Articles
- Page Path
- HOME > Restor Dent Endod > Volume 46(1); 2021 > Article
- Research Article Corrosion resistance assessment of nickel-titanium endodontic files with and without heat treatment
-
Tatiana Dias Costa1
 , Elison da Fonseca e Silva2
, Elison da Fonseca e Silva2 , Paula Liparini Caetano3
, Paula Liparini Caetano3 , Marcio José da Silva Campos3
, Marcio José da Silva Campos3 , Leandro Marques Resende4
, Leandro Marques Resende4 , André Guimarães Machado1
, André Guimarães Machado1 , Antônio Márcio Resende do Carmo4
, Antônio Márcio Resende do Carmo4
-
Restor Dent Endod 2020;46(1):e6.
DOI: https://doi.org/10.5395/rde.2021.46.e6
Published online: December 28, 2020
1Department of Endodontics, Juiz de Fora Estacio University Center, Juiz de Fora, MG, Brazil.
2Department of Metallurgical Engineering, Federal Institute Juiz de Fora, Juiz de Fora, MG, Brazil.
3Department of Orthodontics, Federal University of Juiz de Fora, Juiz de Fora, MG, Brazil.
4Department of Endodontics, Federal University of Juiz de Fora, Juiz de Fora, MG, Brazil.
- Correspondence to Paula Liparini Caetano, DDS, MS. Postgraduate Student, Department of Orthodontics, Federal University of Juiz de Fora, Ivon José Cury 2, Juiz de Fora, MG 36037-467, Brazil. xpaulinha1@hotmail.com
Copyright © 2021. The Korean Academy of Conservative Dentistry
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Objectives The aim of this study was to evaluate the corrosion resistance of heat-treated (Reciproc and WaveOne) and non-heat-treated (ProTaper and Mtwo) superelastic nickel-titanium endodontic files when immersed in a 5.25% sodium hypochlorite solution.
-
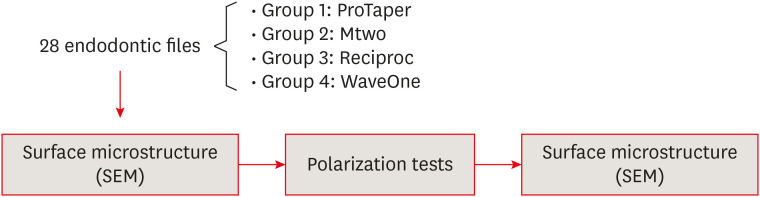
Materials and Methods Anodic polarization curves were obtained with potential sweeps that began at the open circuit potential or corrosion potential (Ecorr). The pitting potential (Epit) was identified on the anodic polarization curve as the potential at which a sudden increase in current was observed. The micromorphology of the 28 tested files was analyzed before and after the electrochemical assay using scanning electron microscope (SEM). The data were analyzed using 1-way analysis of variance with the post hoc Bonferroni test (for Ecorr) and the Student t-test for independent samples (for Epit).
