Articles
- Page Path
- HOME > Restor Dent Endod > Volume 48(2); 2023 > Article
- Review Article Stem cell-derived exosomes for dentin-pulp complex regeneration: a mini-review
-
Dina A. Hammouda1
 , Alaa M Mansour1
, Alaa M Mansour1 , Mahmoud A. Saeed2
, Mahmoud A. Saeed2 , Ahmed R. Zaher1
, Ahmed R. Zaher1 , Mohammed E. Grawish1,3
, Mohammed E. Grawish1,3
-
Restor Dent Endod 2023;48(2):e20.
DOI: https://doi.org/10.5395/rde.2023.48.e20
Published online: May 3, 2023
1Department of Oral Biology, Faculty of Dentistry, Mansoura University, Mansoura, Egypt.
2Department of Oral Biology, Faculty of Dentistry, Menoufia University, Shibin el Kom, Egypt.
3Department of Oral Biology, Faculty of Oral and Dental Medicine, Delta University for Science and Technology, Dakahlia, Egypt.
- Correspondence to Mohammed E. Grawish, BDS, MSc, PhD. Department of Oral Biology, Faculty of Dentistry, Mansoura University, Mansoura 35511, Egypt. grawish2005@yahoo.com
Copyright © 2023. The Korean Academy of Conservative Dentistry
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
- This mini-review was conducted to present an overview of the use of exosomes in regenerating the dentin-pulp complex (DPC). The PubMed and Scopus databases were searched for relevant articles published between January 1, 2013 and January 1, 2023. The findings of basic in vitro studies indicated that exosomes enhance the proliferation and migration of mesenchymal cells, as human dental pulp stem cells, via mitogen-activated protein kinases and Wingless-Int signaling pathways. In addition, they possess proangiogenic potential and contribute to neovascularization and capillary tube formation by promoting endothelial cell proliferation and migration of human umbilical vein endothelial cells. Likewise, they regulate the migration and differentiation of Schwann cells, facilitate the conversion of M1 pro-inflammatory macrophages to M2 anti-inflammatory phenotypes, and mediate immune suppression as they promote regulatory T cell conversion. Basic in vivo studies have indicated that exosomes triggered the regeneration of dentin-pulp–like tissue, and exosomes isolated under odontogenic circumstances are particularly strong inducers of tissue regeneration and stem cell differentiation. Exosomes are a promising regenerative tool for DPC in cases of small pulp exposure or for whole-pulp tissue regeneration.
INTRODUCTION
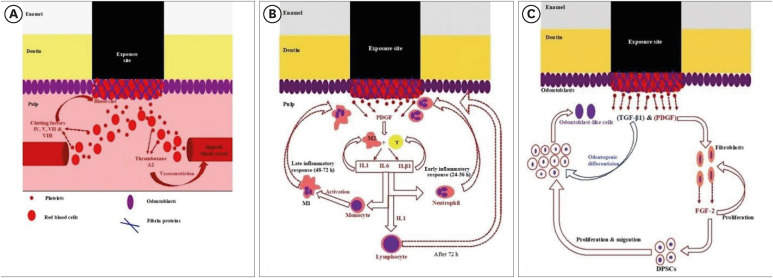
Diagrammatic representation of (A) hemostasis, (B) early/late inflammatory, and (C) regenerative phases of pulp self-healing mechanisms.
MATERIALS AND METHODS
RESULTS
In vitro studies evaluating SC-derived exosomes for dentin-pulp complex regeneration
| Source of exosomes | Study design | Outcomes | Ref. |
|---|---|---|---|
| hDPSCs and odontogenic differentiated hDPSCs | Effect on hDPSCs and hBMSCs | Exosomes triggered the P38 MAPK pathway and increased the expression of genes required for odontogenic differentiation. | Huang et al. (2016) [6] |
| hDPSCs | Effect on hUVECs | HDPSC-exosomes promoted hUVEC proliferation, proangiogenic factor expression, and tube formation; in addition, p38 MAPK signaling inhibition enhanced hDPSC-exosome–induced tube formation. Therefore, hDPSC-exosomes are a promising biomimetic tool to apply in pulp regeneration. | Xian et al. (2018) [30] |
| hDPSCs cultured under growth and odontogenic differentiation conditions | Effect on hDPSCs | Exosomes isolated under odontogenic conditions were better inducers of hDPSC differentiation than those isolated under growth conditions, as exosomal microRNAs promoted odontogenic differentiation via the TGF-β1/Smad signaling pathway by downregulating LTBP1. | Hu et al. (2019) [31] |
| hDPSCs | Through evaluating a fabricated exosome-fibrin gel (an in situ delivery system) composite in monolayers and hydrogels, endothelial cells isolated from hUVECs were co-cultured with hDPSCs in the exosome-fibrin gel. | Exosomes exerted positive effects on the growth of hUVECs in monolayer culture and on 3-dimensional co-cultured hUVECs and hDPSCs in exosome-loaded fibrin gels. Exosomes with fibrin gels facilitated vascular-like structure formation in less than 7 days by increasing the release of VEGF and promoting the deposition of collagen I, III, and IV. | Zhang et al. (2020) [32] |
| hDPSCs | Effect on hBMSCs; in addition, a fibrin gel was assessed as a delivery system for the exosomes. | Exosomes attracted hBMSCs, and the fibrin gel enhanced their effect. Moreover, exosomes improved the proliferation of hBMSCs. | Ivica et al. (2020) [28] |
| hDPSCs and an immortalized murine odontoblast cell line (mDPCs) | Effect on hDPSCs | Exosomes derived from both hDPSCs and mDPCs upregulated bone sialoprotein, dentin sialophosphoprotein, and VEGF odontogenic gene expression and improved mineralization in vitro. | Swanson et al. (2020) [5] |
| Rat HERS cell line from PN8 rat first mandibular molars | Effect on DPSCs isolated from unerupted first molars of 1- to 3-day-old postnatal Sprague-Dawley rats | HERS cell-derived exosomes promoted the migration and proliferation of DPSCs, induced odontogenic differentiation and activation of Wnt/β-catenin signaling, and contributed to tube formation and neural differentiation. | Zhang et al. (2020) [33] |
