Articles
- Page Path
- HOME > Restor Dent Endod > Volume 46(1); 2021 > Article
- Research Article Biomineralization of three calcium silicate-based cements after implantation in rat subcutaneous tissue
-
Ranjdar Mahmood Talabani1
 , Balkees Taha Garib2
, Balkees Taha Garib2 , Reza Masaeli3
, Reza Masaeli3 , Kavosh Zandsalimi4
, Kavosh Zandsalimi4 , Farinaz Ketabat5
, Farinaz Ketabat5
-
Restor Dent Endod 2020;46(1):e1.
DOI: https://doi.org/10.5395/rde.2021.46.e1
Published online: December 2, 2020
1Department of Conservative Dentistry, University of Sulaimani, Sulaimani, Iraq.
2Department of Oral Diagnosis, University of Sulaimani, Sulaimani, Iraq.
3Department of Dental Biomaterial, Tehran University of Medical Sciences, Tehran, Iran.
4Department of Life Sciences Engineering, Faculty of New Sciences and Technologies, University of Tehran, Tehran, Iran.
5Division of Biomedical Engineering, University of Saskatchewan, Saskatoon, Canada.
- Correspondence to Ranjdar Mahmood Talabani, BDS, MSc. Lecturer, Department of Conservative Dentistry, University of Sulaimani, Sulaymaniyah - Dream Land B-25, Sulaymaniyah 46001, Kurdistan Region, Iraq. ranjdar.osman@univsul.edu.iq
Copyright © 2021. The Korean Academy of Conservative Dentistry
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Objectives The aim of this study was to evaluate the dystrophic mineralization deposits from 3 calcium silicate-based cements (Micro-Mega mineral trioxide aggregate [MM-MTA], Biodentine [BD], and EndoSequence Root Repair Material [ESRRM] putty) over time after subcutaneous implantation into rats.
-
Materials and Methods Forty-five silicon tubes containing the tested materials and 15 empty tubes (serving as a control group) were subcutaneously implanted into the backs of 15 Wistar rats. At 1, 4, and 8 weeks after implantation, the animals were euthanized (n = 5 animals/group), and the silicon tubes were removed with the surrounding tissues. Histopathological tissue sections were stained with von Kossa stain to assess mineralization. Scanning electron microscopy and energy-dispersive X-ray spectroscopy (SEM/EDX) were also used to assess the chemical components of the surface precipitates deposited on the implant and the pattern of calcium and phosphorus distribution at the material-tissue interface. The calcium-to-phosphorus ratios were compared using the non-parametric Kruskal-Wallis test at a significance level of 5%.
-
Results The von Kossa staining showed that both BD and ESRRM putty induced mineralization starting at week 1; this mineralization increased further until the end of the study. In contrast, MM-MTA induced dystrophic calcification later, from 4 weeks onward. SEM/EDX showed no statistically significant differences in the calcium- and phosphorus-rich areas among the 3 materials at any time point (p > 0.05).
-
Conclusions After subcutaneous implantation, biomineralization of the 3-calcium silicate-based cements started early and increased over time, and all 3 tested cements generated calcium- and phosphorus-containing surface precipitates.