-
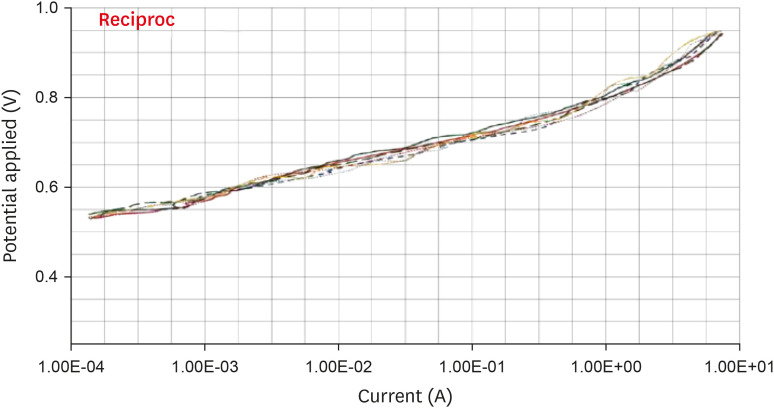
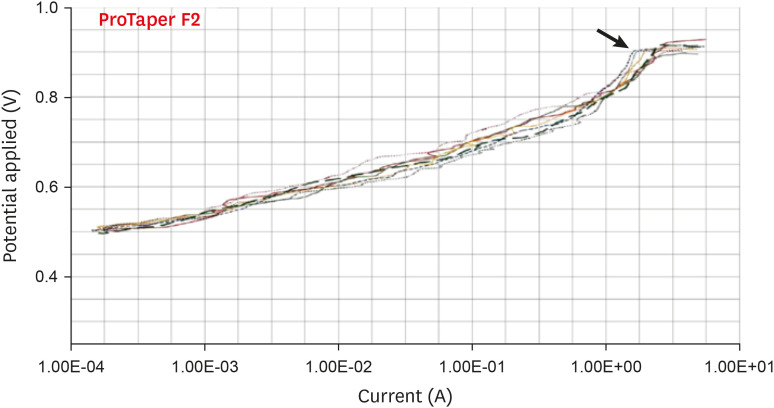
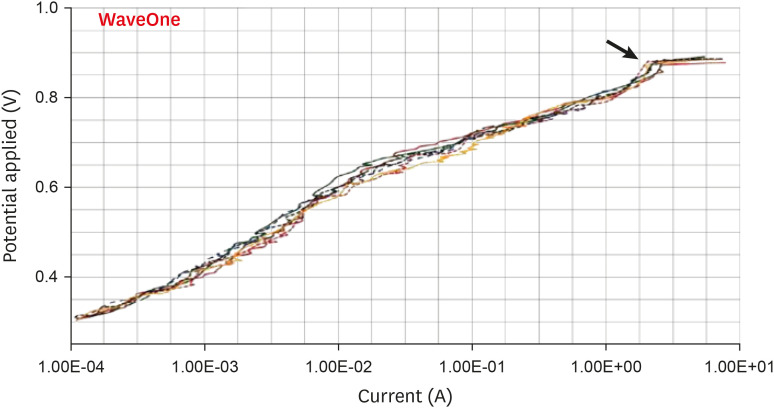
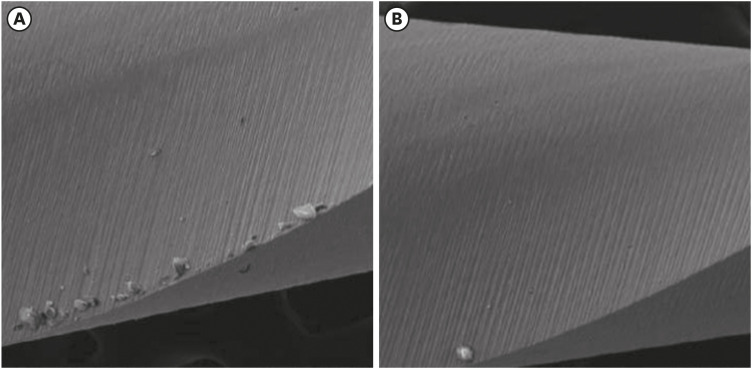
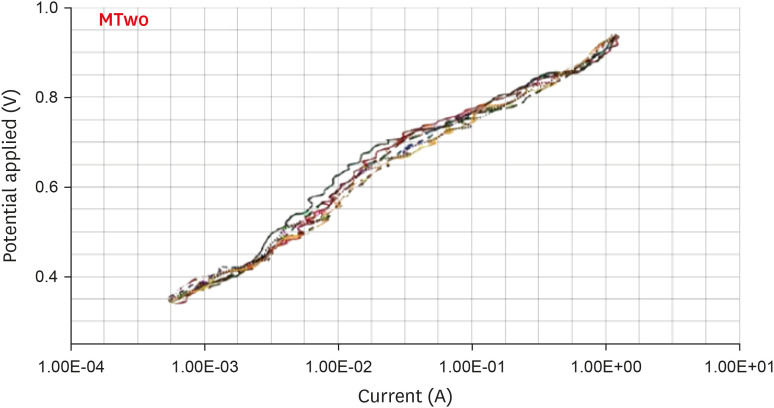
Results The mean Ecorr values were 0.506 V for ProTaper, 0.348 V for Mtwo, 0.542 V for Reciproc, and 0.321 V for WaveOne files. Only WaveOne and Protaper files exhibited pitting corrosion, with Epit values of 0.879 V and 0.904 V, respectively. On the SEM images of the ProTaper and WaveOne files, cavities suggestive of pitting corrosion were detected.