| hSCAPs | Effect on rat BMSCs | The dentinogenesis capacity of BMSCs was enhanced with increased gene and protein expression of dentin sialoprotein and mineralized nodule formation. | Zhuang et al. (2020) [34] |
| LPS-stimulated hDPSCs | Effect on hUVECs | LPS-exosomes activated the angiogenic potential of hUVECs by promoting proliferation, migration, and tube formation by increasing the expression of VEGF and kinase insert domain-containing receptors. | Huang et al. (2021) [35] |
| hDPSCs cultured with or without LPS | Effect on rat BMSCs | Exosomes derived from hDPSCs cultured with or without LPS modulated BMSC proliferation, migration, angiogenesis, and differentiation. | Chen et al. (2021) [36] |
| hSHED aggregates | Pro-angiogenic effects of SHED aggregate-derived exosomes on SHED and hUVECs | SHED aggregate-derived exosomes promoted SHED endothelial differentiation and enhanced the angiogenic ability of hUVECs by regulating TGF-β/Smad2/3 signaling. | Wu et al. (2021) [37] |
| Supernatant of hDPSCs and LPS-preconditioned hDPSCs | Effect on human Schwann cell line migration and differentiation | Exosomes from hDPSCs, especially from LPS-preconditioned hDPSCs, can promote the proliferation, migration, and odontogenic differentiation of Schwann cells. | Li et al. (2021) [38] |
| hUCMSCs and hDPSCs | Effect on LPS-induced inflammation of hDPSCs | Exosomes ameliorate LPS-induced inflammation by decreasing pro-inflammatory cytokines and increasing anti-inflammatory cytokines with better results relative to hUCMSC-exosomes. | Zeng et al. (2022) [39] |
| hSCAP | Effect on Tregs of C57BL/6 female mice | SCAP-exosomes promoted Treg conversion. | Yu et al. (2022) [40] |
In vivo studies evaluating SC-derived exosomes for dentin-pulp complex regeneration
| Source of exosomes | Study design | Outcomes | Ref. |
|---|---|---|---|
| hDPSCs and odontogenic differentiated hDPSCs | The tooth root slice model was filled with primary hDPSCs embedded within either control or exosome-incorporated collagen membranes and implanted subcutaneously on the backs of nude mice for 2 wk. | Exosomes triggered the regeneration of dentin-pulp–like tissue, and exosomes isolated under odontogenic conditions are better inducers of SC differentiation and tissue regeneration than those isolated under growth conditions. | Huang et al. (2016) [6] |
| hDPSCs and an immortalized murine odontoblast cell line (mDPCs) | Subcutaneous implantation was conducted of exosomes containing microspheres with hDPSCs or immortalized murine odontoblast cells and attached to nanofibrous tissue engineering scaffolds. | Exosomes containing microspheres induce hDPSC differentiation in vivo with no inflammation and minimal fibrous capsule formation. | Swanson et al. (2020) [5] |
| A rat molar pulp-capping model using an amphiphilic synthetic polymeric vehicle synthesized from PLGA-PEG-PLGA triblock copolymers encapsulated exosomes containing microspheres to maintain their biologic integrity throughout release up to 8–12 wk. | The controlled release of odontogenic exosomes accelerated tertiary dentin bridge formation without signs of bacterial infection, with results superior to glass-ionomer cement alone after 6 wk. | ||
| Rat HERS cell line from PN8 rat first mandibular molars | DPSCs isolated from unerupted first molars of 1- to 3-day-old postnatal Sprague-Dawley rats were mixed with collagen gel combined with or without exosome-like vesicles and transplanted into the renal capsules of rats or subcutaneously into nude mice. | HERS cell-derived exosomes with DPCs triggered the regeneration of dentin-pulp–like tissue comprised of reparative dentin-like tissue and blood vessels and neurons in soft tissue. | Zhang et al. (2020) [33] |
| hSCAPs | Subcutaneous implantation of root fragment containing rat BMSC- and hSCAP-exosomes in immunodeficient mice. | Dentin-pulp–like tissues with newly formed dentin were observed in the SCAP-exosome group. Odontoblasts were polarized, columnar, and in an ordered arrangement at the junction of pulp and predentin, and their processes extended into the dentinal tubules. | Zhuang et al. (2020) [34] |
| hDPSCs cultured with or without LPS | Exosomes derived from hDPSCs cultured with or without LPS were implanted into a rat pulpless root canal containing PuraMarix peptide hydrogel and BMSCs. | Exosomes derived from hDPSCs cultured with or without LPS enhanced the structure of the regenerated tissue closer to that of normal dental pulp with greater efficiency than exosomes derived from hDPSCs cultured with LPS. | Chen et al. (2021) [36] |
| hSHED aggregates | Tooth fragments containing SHED cell aggregates with or without GW4869 (a sphingomyelinase inhibitor used for blocking exosome generation)/SHED aggregate-derived exosomes were subcutaneously transplanted into the backs of mice for 12 wk. | SHED aggregate-derived exosomes considerably improved angiogenesis and pulp tissue regeneration in vivo. | Wu et al. (2021) [37] |
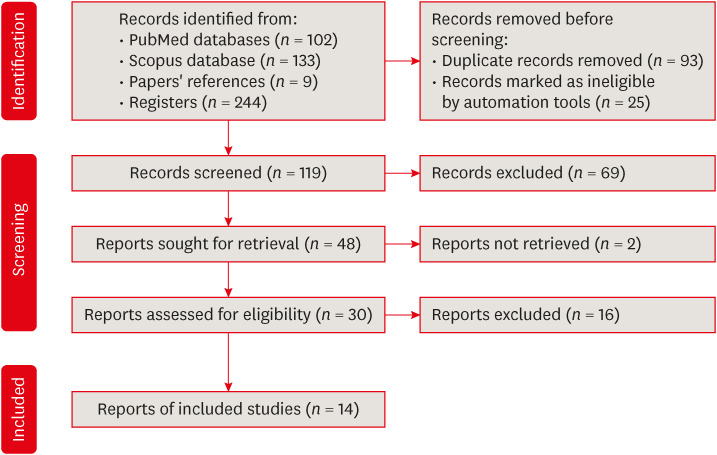
Preferred reporting items for systematic reviews and meta-analyses flow diagram for article selection.