INTRODUCTION
MATERIALS AND METHODS
Tested cements
RESULTS
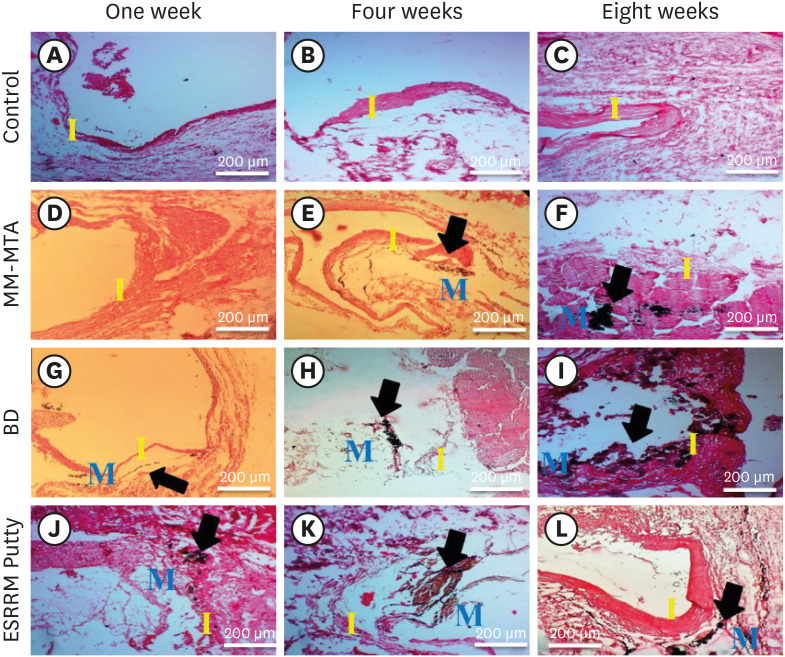
Histologic tissue sections demonstrating the biomineralization (calcium deposition) in the experimental groups at the open ends of the subcutaneously-implanted silicon tubes at the 3 time points (weeks 1, 4, and 8). (A-C) Empty tubes: no dystrophic calcification was seen at any time point (×10). (D) MM-MTA containing tubes: no calcification were seen at week 1 (×10). (E and F) Black dystrophic calcifications were seen at weeks 4 and 8 (arrows, ×10). (G) BD-containing tubes: discrete deposits were seen at week 1 (arrow, ×10), and (H and I) more obvious dystrophic calcifications were seen at weeks 4 and 8 (arrows, ×10). (J-L) ESRRM putty-containing tubes: noticeable black dystrophic calcifications were obvious at all periods (arrows, ×10) (von Kossa stain, scale bar = 200 μm).
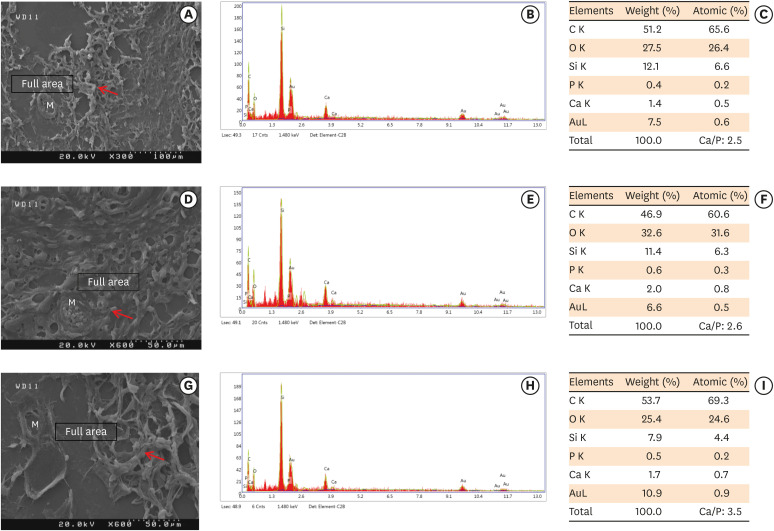
Scanning electron microscopy observations and elemental composition obtained using energy-dispersive X-ray analysis of the surface precipitates produced by (A-C) MM-MTA, (D-F) BD, and (G-I) ESRRM putty at 7 days after the subcutaneous implantation of the materials. Mineralization is indicated by the red arrows.
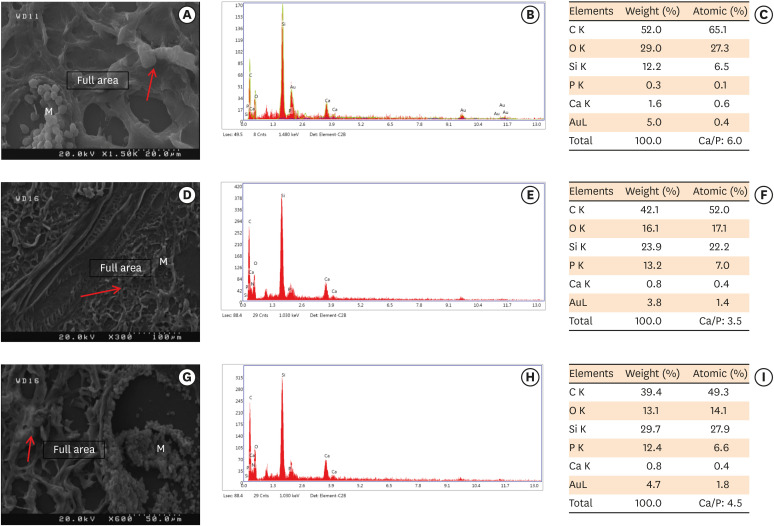
Scanning electron microscopy observations and elemental composition obtained using energy-dispersive X-ray analysis of the surface precipitates produced by (A-C) MM-MTA, (D-F) BD, and (G-I) ESRRM putty at 4 weeks after the subcutaneous implantation of the materials. Mineralization is indicated by the red arrows.