-
Conclusions Signs of corrosion were observed in both heat-treated and non-heat-treated files. Of the evaluated files, WaveOne (a heat-treated file) and ProTaper (a non-heat-treated file) exhibited the lowest corrosion resistance.
INTRODUCTION
MATERIALS AND METHODS
RESULTS
Mean ± standard deviation (SD) of the corrosion potential (Ecorr) and pitting potential (Epit) values of ProTaper, Mtwo, Reciproc, and WaveOne files
| Files | Ecorr* | Epit† | |
|---|---|---|---|
| Heat-treated | |||
| ProTaper | 0.506 ± 0.013 | 0.904 ± 0.010 | |
| Mtwo | 0.348 ± 0.006 | - | |
| Non-heat-treated | |||
| Reciproc | 0.542 ± 0.001 | - | |
| WaveOne | 0.321 ± 0.001 | 0.879 ± 0.005 | |
| p value | < 0.001* | 0.004† | |
A p values for the comparisons of corrosion potential (Ecorr) between ProTaper, Mtwo, Reciproc, and WaveOne files
| Comparisons of Ecorr | ProTaper | Mtwo | Reciproc | WaveOne |
|---|---|---|---|---|
| ProTaper | - | < 0.05 | < 0.05 | < 0.05 |
| Mtwo | < 0.05 | - | < 0.05 | < 0.05 |
| Reciproc | < 0.05 | < 0.05 | - | < 0.05 |
| WaveOne | < 0.05 | < 0.05 | < 0.05 | - |
Anodic polarization curves of the Reciproc files in 5.25% sodium hypochlorite (NaOCl) solution.
Anodic polarization curves of the ProTaper files in 5.25% sodium hypochlorite (NaOCl) solution. The arrow indicates the cavity of pitting.
Anodic polarization curves of the Mtwo files in 5.25% sodium hypochlorite (NaOCl) solution. The arrow indicates the cavity of pitting.
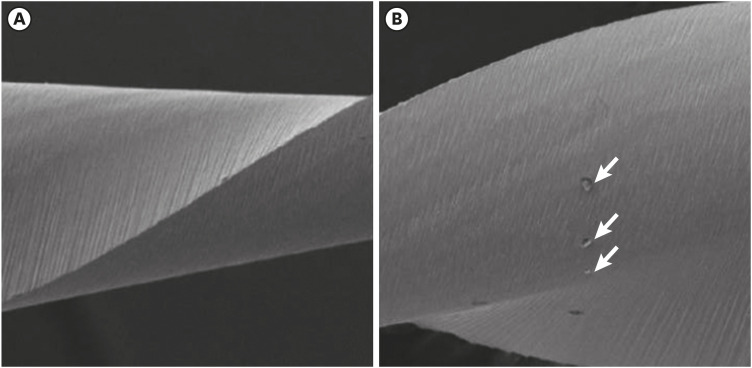
Scanning electron microscopic image of Reciproc files. (A) Before (×304) and (B) after the electrochemical test (×306).
Scanning electron microscopic image of Mtwo files. (A) Before (×296) and (B) after the electrochemical test (×298).
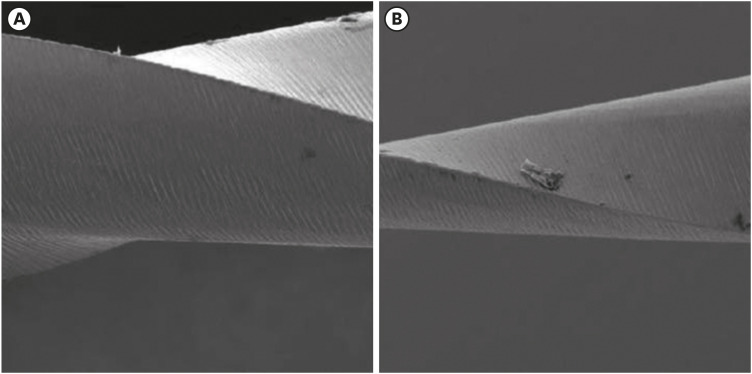
Scanning electron microscopic image of ProTaper files. (A) Before (×256) and (B) after the electrochemical test, with the presence of corrosion by pitting (×250).