CONCLUDING REMARKS
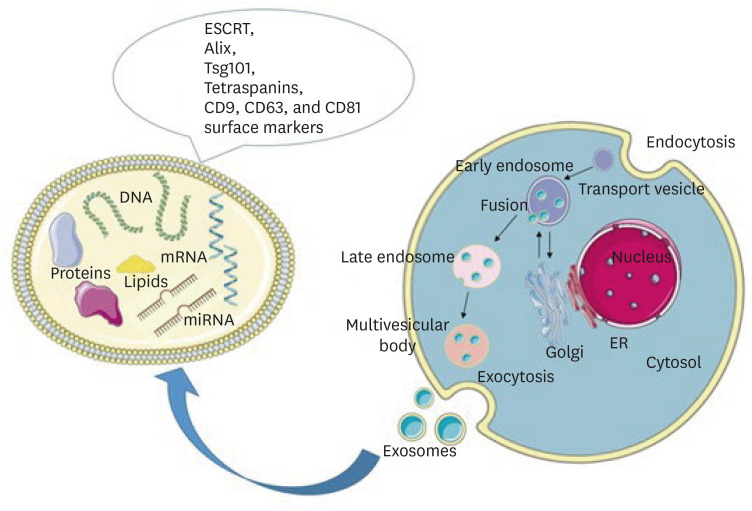
Schematic representation of exosome biogenesis and molecular cargo. Exosomes are formed through the inward budding of the endosomal membrane resulting in the formation of early endosomes, late endosomes, and multivesicular bodies (MVBs). Upon fusion of MVBs with the plasma membrane, exosomes are released into the extracellular space. The molecular cargo of exosomes consists of lipids, DNA, mRNA, microRNA, and proteins. On their surface, they carry the RNA ESCRT, Alix, Tsg101, and tetraspanins CD9, CD63, and CD81 markers. Parts of the figure were drawn using pictures from Servier Medical Art. Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/).
THERAPEUTIC EFFECTS OF STEM CELL-DERIVED EXOSOMES IN DPC REGENERATION
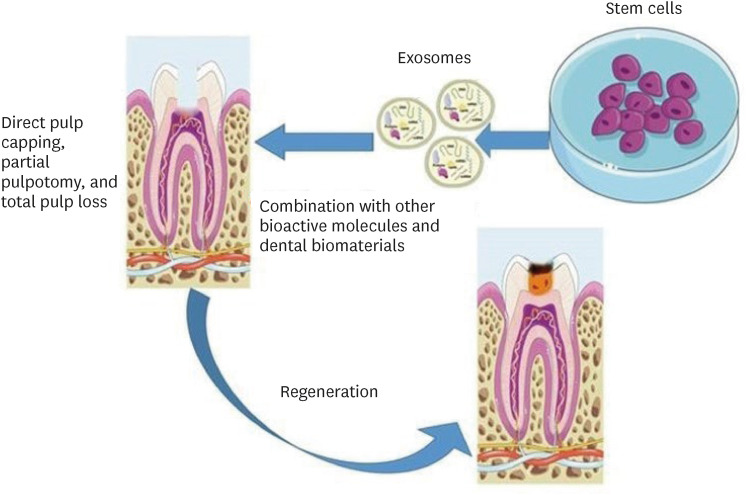
Schematic representation of the suggested methods for the use of exosomes in dentin-pulp complex (DPC) regeneration. Parts of the figure were drawn using pictures from Servier Medical Art. Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/).
CONCLUDING REMARKS
-
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
-
Author Contributions:
Conceptualization: Grawish ME.
Data curation: Mansour AM, Grawish ME.
Formal analysis: Zaher AR.
Methodology: Saeed MA, Hammouda DA.
Supervision: Zaher AR, Grawish ME, Mansour AM.
Validation: Zaher AR.
Writing - original draft: Grawish ME, Mansour AM, Hammouda DA.
Writing - review & editing: Zaher AR, Grawish ME.