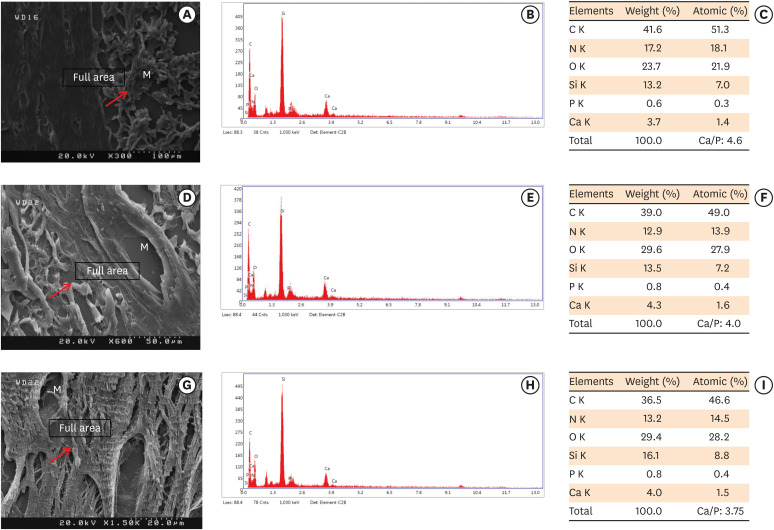
Scanning electron microscopy observations and elemental composition obtained using energy-dispersive X-ray analysis of the surface precipitates produced by (A-C) MM-MTA, (D-F) BD, and (G-I) EndoSequence Root Repair Material putty at 8 weeks after the subcutaneous implantation of the materials. Mineralization is indicated by the red arrows.
Comparative semi-quantitative analysis of calcium- and phosphorus-rich areas of the 3 calcium silicate-based cements at 3 different time periods
DISCUSSION
CONCLUSIONS
ACKNOWLEDGEMENTS
-
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
-
Author Contributions:
Conceptualization: Talabani RM, Garib BT, Masaeli R.
Data curation: Talabani RM.
Formal analysis: Talabani RM.
Investigation: Talabani RM, Zandsalimi K, Ketabat F.
Methodology: Garib BT, Masaeli RM.
Project administration: Talabani RM.
Software: Ketabat F.
Supervision: Garib BT, Masaeli R.
Validation: Zandsalimi K.
Visualization: Garib BT.
Writing - original draft: Talabani RM.
Writing - review & editing: Garib BT.
- 1. Wang Z. Bioceramic materials in endodontics. Endod Topics 2015;32:3-30.Article
- 2. Koch KA, Brave DG. Bioceramics, part I: the clinician's viewpoint. Dent Today 2012;31:130-135.