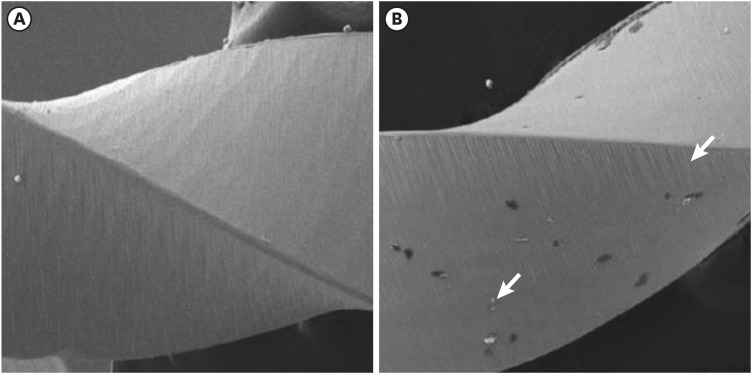
Scanning electron microscopic image of WaveOne files. (A) Before (×304) and (B) after the electrochemical test, with the presence of corrosion by pitting (×306).
DISCUSSION
CONCLUSIONS
-
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
-
Author Contributions:
Conceptualization: Silva EF, Resende L, Machado AG, Carmo AMR.
Data curation: Costa TD.
Formal analysis: Silva EF, Caetano PL, Campos MJS, Carmo AMR.
Funding acquisition: Costa T, Machado AG.
Investigation: Costa TD, Machado AG.
Methodology: Costa TD, Silva EF, Machado AG, Carmo AMR.
Project administration: Silva EF, Resende LM, Machado AG, Carmo AMR.
Resources: Silva EF.
Software: Silva EF.
Supervision: Silva EF, Campos MJS.
Validation: Silva EF.
Visualization: Silva EF, Machado AG.
Writing - original draft: Costa TD, Silva EF, Caetano PL, Campos MJS, Resende LM, Machado AG, Carmo AMR.
Writing - review & editing: Caetano PL, Campos MJS.
- 1. Walia HM, Brantley WA, Gerstein H. An initial investigation of the bending and torsional properties of Nitinol root canal files. J Endod 1988;14:346-351.ArticlePubMed
- 2. Gavini G, Santos MD, Caldeira CL, Machado MEL, Freire LG, Iglecias EF, Peters OA, Candeiro GTM. Nickel-titanium instruments in endodontics: a concise review of the state of the art. Braz Oral Res 2018;32:e67.ArticlePubMed
- 3. Hilt BR, Cunningham CJ, Shen C, Richards N. Torsional properties of stainless-steel and nickel-titanium files after multiple autoclave sterilizations. J Endod 2000;26:76-80.ArticlePubMed
- 4. Darabara M, Bourithis L, Zinelis S, Papadimitriou GD. Susceptibility to localized corrosion of stainless steel and NiTi endodontic instruments in irrigating solutions. Int Endod J 2004;37:705-710.ArticlePubMed
- 5. Talha M, Behera CK, Sinha OP. A review on nickel-free nitrogen containing austenitic stainless steels for biomedical applications. Mater Sci Eng C 2013;33:3563-3575.Article
- 6. Wright PP, Kahler B, Walsh LJ. Alkaline sodium hypochlorite irrigant and its chemical interactions. Materials (Basel) 2017;10:1147-1155.ArticlePubMedPMC
- 7. Pedullà E, Benites A, La Rosa GM, Plotino G, Grande NM, Rapisarda E, Generali L. Cyclic fatigue resistance of heat-treated nickel-titanium instruments after immersion in sodium hypochlorite and/or sterilization. J Endod 2018;44:648-653.ArticlePubMed
- 8. Ametrano G, D'Antò V, Di Caprio MP, Simeone M, Rengo S, Spagnuolo G. Effects of sodium hypochlorite and ethylenediaminetetraacetic acid on rotary nickel-titanium instruments evaluated using atomic force microscopy. Int Endod J 2011;44:203-209.ArticlePubMed
- 9. Bonaccorso A, Tripi TR, Rondelli G, Condorelli GG, Cantatore G, Schäfer E. Pitting corrosion resistance of nickel-titanium rotary instruments with different surface treatments in seventeen percent ethylenediaminetetraacetic acid and sodium chloride solutions. J Endod 2008;34:208-211.ArticlePubMed