- 1. Yu C, Abbott PV. An overview of the dental pulp: its functions and responses to injury. Aust Dent J 2007;52(Supplement):S4-SS16.Article
- 2. Goldberg M, Kulkarni AB, Young M, Boskey A. Dentin: structure, composition and mineralization. Front Biosci (Elite Ed) 2011;3:711-735.ArticlePubMedPMC
- 3. Cordeiro MM, Dong Z, Kaneko T, Zhang Z, Miyazawa M, Shi S, Smith AJ, Nör JE. Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J Endod 2008;34:962-969.ArticlePubMed
- 4. Duncan HF, Yamauchi Y. Pulp exposure management. Clin Dent Rev 2019;3:4.ArticlePDF
- 5. Swanson WB, Gong T, Zhang Z, Eberle M, Niemann D, Dong R, Rambhia KJ, Ma PX. Controlled release of odontogenic exosomes from a biodegradable vehicle mediates dentinogenesis as a novel biomimetic pulp capping therapy. J Control Release 2020;324:679-694.ArticlePubMedPMC
- 6. Huang CC, Narayanan R, Alapati S, Ravindran S. Exosomes as biomimetic tools for stem cell differentiation: applications in dental pulp tissue regeneration. Biomaterials 2016;111:103-115.ArticlePubMedPMC
- 7. Li Z, Liu L, Wang L, Song D. The effects and potential applications of concentrated growth factor in dentin-pulp complex regeneration. Stem Cell Res Ther 2021;12:357.ArticlePubMedPMCPDF
- 8. Simon SR, Berdal A, Cooper PR, Lumley PJ, Tomson PL, Smith AJ. Dentin-pulp complex regeneration: from lab to clinic. Adv Dent Res 2011;23:340-345.ArticlePubMedPDF
- 9. Asgary S, Parirokh M, Eghbal MJ, Ghoddusi J. SEM evaluation of pulp reaction to different pulp capping materials in dog’s teeth. Iran Endod J 2006;1:117-123.PubMed
- 10. Aljandan B, AlHassan H, Saghah A, Rasheed M, Ali AA. The effectiveness of using different pulp-capping agents on the healing response of the pulp. Indian J Dent Res 2012;23:633-637.ArticlePubMed
- 11. Boland GM, Perkins G, Hall DJ, Tuan RS. Wnt 3a promotes proliferation and suppresses osteogenic differentiation of adult human mesenchymal stem cells. J Cell Biochem 2004;93:1210-1230.ArticlePubMed
- 12. Giuroiu CL, Căruntu ID, Lozneanu L, Melian A, Vataman M, Andrian S. Dental pulp: correspondences and contradictions between clinical and histological diagnosis. BioMed Res Int 2015;2015:960321.ArticlePubMedPMCPDF
- 13. Imura N, Pinheiro ET, Gomes BP, Zaia AA, Ferraz CC, Souza-Filho FJ. The outcome of endodontic treatment: a retrospective study of 2000 cases performed by a specialist. J Endod 2007;33:1278-1282.ArticlePubMed
- 14. Cvek M. Prognosis of luxated non-vital maxillary incisors treated with calcium hydroxide and filled with gutta-percha. A retrospective clinical study. Endod Dent Traumatol 1992;8:45-55.ArticlePubMed
- 15. Kim JY, Xin X, Moioli EK, Chung J, Lee CH, Chen M, Fu SY, Koch PD, Mao JJ. Regeneration of dental-pulp-like tissue by chemotaxis-induced cell homing. Tissue Eng Part A 2010;16:3023-3031.ArticlePubMedPMC
- 16. Garcia-Godoy F, Murray PE. Recommendations for using regenerative endodontic procedures in permanent immature traumatized teeth. Dent Traumatol 2012;28:33-41.ArticlePubMed
- 17. Palma PJ, Ramos JC, Martins JB, Diogenes A, Figueiredo MH, Ferreira P, Viegas C, Santos JM. Histologic evaluation of regenerative endodontic procedures with the use of chitosan scaffolds in immature dog teeth with apical periodontitis. J Endod 2017;43:1279-1287.ArticlePubMed
- 18. Palma PJ, Martins J, Diogo P, Sequeira D, Ramos JC, Diogenes A, Sequeira D, Ramos JC, Diogenes A, Santos JM. Does apical papilla survive and develop in apical periodontitis presence after regenerative endodontic procedures? Appl Sci 2019;9:3942-3955.Article
- 19. Chrepa V, Pitcher B, Henry MA, Diogenes A. Survival of the apical papilla and its resident stem cells in a case of advanced pulpal necrosis and apical periodontitis. J Endod 2017;43:561-567.ArticlePubMed
- 20. Smith AJ, Cassidy N, Perry H, Bègue-Kirn C, Ruch JV, Lesot H. Reactionary dentinogenesis. Int J Dev Biol 1995;39:273-280.PubMed
- 21. Simon S, Smith AJ, Berdal A, Lumley PJ, Cooper PR. The MAP kinase pathway is involved in odontoblast stimulation via p38 phosphorylation. J Endod 2010;36:256-259.ArticlePubMed