- 3. Bueno CR, Valentim D, Marques VA, Gomes-Filho JE, Cintra LT, Jacinto RC, Dezan-Junior E. Biocompatibility and biomineralization assessment of bioceramic-, epoxy-, and calcium hydroxide-based sealers. Braz Oral Res 2016;30:S1806-83242016000100267.ArticlePubMed
- 4. Jitaru S, Hodisan I, Timis L, Lucian A, Bud M. The use of bioceramics in endodontics - literature review. Clujul Med 2016;89:470-473.ArticlePubMedPMCPDF
- 5. Prati C, Gandolfi MG. Calcium silicate bioactive cements: biological perspectives and clinical applications. Dent Mater 2015;31:351-370.ArticlePubMed
- 6. Gandolfi MG, Siboni F, Botero T, Bossù M, Riccitiello F, Prati C. Calcium silicate and calcium hydroxide materials for pulp capping: biointeractivity, porosity, solubility and bioactivity of current formulations. J Appl Biomater Funct Mater 2015;13:43-60.ArticlePubMedPDF
- 7. Reyes-Carmona JF, Felippe MS, Felippe WT. Biomineralization ability and interaction of mineral trioxide aggregate and white Portland cement with dentin in a phosphate-containing fluid. J Endod 2009;35:731-736.ArticlePubMed
- 8. Torabinejad M, Parirokh M, Dummer PM. Mineral trioxide aggregate and other bioactive endodontic cements: an updated overview - part II: other clinical applications and complications. Int Endod J 2018;51:284-317.ArticlePubMedPDF
- 9. Köseoğlu S, Pekbağr Yan K T, Kucukyilmaz E, Sağlam M, Enhos S, Akgün A. Biological response of commercially available different tricalcium silicate-based cements and pozzolan cement. Microsc Res Tech 2017;80:994-999.ArticlePubMedPDF
- 10. Raghavendra SS, Jadhav GR, Gathani KM, Kotadia P. Bioceramics in endodontics - a review. J Istanb Univ Fac Dent 2017;51(Supplement 1):S128-S137.PubMedPMC
- 11. Köseoğlu S, Pekbağr Yan K T, Kucukyilmaz E, Sağlam M, Enhos S, Akgün A. Biological response of commercially available different tricalcium silicate-based cements and pozzolan cement. Microsc Res Tech 2017;80:994-999.ArticlePubMedPDF
- 12. Escobar-García DM, Aguirre-López E, Méndez-González V, Pozos-Guillén A. Cytotoxicity and initial biocompatibility of endodontic biomaterials (MTA and Biodentine) used as root-end filling materials. BioMed Res Int 2016;2016:7926961.PubMedPMC
- 13. Han L, Okiji T. Uptake of calcium and silicon released from calcium silicate-based endodontic materials into root canal dentine. Int Endod J 2011;44:1081-1087.ArticlePubMed
- 14. Atmeh AR, Chong EZ, Richard G, Festy F, Watson TF. Dentin-cement interfacial interaction: calcium silicates and polyalkenoates. J Dent Res 2012;91:454-459.ArticlePubMedPMCPDF
- 15. Charland T, Hartwell GR, Hirschberg C, Patel R. An evaluation of setting time of mineral trioxide aggregate and EndoSequence Root Repair Material in the presence of human blood and minimal essential media. J Endod 2013;39:1071-1072.ArticlePubMed
- 16. Tran D, He J, Glickman GN, Woodmansey KF. Comparative analysis of calcium silicate-based root filling materials using an open apex model. J Endod 2016;42:654-658.ArticlePubMed
- 17. Gomes Filho JE, Queiroz ÍO, Watanabe S, Cintra LT, Ervolino E. Influence of diabetes mellitus on the mineralization ability of two endodontic materials. Braz Oral Res 2016;30:S1806-83242016000100218.PubMed
- 18. Holland R, de Souza V, Nery MJ, Otoboni Filho JA, Bernabé PF, Dezan Júnior E. Reaction of rat connective tissue to implanted dentin tubes filled with mineral trioxide aggregate or calcium hydroxide. J Endod 1999;25:161-166.ArticlePubMed
- 19. Cintra LT, Ribeiro TA, Gomes-Filho JE, Bernabé PF, Watanabe S, Facundo AC, Samuel RO, Dezan-Junior E. Biocompatibility and biomineralization assessment of a new root canal sealer and root-end filling material. Dent Traumatol 2013;29:145-150.ArticlePubMed
- 20. Hench LL. Bioceramics: from concept to clinic. J Am Ceram 1991;74:1487-1510.Article
- 21. Bonewald LF, Harris SE, Rosser J, Dallas MR, Dallas SL, Camacho NP, Boyan B, Boskey A. von Kossa staining alone is not sufficient to confirm that mineralization in vitro represents bone formation. Calcif Tissue Int 2003;72:537-547.ArticlePubMedPDF
- 22. Camilleri J. Characterization of hydration products of mineral trioxide aggregate. Int Endod J 2008;41:408-417.ArticlePubMed
- 23. Yang WK, Ko HJ, Kim MR. Evaluation of the rat tissue reaction to experimental new resin cement and mineral trioxide aggregate cement. Restor Dent Endod 2012;37:194-200.ArticlePubMedPMC
- 24. Bósio CC, Felippe GS, Bortoluzzi EA, Felippe MC, Felippe WT, Rivero ER. Subcutaneous connective tissue reactions to iRoot SP, mineral trioxide aggregate (MTA) Fillapex, DiaRoot BioAggregate and MTA. Int Endod J 2014;47:667-674.PubMed
- 25. Clark G. Staining procedures. Baltimore, MD: Williams and Wilkins; 1981. p. 187.
