of a newly developed fast-setting pozzolan cement. J Endod 2013;39:467-472.ArticlePubMed
- 29. Możyńska J, Metlerski M, Lipski M, Nowicka A. Tooth discoloration induced by different calcium silicate-based cements: a systematic review of in vitro studies. J Endod 2017;43:1593-1601.PubMed
- 30. Kang SH, Shin YS, Lee HS, Kim SO, Shin Y, Jung IY, Song JS. Color changes of teeth after treatment with various mineral trioxide aggregate-based materials: an ex vivo study. J Endod 2015;41:737-741.PubMed
- 31. Camilleri J. Staining potential of Neo MTA Plus, MTA Plus, and Biodentine used for pulpotomy procedures. J Endod 2015;41:1139-1145.ArticlePubMed
- 32. Neelakantan P, Berger T, Primus C, Shemesh H, Wesselink PR. Acidic and alkaline chemicals' influence on a tricalcium silicate-based dental biomaterial. J Biomed Mater Res B Appl Biomater 2019;107:377-387.ArticlePubMedPDF
- 33. Jacinto RC, Linhares-Farina G, Sposito OS, Zanchi CH, Cenci MS. Influence of 2% chlorhexidine on pH, calcium release and setting time of a resinous MTA-based root-end filling material. Braz Oral Res 2015;29:1-6.Article
- 34. Han L, Okiji T. Uptake of calcium and silicon released from calcium silicate-based endodontic materials into root canal dentine. Int Endod J 2011;44:1081-1087.ArticlePubMed
- 35. Han L, Okiji T. Bioactivity evaluation of three calcium silicate-based endodontic materials. Int Endod J 2013;46:808-814.ArticlePubMed
- 36. Elnaghy AM. Influence of acidic environment on properties of biodentine and white mineral trioxide aggregate: a comparative study. J Endod 2014;40:953-957.ArticlePubMed
- 37. Wang Z, Ma J, Shen Y, Haapasalo M. Acidic pH weakens the microhardness and microstructure of three tricalcium silicate materials. Int Endod J 2015;48:323-332.PubMed
- 38. Yan P, Peng B, Fan B, Fan M, Bian Z. The effects of sodium hypochlorite (5.25%), Chlorhexidine (2%), and Glyde File Prep on the bond strength of MTA-dentin. J Endod 2006;32:58-60.ArticlePubMed
- 39. Barbin LE, Saquy PC, Guedes DF, Sousa-Neto MD, Estrela C, Pécora JD. Determination of para-chloroaniline and reactive oxygen species in chlorhexidine and chlorhexidine associated with calcium hydroxide. J Endod 2008;34:1508-1514.ArticlePubMed
- 40. Barbin LE, Estrela C, Guedes DF, Spanó JC, Sousa-Neto MD, Pécora JD. Detection of para-chloroaniline, reactive oxygen species, and 1-chloro-4-nitrobenzene in high concentrations of chlorhexidine and in a mixture of chlorhexidine and calcium hydroxide. J Endod 2013;39:664-668.ArticlePubMed
 , Sevinc Askerbeyli Örs1
, Sevinc Askerbeyli Örs1 , Hacer Aksel2
, Hacer Aksel2 , Şenay Canay3
, Şenay Canay3 , Duygu Karasan4
, Duygu Karasan4



 KACD
KACD



 ePub Link
ePub Link Cite
Cite