- 22. Bai Y, Cheng X, Liu X, Guo Q, Wang Z, Fu Y, He W, Yu Q. Transforming growth factor-β1 promotes early odontoblastic differentiation of dental pulp stem cells via activating AKT, Erk1/2 and p38 MAPK pathways. J Dent Sci 2023;18:87-94.ArticlePubMed
- 23. Guo W, Fan Z, Wang S, Du J. ALK5 is essential for tooth germ differentiation during tooth development. Biotech Histochem 2019;94:481-490.ArticlePubMed
- 24. Nakashima M, Iohara K. Mobilized dental pulp stem cells for pulp regeneration: initiation of clinical trial. J Endod 2014;40(Supplement):S26-S32.ArticlePubMed
- 25. Nakashima M, Iohara K, Murakami M, Nakamura H, Sato Y, Ariji Y, Matsushita K. Pulp regeneration by transplantation of dental pulp stem cells in pulpitis: a pilot clinical study. Stem Cell Res Ther 2017;8:61.ArticlePubMedPMCPDF
- 26. Mitsiadis TA, Orsini G, Jimenez-Rojo L. Stem cell-based approaches in dentistry. Eur Cell Mater 2015;30:248-257.ArticlePubMed
- 27. Nassar W, El-Ansary M, Sabry D, Mostafa MA, Fayad T, Kotb E, Temraz M, Saad AN, Essa W, Adel H. Umbilical cord mesenchymal stem cells derived extracellular vesicles can safely ameliorate the progression of chronic kidney diseases. Biomater Res 2016;20:21.ArticlePubMedPMC
- 28. Ivica A, Ghayor C, Zehnder M, Valdec S, Weber FE. Pulp-derived exosomes in a fibrin-based regenerative root filling material. J Clin Med 2020;9:491.ArticlePubMedPMC
- 29. Cooper LF, Ravindran S, Huang CC, Kang M. A role for exosomes in craniofacial tissue engineering and regeneration. Front Physiol 2020;10:1569.ArticlePubMedPMC
- 30. Xian X, Gong Q, Li C, Guo B, Jiang H. Exosomes with highly angiogenic potential for possible use in pulp regeneration. J Endod 2018;44:751-758.ArticlePubMed
- 31. Hu X, Zhong Y, Kong Y, Chen Y, Feng J, Zheng J. Lineage-specific exosomes promote the odontogenic differentiation of human dental pulp stem cells (DPSCs) through TGFβ1/smads signaling pathway via transfer of microRNAs. Stem Cell Res Ther 2019;10:170.ArticlePubMedPMCPDF
- 32. Zhang S, Thiebes AL, Kreimendahl F, Ruetten S, Buhl EM, Wolf M, Jockenhoevel S, Apel C. Extracellular vesicles-loaded fibrin gel supports rapid neovascularization for dental pulp regeneration. Int J Mol Sci 2020;21:4226.ArticlePubMedPMC
- 33. Zhang S, Yang Y, Jia S, Chen H, Duan Y, Li X, Wang S, Wang T, Lyu Y, Chen G, Tian W. Exosome-like vesicles derived from Hertwig’s epithelial root sheath cells promote the regeneration of dentin-pulp tissue. Theranostics 2020;10:5914-5931.ArticlePubMedPMC
- 34. Zhuang X, Ji L, Jiang H, Liu Y, Liu X, Bi J, Zhao W, Ding Z, Chen X. Exosomes derived from stem cells from the apical papilla promote dentine-pulp complex regeneration by inducing specific dentinogenesis. Stem Cells Int 2020;2020:5816723.ArticlePubMedPMCPDF
- 35. Huang X, Qiu W, Pan Y, Li J, Chen Z, Zhang K, Luo Y, Wu B, Xu W. Exosomes from LPS-stimulated hDPSCs activated the angiogenic potential of HUVECs in vitro. Stem Cells Int 2021;2021:6685307.PubMedPMC
- 36. Chen WJ, Xie J, Lin X, Ou MH, Zhou J, Wei XL, Chen WX. The role of small extracellular vesicles derived from lipopolysaccharide-preconditioned human dental pulp stem cells in dental pulp regeneration. J Endod 2021;47:961-969.ArticlePubMed
- 37. Wu M, Liu X, Li Z, Huang X, Guo H, Guo X, Yang X, Li B, Xuan K, Jin Y. SHED aggregate exosomes shuttled miR-26a promote angiogenesis in pulp regeneration via TGF-β/SMAD2/3 signalling. Cell Prolif 2021;54:e13074.ArticlePubMedPMCPDF
- 38. Li J, Ju Y, Liu S, Fu Y, Zhao S. Exosomes derived from lipopolysaccharide-preconditioned human dental pulp stem cells regulate Schwann cell migration and differentiation. Connect Tissue Res 2021;62:277-286.ArticlePubMed
- 39. Zeng J, He K, Mai R, Lin T, Wei R, Nong J, Wu Y. Exosomes from human umbilical cord mesenchymal stem cells and human dental pulp stem cells ameliorate lipopolysaccharide-induced inflammation in human dental pulp stem cells. Arch Oral Biol 2022;138:105411.ArticlePubMed
- 40. Yu S, Chen X, Liu Y, Zhuang XY, Wang AC, Liu XM, Zhu S. Exosomes derived from stem cells from the apical papilla alleviate inflammation in rat pulpitis by upregulating regulatory T cells. Int Endod J 2022;55:517-530.ArticlePubMedPDF