- 26. Gerstenfeld LC, Chipman SD, Glowacki J, Lian JB. Expression of differentiated function by mineralizing cultures of chicken osteoblasts. Dev Biol 1987;122:49-60.ArticlePubMed
- 27. Cosme-Silva L, Dal-Fabbro R, Gonçalves LO, Prado AS, Plazza FA, Viola NV, Cintra LT, Gomes Filho JE. Hypertension affects the biocompatibility and biomineralization of MTA, High-plasticity MTA, and Biodentine® . Braz Oral Res 2019;33:e060.ArticlePubMed
- 28. Han L, Okiji T. Bioactivity evaluation of three calcium silicate-based endodontic materials. Int Endod J 2013;46:808-814.ArticlePubMed
- 29. Hinata G, Yoshiba K, Han L, Edanami N, Yoshiba N, Okiji T. Bioactivity and biomineralization ability of calcium silicate-based pulp-capping materials after subcutaneous implantation. Int Endod J 2017;50(Supplement 2):e40-e51.ArticlePubMedPDF
- 30. Yaltirik M, Ozbas H, Bilgic B, Issever H. Reactions of connective tissue to mineral trioxide aggregate and amalgam. J Endod 2004;30:95-99.ArticlePubMed
- 31. Ozbas H, Yaltirik M, Bilgic B, Issever H. Reactions of connective tissue to compomers, composite and amalgam root-end filling materials. Int Endod J 2003;36:281-287.ArticlePubMedPDF
- 32. Bueno CR, Vasques AM, Cury MT, Sivieri-Araújo G, Jacinto RC, Gomes-Filho JE, Cintra LT, Dezan-Júnior E. Biocompatibility and biomineralization assessment of mineral trioxide aggregate flow. Clin Oral Investig 2019;23:169-177.ArticlePubMedPDF
- 33. Cintra LT, Benetti F, de Azevedo Queiroz ÍO, de Araújo Lopes JM, Penha de Oliveira SH, Sivieri Araújo G, Gomes-Filho JE. Cytotoxicity, biocompatibility, and biomineralization of the new high-plasticity MTA material. J Endod 2017;43:774-778.ArticlePubMed
- 34. Tziafas D, Pantelidou O, Alvanou A, Belibasakis G, Papadimitriou S. The dentinogenic effect of mineral trioxide aggregate (MTA) in short-term capping experiments. Int Endod J 2002;35:245-254.ArticlePubMed