- 10. Lee JB, Yoon SI. Effect of nitrogen alloying on the semiconducting properties of passive films and metastable pitting susceptibility of 316L and 316LN stainless steels. Mater Chem Phys 2010;122:194-199.Article
- 11. Prymak O, Klocke A, Kahl-Nieke B, Epple M. Fatigue of orthodontic nickel-titanium (NiTi) wires in different fluids under constant mechanical stress. Mater Sci Eng A 2004;378:110-114.Article
- 12. Stokes OW, Fiore PM, Barss JT, Koerber A, Gilbert JL, Lautenschlager EP. Corrosion in stainless-steel and nickel-titanium files. J Endod 1999;25:17-20.ArticlePubMed
- 13. Yum JW, Park JK, Hur B, Kim HC. Comparative analysis of various corrosive environmental conditions for NiTi rotary files. J Korean Acad Conserv Dent 2008;33:377-388.Article
- 14. Berutti E, Angelini E, Rigolone M, Migliaretti G, Pasqualini D. Influence of sodium hypochlorite on fracture properties and corrosion of ProTaper Rotary instruments. Int Endod J 2006;39:693-699.ArticlePubMed
- 15. Pereira ES, Peixoto IF, Viana AC, Oliveira II, Gonzalez BM, Buono VT, Bahia MG. Physical and mechanical properties of a thermomechanically treated NiTi wire used in the manufacture of rotary endodontic instruments. Int Endod J 2012;45:469-474.ArticlePubMed
- 16. Ye J, Gao Y. Metallurgical characterization of M-Wire nickel-titanium shape memory alloy used for endodontic rotary instruments during low-cycle fatigue. J Endod 2012;38:105-107.ArticlePubMed
- 17. Aoun CM, Nehme WB, Naaman AS, Khalil IT. Review and classification of heat treatment procedures and their impact on mechanical behavior of endodontic files. Int J Curr Res 2017;9:51300-51306.
- 18. Gutmann JL, Gao Y. Alteration in the inherent metallic and surface properties of nickel-titanium root canal instruments to enhance performance, durability and safety: a focused review. Int Endod J 2012;45:113-128.ArticlePubMed
- 19. Prasad PS, Sam JE, Kumar A, Kannan . The effect of 5% sodium hypochlorite, 17% EDTA and triphala on two different rotary Ni-Ti instruments: an AFM and EDS analysis. J Conserv Dent 2014;17:462-466.ArticlePubMedPMC
- 20. Ramires I, Guastaldi AC. Study of Ti-6Al-4V biomaterial using electrochemistry and XPS techniques. Quim Nova 2002;25:10-14.
- 21. Olmedo DG, Tasat DR, Duffó G, Guglielmotti MB, Cabrini RL. The issue of corrosion in dental implants: a review. Acta Odontol Latinoam 2009;22:3-9.PubMed
- 22. Silva EF, Oliveira LF. Chemical and metallographic characterization of stainless steel in implants removed from patients. Acta Ortop Bras 2011;19:280-285.Article
- 23. Sonntag RE, Gordon JV. Introduction to thermodynamics: classical and statistical. 3rd ed. Hoboken, NJ: John Wiley & Sons, Inc; 1991. p. 800.