- 41. Stanko P, Altanerova U, Jakubechova J, Repiska V, Altaner C. Dental mesenchymal stem/stromal cells and their exosomes. Stem Cells Int 2018;2018:8973613.ArticlePubMedPMCPDF
- 42. Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol 2002;2:569-579.ArticlePubMedPDF
- 43. Février B, Raposo G. Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr Opin Cell Biol 2004;16:415-421.ArticlePubMed
- 44. Qian X, An N, Ren Y, Yang C, Zhang X, Li L. Immunosuppressive effects of mesenchymal stem cells-derived exosomes. Stem Cell Rev Rep 2021;17:411-427.ArticlePubMedPDF
- 45. Zarà M, Amadio P, Campodonico J, Sandrini L, Barbieri SS. Exosomes in cardiovascular diseases. Diagnostics (Basel) 2020;10:943.ArticlePubMedPMC
- 46. Guo SC, Tao SC, Yin WJ, Qi X, Yuan T, Zhang CQ. Exosomes derived from platelet-rich plasma promote the re-epithelization of chronic cutaneous wounds via activation of YAP in a diabetic rat model. Theranostics 2017;7:81-96.ArticlePubMedPMC
- 47. Brunello G, Zanotti F, Trentini M, Zanolla I, Pishavar E, Favero V, Favero R, Favero L, Bressan E, Bonora M, Sivolella S, Zavan B. Exosomes derived from dental pulp stem cells show different angiogenic and osteogenic properties in relation to the age of the donor. Pharmaceutics 2022;14:908.ArticlePubMedPMC
REFERENCES
Tables & Figures
REFERENCES
Citations

- Extracellular vesicles derived from dental mesenchymal stem cells for regenerative medicine: a scoping review
Maria Emília Mota, Márcia Martins Marques, Thaís Gimenez, Suely Kunimi Kubo Ariga, Tiago Góss dos Santos, Fábio Abreu Alves, Maria Stella Moreira
Molecular Biology Reports.2026;[Epub] CrossRef - Cell Homing Strategies in Regenerative Endodontic Therapy
David Kim, Sahng G. Kim
Cells.2025; 14(3): 201. CrossRef - Impact of dental pulp cells-derived small extracellular vesicles on the properties and behavior of dental pulp cells: an in-vitro study
Dina A. Hammouda, Alaa M. Mansour, Ahmed R. Zaher, Mohammed E. Grawish
BMC Oral Health.2025;[Epub] CrossRef - Methodological Approaches for Economic Comparison of Mesenchymal Stem Cell and Exosome-based Therapies with Conventional Endodontic Treatments in Regenerative Endodontics
Madina A. Kurmanalina Kurmanalina, Nadiar M. Mussin, Aigul M. Sumanova, Violetta R. Detochkina, Maryam Mardani, Nader Tanideh, Amin Tamadon
West Kazakhstan Medical Journal.2025; 67(2): 188. CrossRef - Exosomal circ_0003057 promotes osteo/odontogenic differentiation of hDPSCs by binding with EIF4A3 through upregulated parental gene ANKH
Bingtao Wang, Yuanyuan Kong, Huixian Dong, Feng Lai, Zixin Guo, Liecong Lin, Jingyi Xu, Jingkun Zhang, Yiguo Jiang, Qianzhou Jiang
International Endodontic Journal.2025; 58(9): 1433. CrossRef - Mechanistic insights into dental stem cells‐derived exosomes in regenerative endodontics
Paras Ahmad, Nathan Estrin, Nima Farshidfar, Yufeng Zhang, Richard J. Miron
International Endodontic Journal.2025; 58(9): 1384. CrossRef - Development and characterization of an exosome-loaded biomimetic hydroxyapatite/gelatin scaffold for enhanced dental pulp regeneration
Yuen-Shan Tsai, Shih-Jung Cheng, Tsao-Li Chuang, Shu-Fang Chang, Feng-Huei Lin, Chun-Pin Lin
Journal of Dental Sciences.2025;[Epub] CrossRef - Exosomes as Promising Therapeutic Tools for Regenerative Endodontic Therapy
Qingyue Kong, Yujie Wang, Nan Jiang, Yifan Wang, Rui Wang, Xiaohan Hu, Jing Mao, Xin Shi
Biomolecules.2024; 14(3): 330. CrossRef - Role and Molecular Mechanism of miR-586 in the Differentiation of Dental Pulp Stem Cells into Odontoblast-like Cells
Gang Pan, Qianwen Zhou, Chenhua Pan, Yingxue Zhang
Cell Biochemistry and Biophysics.2024; 83(1): 507. CrossRef
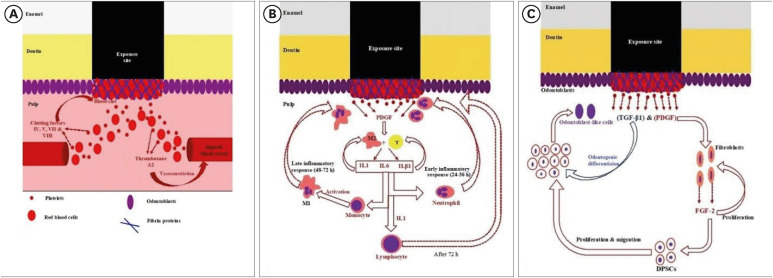
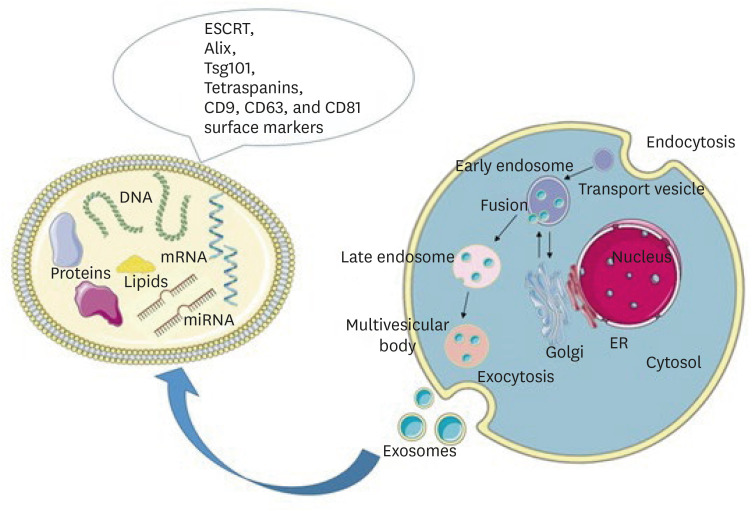
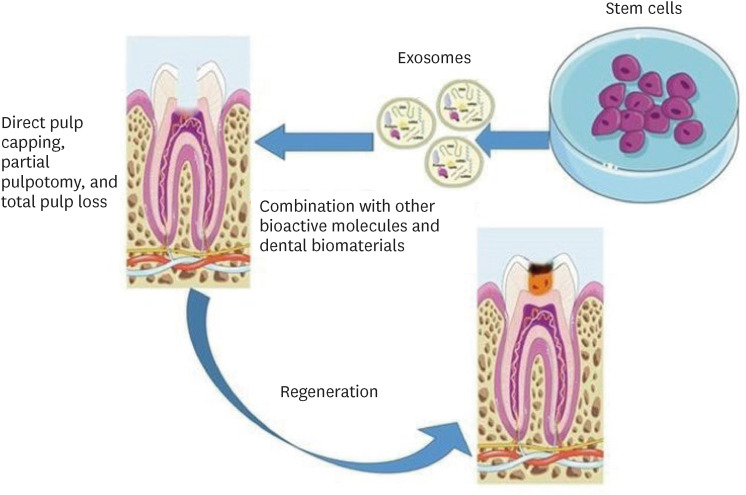
Figure 1
Figure 2
Figure 3
Figure 4
In vitro studies evaluating SC-derived exosomes for dentin-pulp complex regeneration
| Source of exosomes | Study design | Outcomes | Ref. |
|---|---|---|---|
| hDPSCs and odontogenic differentiated hDPSCs | Effect on hDPSCs and hBMSCs | Exosomes triggered the P38 MAPK pathway and increased the expression of genes required for odontogenic differentiation. | Huang |