- 35. Dominguez MS, Witherspoon DE, Gutmann JL, Opperman LA. Histological and scanning electron microscopy assessment of various vital pulp-therapy materials. J Endod 2003;29:324-333.ArticlePubMed
- 36. Sarkar NK, Caicedo R, Ritwik P, Moiseyeva R, Kawashima I. Physicochemical basis of the biologic properties of mineral trioxide aggregate. J Endod 2005;31:97-100.ArticlePubMed
- 37. Seux D, Couble ML, Hartmann DJ, Gauthier JP, Magloire H. Odontoblast-like cytodifferentiation of human dental pulp cells in vitro in the presence of a calcium hydroxide-containing cement. Arch Oral Biol 1991;36:117-128.ArticlePubMed
- 38. Schröder U. Effects of calcium hydroxide-containing pulp-capping agents on pulp cell migration, proliferation, and differentiation. J Dent Res 1985;64:541-548.ArticlePubMedPDF
- 39. Camilleri J, Sorrentino F, Damidot D. Investigation of the hydration and bioactivity of radiopacified tricalcium silicate cement, Biodentine and MTA Angelus. Dent Mater 2013;29:580-593.ArticlePubMed
- 40. Camilleri J. Characterization and hydration kinetics of tricalcium silicate cement for use as a dental biomaterial. Dent Mater 2011;27:836-844.ArticlePubMed
- 41. Setbon HM, Devaux J, Iserentant A, Leloup G, Leprince JG. Influence of composition on setting kinetics of new injectable and/or fast setting tricalcium silicate cements. Dent Mater 2014;30:1291-1303.ArticlePubMed
- 42. Murphy S, Wren AW, Towler MR, Boyd D. The effect of ionic dissolution products of Ca-Sr-Na-Zn-Si bioactive glass on in vitro cytocompatibility. J Mater Sci Mater Med 2010;21:2827-2834.ArticlePubMedPDF
- 43. Kim EJ, Bu SY, Sung MK, Choi MK. Effects of silicon on osteoblast activity and bone mineralization of MC3T3-E1 cells. Biol Trace Elem Res 2013;152:105-112.ArticlePubMedPDF
- 44. Shokouhinejad N, Nekoofar MH, Razmi H, Sajadi S, Davies TE, Saghiri MA, Gorjestani H, Dummer PM. Bioactivity of EndoSequence root repair material and bioaggregate. Int Endod J 2012;45:1127-1134.ArticlePubMed
- 45. Ma J, Shen Y, Stojicic S, Haapasalo M. Biocompatibility of two novel root repair materials. J Endod 2011;37:793-798.ArticlePubMed