- 24. Lambert FL. Configurational entropy revisited. J Chem Educ 2007;84:1548-1550.Article
- 25. Es-Souni M, Es-Souni M, Fischer-Brandies H. On the properties of two binary NiTi shape memory alloys. Effects of surface finish on the corrosion behaviour and in vitro biocompatibility. Biomaterials 2002;23:2887-2894.ArticlePubMed
- 26. Thompson SA. An overview of nickel-titanium alloys used in dentistry. Int Endod J 2000;33:297-310.ArticlePubMed
REFERENCES
Tables & Figures
REFERENCES
Citations

- A novel approach to assess surface roughness and EDX profiling of blue rotary NiTi files following dynamic immersion in various hypochlorite concentrations
Hebatullah Ahmad Safwat, Nesreen Y. Mohammed, Asmaa Abd El-Hady
BMC Oral Health.2025;[Epub] CrossRef - Effect of phytic acid on chemical, structural, and mechanical characteristics of nickel–titanium endodontic files
Mai Samara, Mohannad Nassar, Abdullah Alqedairi, Hussam Alfawaz, Ahmed Jamleh
Scientific Reports.2024;[Epub] CrossRef - Investigating the Tribocorrosion Behaviour of NiTiNOL60 Alloy in Engineering and Biomedical Applications—An Overview
Anthony O. Okoani, Ashveen Nand, Cho-Pei Jiang, Maziar Ramezani
Metals.2024; 14(12): 1334. CrossRef - Electrochemical Properties of Nickel-Titanium Rotary Endodontic Instruments