| hDPSCs | Effect on hUVECs | HDPSC-exosomes promoted hUVEC proliferation, proangiogenic factor expression, and tube formation; in addition, p38 MAPK signaling inhibition enhanced hDPSC-exosome–induced tube formation. Therefore, hDPSC-exosomes are a promising biomimetic tool to apply in pulp regeneration. | Xian |
| hDPSCs cultured under growth and odontogenic differentiation conditions | Effect on hDPSCs | Exosomes isolated under odontogenic conditions were better inducers of hDPSC differentiation than those isolated under growth conditions, as exosomal microRNAs promoted odontogenic differentiation via the TGF-β1/Smad signaling pathway by downregulating LTBP1. | Hu |
| hDPSCs | Through evaluating a fabricated exosome-fibrin gel (an | Exosomes exerted positive effects on the growth of hUVECs in monolayer culture and on 3-dimensional co-cultured hUVECs and hDPSCs in exosome-loaded fibrin gels. Exosomes with fibrin gels facilitated vascular-like structure formation in less than 7 days by increasing the release of VEGF and promoting the deposition of collagen I, III, and IV. | Zhang |
| hDPSCs | Effect on hBMSCs; in addition, a fibrin gel was assessed as a delivery system for the exosomes. | Exosomes attracted hBMSCs, and the fibrin gel enhanced their effect. Moreover, exosomes improved the proliferation of hBMSCs. | Ivica |
| hDPSCs and an immortalized murine odontoblast cell line (mDPCs) | Effect on hDPSCs | Exosomes derived from both hDPSCs and mDPCs upregulated bone sialoprotein, dentin sialophosphoprotein, and VEGF odontogenic gene expression and improved mineralization | Swanson |
| Rat HERS cell line from PN8 rat first mandibular molars | Effect on DPSCs isolated from unerupted first molars of 1- to 3-day-old postnatal Sprague-Dawley rats | HERS cell-derived exosomes promoted the migration and proliferation of DPSCs, induced odontogenic differentiation and activation of Wnt/β-catenin signaling, and contributed to tube formation and neural differentiation. | Zhang |
| hSCAPs | Effect on rat BMSCs | The dentinogenesis capacity of BMSCs was enhanced with increased gene and protein expression of dentin sialoprotein and mineralized nodule formation. | Zhuang |
| LPS-stimulated hDPSCs | Effect on hUVECs | LPS-exosomes activated the angiogenic potential of hUVECs by promoting proliferation, migration, and tube formation by increasing the expression of VEGF and kinase insert domain-containing receptors. | Huang |
| hDPSCs cultured with or without LPS | Effect on rat BMSCs | Exosomes derived from hDPSCs cultured with or without LPS modulated BMSC proliferation, migration, angiogenesis, and differentiation. | Chen |
| hSHED aggregates | Pro-angiogenic effects of SHED aggregate-derived exosomes on SHED and hUVECs | SHED aggregate-derived exosomes promoted SHED endothelial differentiation and enhanced the angiogenic ability of hUVECs by regulating TGF-β/Smad2/3 signaling. | Wu |
| Supernatant of hDPSCs and LPS-preconditioned hDPSCs | Effect on human Schwann cell line migration and differentiation | Exosomes from hDPSCs, especially from LPS-preconditioned hDPSCs, can promote the proliferation, migration, and odontogenic differentiation of Schwann cells. | Li |
| hUCMSCs and hDPSCs | Effect on LPS-induced inflammation of hDPSCs | Exosomes ameliorate LPS-induced inflammation by decreasing pro-inflammatory cytokines and increasing anti-inflammatory cytokines with better results relative to hUCMSC-exosomes. | Zeng |
| hSCAP | Effect on Tregs of C57BL/6 female mice | SCAP-exosomes promoted Treg conversion. | Yu |
SC, stem cell; Ref., reference; hDPSC, human dental pulp stem cell; hBMSC, human bone marrow mesenchymal stem cell; MAPK, mitogen-activated protein kinase; hUVEC, human umbilical vein endothelial cell; TGF-β, transforming growth factor beta; LTBP1, latent transforming growth factor beta binding protein 1; VEGF, vascular endothelial growth factor; HERS, rat epithelial root sheath of Hertwig; Wnt, Wingless-Int; hSCAP, human stem cell from the apical papilla; LPS, lipopolysaccharide; hSHED, human exfoliated deciduous tooth; hUCMSC, human umbilical cord mesenchymal stem cell; Treg, regulatory T cell.
