Articles
- Page Path
- HOME > Restor Dent Endod > Volume 45(1); 2020 > Article
- Research Article Effect of hydrofluoric acid-based etchant at an elevated temperature on the bond strength and surface topography of Y-TZP ceramics
-
Mi-Kyung Yu
 , Myung-Jin Lim
, Myung-Jin Lim , Noo-Ri Na
, Noo-Ri Na , Kwang-Won Lee
, Kwang-Won Lee
-
Restor Dent Endod 2019;45(1):e6.
DOI: https://doi.org/10.5395/rde.2020.45.e6
Published online: December 3, 2019
Department of Conservative Dentistry, School of Dentistry, Chonbuk National University, Jeonju, Korea.
- Correspondence to Kwang-Won Lee, DDS, PhD. Professor, Department of Conservative Dentistry, School of Dentistry, Chonbuk National University, 567 Baekje-daero, Deokjin-gu, Jeonju 54896, Korea. lkw@jbnu.ac.kr
Copyright © 2020. The Korean Academy of Conservative Dentistry
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 2,204 Views
- 11 Download
- 12 Crossref
Abstract
-
Objectives This study investigated the effects of a hydrofluoric acid (HA; solution of hydrogen fluoride [HF] in water)-based smart etching (SE) solution at an elevated temperature on yttria-stabilized tetragonal zirconia polycrystal (Y-TZP) ceramics in terms of bond strength and morphological changes.
-
Materials and Methods Eighty sintered Y-TZP specimens were prepared for shear bond strength (SBS) testing. The bonding surface of the Y-TZP specimens was treated with 37% phosphoric acid etching at 20°C–25°C, 4% HA etching at 20°C–25°C, or HA-based SE at 70°C–80°C. In all groups, zirconia primers were applied to the bonding surface of Y-TZP. For each group, 2 types of resin cement (with or without methacryloyloxydecyl dihydrogen phosphate [MDP]) were used. SBS testing was performed. Topographic changes of the etched Y-TZP surface were analyzed using scanning electron microscopy and atomic force microscopy. The results were analyzed and compared using 2-way analysis of variance.
-
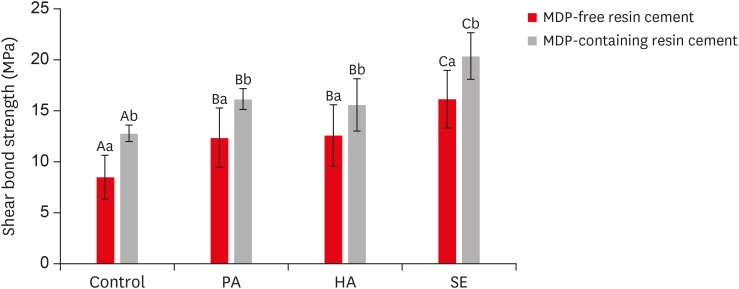
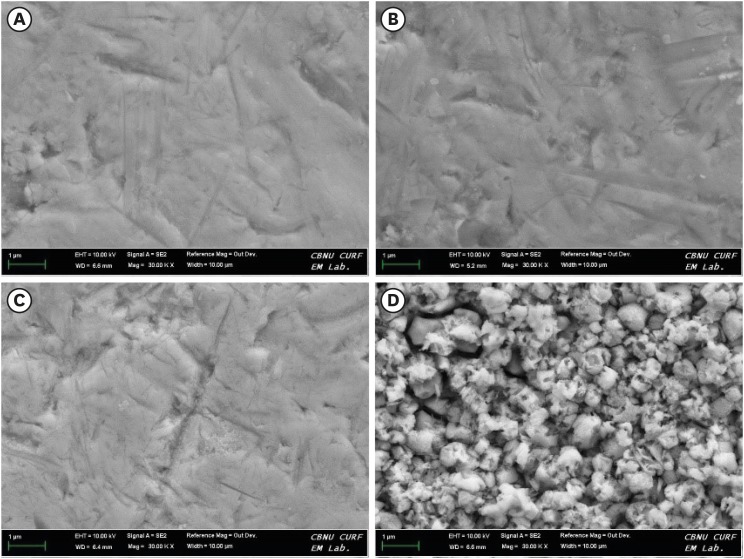
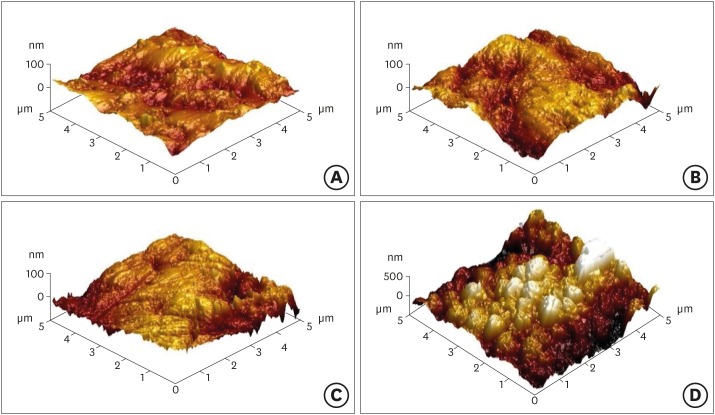
Results Regardless of the type of resin cement, the highest bond strength was measured in the SE group, with significant differences compared to the other groups (p < 0.05). In all groups, MDP-containing resin cement yielded significantly higher bond strength values than MDP-free resin cement (p < 0.05). It was also shown that the Y-TZP surface was etched by the SE solution, causing a large change in the surface topography.