- 46. Dawood AE, Parashos P, Wong RH, Reynolds EC, Manton DJ. Calcium silicate-based cements: composition, properties, and clinical applications. J Investig Clin Dent 2017;8:e12195.ArticlePDF
- 47. Hansen SW, Marshall JG, Sedgley CM. Comparison of intracanal EndoSequence Root Repair Material and ProRoot MTA to induce pH changes in simulated root resorption defects over 4 weeks in matched pairs of human teeth. J Endod 2011;37:502-506.ArticlePubMed
REFERENCES
Tables & Figures
REFERENCES
Citations

- Evaluating Retrieval-Augmented Large Language Models on External Cervical Resorption: A Comparative Study of Gemini and NotebookLM
Marc Garcia-Font, Nicolás Dufey-Portilla, Fernando Durán-Sindreu, José Antonio González Sánchez, Gustavo Rodríguez Millán, Venkateshbabu Nagendrababu, Paul M.H. Dummer, Francesc Abella Sans
Journal of Endodontics.2025;[Epub] CrossRef - Antibacterial, biocompatible, and mineralization‐inducing properties of calcium silicate‐based cements
Taimy Cruz Hondares, Xiaoxiao Hao, Yanfang Zhao, Yuyin Lin, Dobrawa Napierala, Janice G. Jackson, Ping Zhang
International Journal of Paediatric Dentistry.2024; 34(6): 843. CrossRef - Bioactive potential of Bio‐C Pulpo is evidenced by presence of birefringent calcite and osteocalcin immunoexpression in the rat subcutaneous tissue
Marcela Borsatto Queiroz, Rafaela Nanami Handa Inada, Camila Soares Lopes, Juliane Maria Guerreiro‐Tanomaru, Estela Sasso‐Cerri, Mário Tanomaru‐Filho, Paulo Sérgio Cerri
Journal of Biomedical Materials Research Part B: Applied Biomaterials.2022; 110(10): 2369. CrossRef
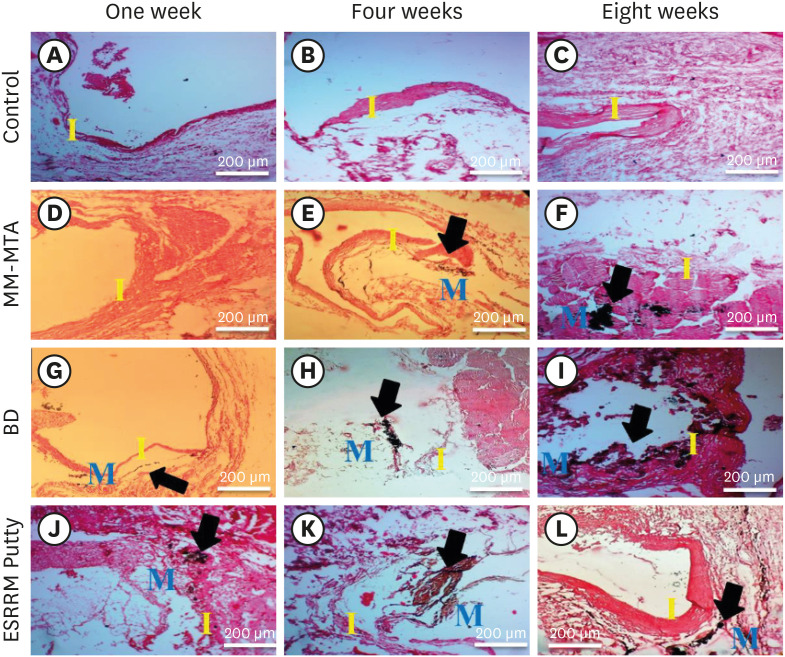
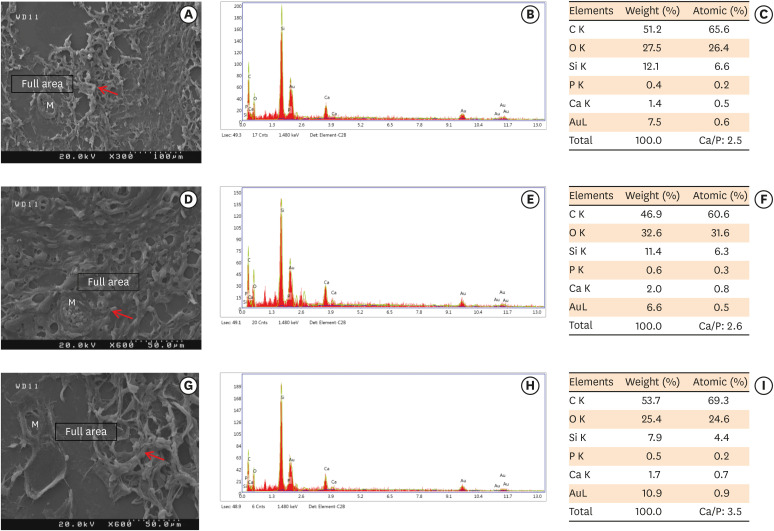
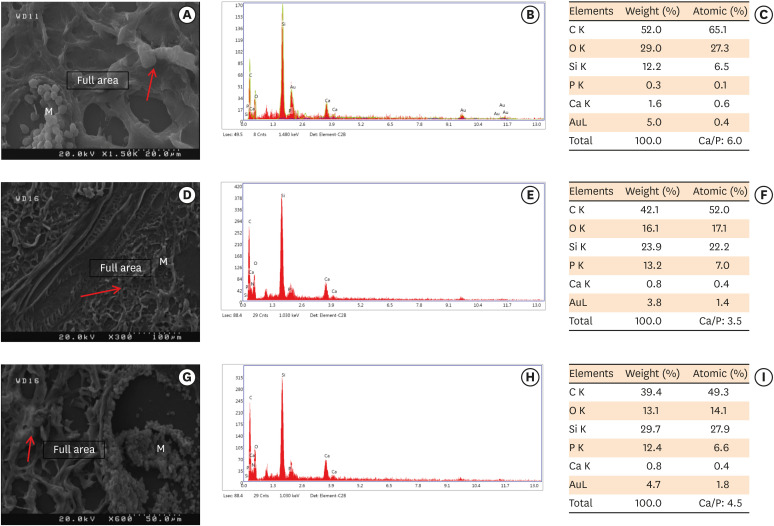
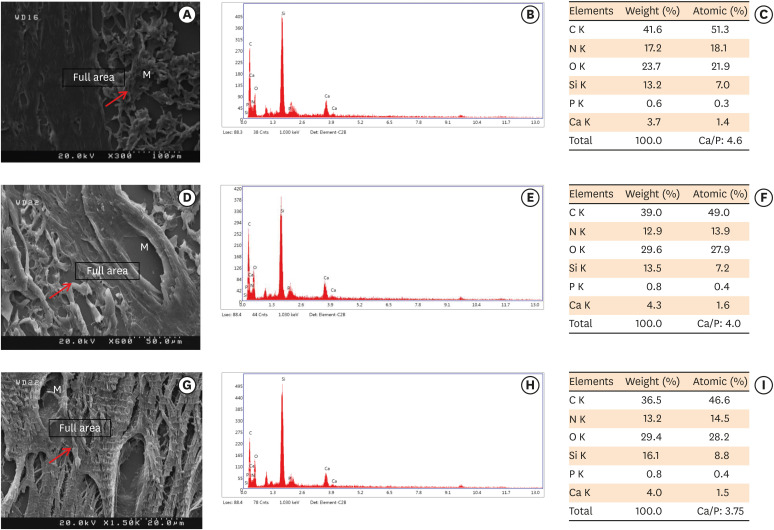
Figure 1
Figure 2
Figure 3
Figure 4
Tested cements
| Cements | Manufacturer | Composition | Lot No. |
|---|---|---|---|