Vidyalakshmi Subramanian, Howard W. Roberts, Shengtong Han, Stephanie J. Sidow, David W. Berzins
Journal of Endodontics.2024; 50(8): 1143. CrossRef - Effects of sodium hypochlorite on corrosion of the rotary nickel-titanium endodontic instruments - SEM analysis
Milica Jovanovic-Medojevic, Jelena Neskovic, Marijana Popovic-Bajic, Djordje Stratimirovic, Slavoljub Zivkovic
Srpski arhiv za celokupno lekarstvo.2022; 150(5-6): 254. CrossRef - Economic analysis of the different endodontic instrumentation techniques used in the Unified Health System
Laura Paredes Merchan, Livia Fernandes Probst, Ana Clara Correa Duarte Simões, Augusto Cesar Santos Raimundo, Yuri Wanderley Cavalcanti, Denise de Fátima Barros Cavalcante, João Victor Frazão Câmara, Antonio Carlos Pereira
BMC Oral Health.2022;[Epub] CrossRef
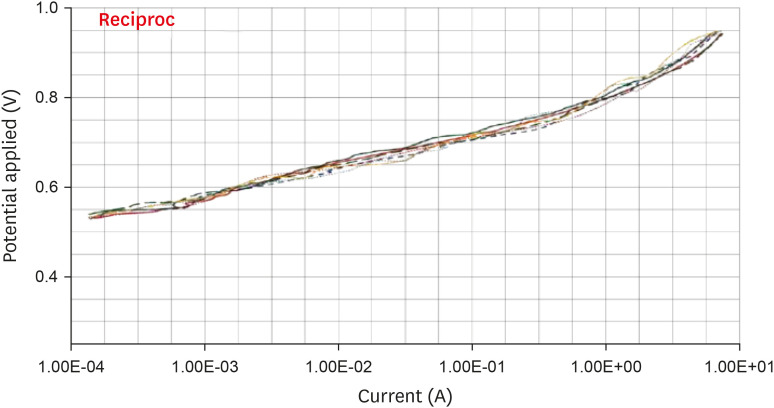
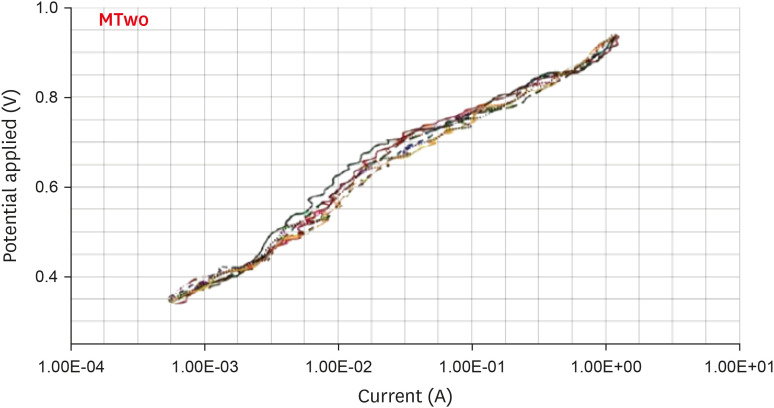
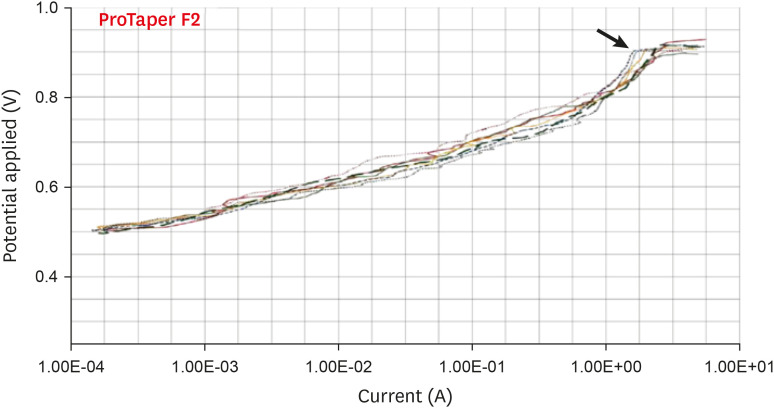
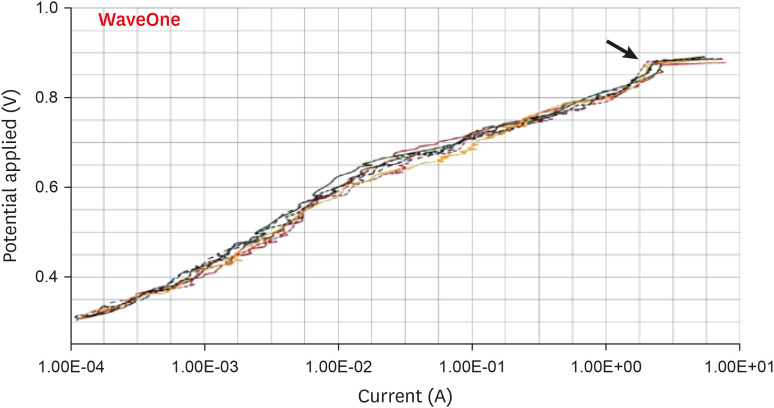
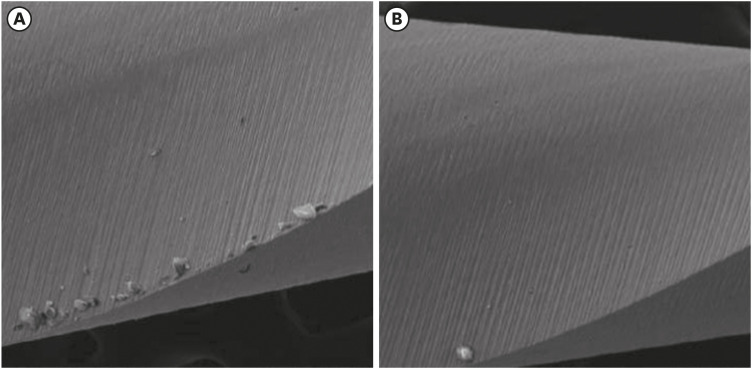
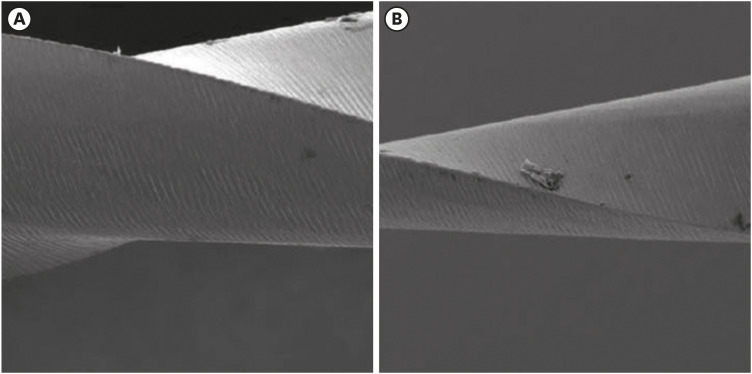
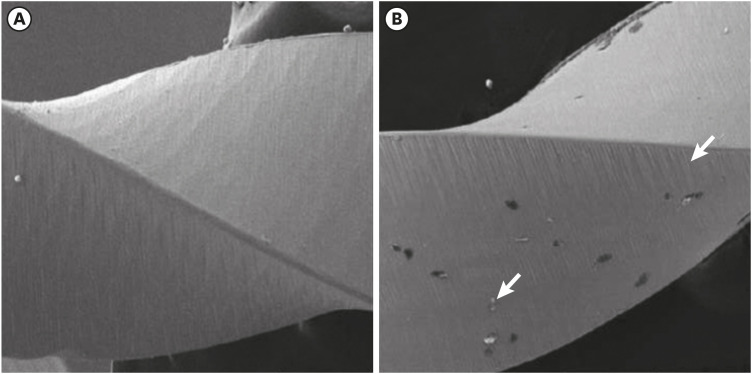
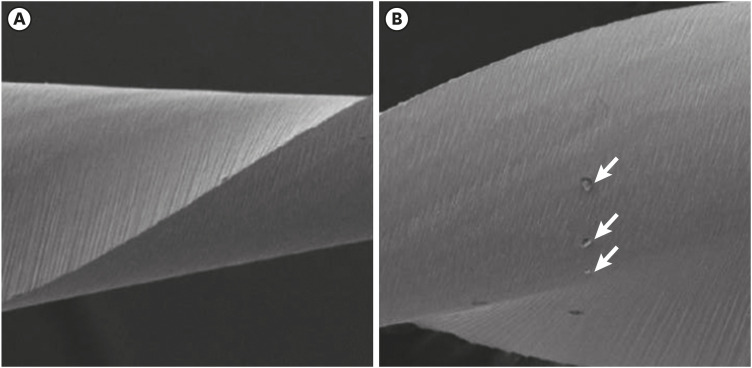
Figure 1
Figure 2
Figure 3
Figure 4
Figure 5
Figure 6
Figure 7