In vivo studies evaluating SC-derived exosomes for dentin-pulp complex regeneration
| Source of exosomes | Study design | Outcomes | Ref. |
|---|---|---|---|
| hDPSCs and odontogenic differentiated hDPSCs | The tooth root slice model was filled with primary hDPSCs embedded within either control or exosome-incorporated collagen membranes and implanted subcutaneously on the backs of nude mice for 2 wk. | Exosomes triggered the regeneration of dentin-pulp–like tissue, and exosomes isolated under odontogenic conditions are better inducers of SC differentiation and tissue regeneration than those isolated under growth conditions. | Huang |
| hDPSCs and an immortalized murine odontoblast cell line (mDPCs) | Subcutaneous implantation was conducted of exosomes containing microspheres with hDPSCs or immortalized murine odontoblast cells and attached to nanofibrous tissue engineering scaffolds. | Exosomes containing microspheres induce hDPSC differentiation | Swanson |
| A rat molar pulp-capping model using an amphiphilic synthetic polymeric vehicle synthesized from PLGA-PEG-PLGA triblock copolymers encapsulated exosomes containing microspheres to maintain their biologic integrity throughout release up to 8–12 wk. | The controlled release of odontogenic exosomes accelerated tertiary dentin bridge formation without signs of bacterial infection, with results superior to glass-ionomer cement alone after 6 wk. | ||
| Rat HERS cell line from PN8 rat first mandibular molars | DPSCs isolated from unerupted first molars of 1- to 3-day-old postnatal Sprague-Dawley rats were mixed with collagen gel combined with or without exosome-like vesicles and transplanted into the renal capsules of rats or subcutaneously into nude mice. | HERS cell-derived exosomes with DPCs triggered the regeneration of dentin-pulp–like tissue comprised of reparative dentin-like tissue and blood vessels and neurons in soft tissue. | Zhang |
| hSCAPs | Subcutaneous implantation of root fragment containing rat BMSC- and hSCAP-exosomes in immunodeficient mice. | Dentin-pulp–like tissues with newly formed dentin were observed in the SCAP-exosome group. Odontoblasts were polarized, columnar, and in an ordered arrangement at the junction of pulp and predentin, and their processes extended into the dentinal tubules. | Zhuang |
| hDPSCs cultured with or without LPS | Exosomes derived from hDPSCs cultured with or without LPS were implanted into a rat pulpless root canal containing PuraMarix peptide hydrogel and BMSCs. | Exosomes derived from hDPSCs cultured with or without LPS enhanced the structure of the regenerated tissue closer to that of normal dental pulp with greater efficiency than exosomes derived from hDPSCs cultured with LPS. | Chen |
| hSHED aggregates | Tooth fragments containing SHED cell aggregates with or without GW4869 (a sphingomyelinase inhibitor used for blocking exosome generation)/SHED aggregate-derived exosomes were subcutaneously transplanted into the backs of mice for 12 wk. | SHED aggregate-derived exosomes considerably improved angiogenesis and pulp tissue regeneration | Wu |
SC, stem cell; Ref., reference; hDPSC, human dental pulp stem cell; PLGA, poly (lactic-co-glycolic acid); PEG, polyethylene glycol; HERS, rat epithelial root sheath of Hertwig; hSCAP, human stem cell from the apical papilla; BMSC, bone marrow mesenchymal stem cell; LPS, lipopolysaccharide; SHED, exfoliated deciduous tooth.
SC, stem cell; Ref., reference; hDPSC, human dental pulp stem cell; hBMSC, human bone marrow mesenchymal stem cell; MAPK, mitogen-activated protein kinase; hUVEC, human umbilical vein endothelial cell; TGF-β, transforming growth factor beta; LTBP1, latent transforming growth factor beta binding protein 1; VEGF, vascular endothelial growth factor; HERS, rat epithelial root sheath of Hertwig; Wnt, Wingless-Int; hSCAP, human stem cell from the apical papilla; LPS, lipopolysaccharide; hSHED, human exfoliated deciduous tooth; hUCMSC, human umbilical cord mesenchymal stem cell; Treg, regulatory T cell.
SC, stem cell; Ref., reference; hDPSC, human dental pulp stem cell; PLGA, poly (lactic-co-glycolic acid); PEG, polyethylene glycol; HERS, rat epithelial root sheath of Hertwig; hSCAP, human stem cell from the apical papilla; BMSC, bone marrow mesenchymal stem cell; LPS, lipopolysaccharide; SHED, exfoliated deciduous tooth.

 KACD
KACD
 ePub Link
ePub Link Cite
Cite