| MM-MTA | Micro-Mega SA, Besançon, France | Powder: tricalcium silicate, dicalcium silicate, tricalcium aluminate, bismuth oxide, calcium sulfate dehydrate, and magnesium oxide Liquid: calcium carbonate | 71708614 |
| ESRRM putty | Brasseler USA, Savannah, GA, USA | Powder: tricalcium silicate, dicalcium silicate, calcium phosphate monobasic, calcium hydroxide, colloidal silica, water-free thickening agent | B19585 |
| BD | Septodont, Saint Maur des Fosses, France | Powder: tricalcium silicate, dicalcium silicate, calcium carbonate, iron oxide, and zirconium oxide | 5024200U0 |
| Liquid: water with calcium chloride and soluble polymer (polycarboxylate) |
MM-MTA, Micro-Mega mineral trioxide aggregate; ESRRM, EndoSequence Root Repair Material; BD, Biodentine.
Comparative semi-quantitative analysis of calcium- and phosphorus-rich areas of the 3 calcium silicate-based cements at 3 different time periods
| Period | MM-MTA (atomic concentrations, %) | BD (atomic concentrations, %) | ESRRM putty (atomic concentrations, %) | p value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ca | P | Ca/P | Ca | P | Ca/P | Ca | P | Ca/P | ||
| Week 1 | 0.5 ± 0.00 | 0.2 ± 0.00 | 2.5 | 0.633 ± 0.1527 | 0.266 ± 0.0577 | 2.4 | 0.667 ± 0.0577 | 0.3 ± 0.1732 | 2.63 | > 0.05 |
| Week 4 | 1.2333 ± 0.551 | 0.2667 ± 0.0577 | 4.433 | 1.1333 ± 0.4618 | 0.4667 ± 0.11547 | 3.433 | 1.75 ± 0.2516 | 0.425 ± 0.0957 | 4.32 | > 0.05 |
| Week 8 | 1.9 ± 0.707 | 0.45 ± 0.2121 | 4.3 | 2.2 ± 0.8485 | 0.5 ± 0.14142 | 4.3 | 2.2 ± 0.98995 | 0.5 ± 0.1414 | 4.27 | > 0.05 |
Values are expressed as mean ± standard deviation.
Ca, calcium; P, phosphorus; MM-MTA, MicroMega mineral trioxide aggregate; BD, Biodentine; ESRRM, EndoSequence Root Repair Material.
MM-MTA, Micro-Mega mineral trioxide aggregate; ESRRM, EndoSequence Root Repair Material; BD, Biodentine.
Values are expressed as mean ± standard deviation.
Ca, calcium; P, phosphorus; MM-MTA, MicroMega mineral trioxide aggregate; BD, Biodentine; ESRRM, EndoSequence Root Repair Material.

 KACD
KACD
 ePub Link
ePub Link Cite
Cite