Figure 8
Figure 9
Mean ± standard deviation (SD) of the corrosion potential (Ecorr) and pitting potential (Epit) values of ProTaper, Mtwo, Reciproc, and WaveOne files
| Files | Ecorr* | Epit† | |
|---|---|---|---|
| Heat-treated | |||
| ProTaper | 0.506 ± 0.013 | 0.904 ± 0.010 | |
| Mtwo | 0.348 ± 0.006 | - | |
| Non-heat-treated | |||
| Reciproc | 0.542 ± 0.001 | - | |
| WaveOne | 0.321 ± 0.001 | 0.879 ± 0.005 | |
| p value | < 0.001* | 0.004† | |
Values are presented as mean ± SD. Data were analyzed using analysis of variance.
*Comparison between 4 groups; †Comparison between 2 groups.
A p values for the comparisons of corrosion potential (Ecorr) between ProTaper, Mtwo, Reciproc, and WaveOne files
| Comparisons of Ecorr | ProTaper | Mtwo | Reciproc | WaveOne |
|---|---|---|---|---|
| ProTaper | - | < 0.05 | < 0.05 | < 0.05 |
| Mtwo | < 0.05 | - | < 0.05 | < 0.05 |
| Reciproc | < 0.05 | < 0.05 | - | < 0.05 |
| WaveOne | < 0.05 | < 0.05 | < 0.05 | - |
Data were analyzed using the post hoc Bonferroni test.
Values are presented as mean ± SD. Data were analyzed using analysis of variance.
*Comparison between 4 groups; †Comparison between 2 groups.
Data were analyzed using the

 KACD
KACD
 ePub Link
ePub Link Cite
Cite