-
Conclusions Bond strength significantly improved when a heated HA-based SE solution was applied to the Y-TZP surface, and the etched Y-TZP surface was more irregular and had higher surface roughness.
INTRODUCTION
MATERIALS AND METHODS
1) Control (n = 20): not etched.
2) PA group (n = 20): 37% phosphate etchant was placed on the Y-TZP surface for 10 minutes at 20°C–25°C. The Y-TZP specimen was then rinsed with an air-water spray and dried.
3) HA group (n = 20): 4% porcelain etchant was placed on the Y-TZP surface for 10 minutes at 20°C–25°C. The Y-TZP specimen was then rinsed with an air-water spray and dried.
4) SE group (n = 20): the SE solution was heated to 70°C–80°C in a water bath. Following the manufacturer's instructions, the Y-TZP specimen was placed in a heated SE solution bath for 10 minutes. The Y-TZP specimen was then rinsed with an air-water spray and dried.
Materials used, manufacturers, and major components
RESULTS
Shear bond strength values of each experimental group according to the use of methacryloyloxydecyl dihydrogen phosphate (MDP)-free or MDP-containing resin cement after etching the yttria-stabilized tetragonal zirconia polycrystal (Y-TZP) surface under various conditions
Shear bond strength values of yttria-stabilized tetragonal zirconia polycrystal (Y-TZP) in each group etched under various conditions with different resin cements.
Scanning electron microscopy (SEM) images at 30,000 times magnification of yttria-stabilized tetragonal zirconia polycrystal (Y-TZP) surfaces etched under different conditions. (A) Etching-free specimen (control); (B) 37% phosphoric acid (PA)-etched specimen; (C) 4% hydrofluoric acid (HA)-etched specimen; (D) HA-based smart etching (SE)-etched specimen.
Atomic force microscopy images of yttria-stabilized tetragonal zirconia polycrystal (Y-TZP) surfaces etched under different conditions. (A) Etching-free specimen (control); (B) 37% phosphoric acid (PA)-etched specimen; (C) 4% hydrofluoric acid (HA)-etched specimen; (D) HA-based smart etching (SE)-etched specimen.
DISCUSSION
CONCLUSIONS
-
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
-
Author Contributions:
Conceptualization: Yu MK, Lee KW.
Data curation: Lim MJ, Na NR.
Formal analysis: Lim MJ, Lee KW.
Methodology: Lim MJ, Na NR.
Project administration: Yu MK, Lee KW.
Resources: Yu MK, Lee KW.
Software: Lim MJ, Na NR.
Supervision: Yu MK, Lee KW.
Validation: Lim MJ, Lee KW.
Writing - original draft: Lim MJ, Lee KW.
Writing - review & editing: Yu MK, Lee KW.
- 1. Chen L, Suh BI, Brown D, Chen X. Bonding of primed zirconia ceramics: evidence of chemical bonding and improved bond strengths. Am J Dent 2012;25:103-108.PubMed
- 2. Pilo R, Kaitsas V, Zinelis S, Eliades G. Interaction of zirconia primers with yttria-stabilized zirconia surfaces. Dent Mater 2016;32:353-362.ArticlePubMed
- 3. Nagaoka N, Yoshihara K, Feitosa VP, Tamada Y, Irie M, Yoshida Y, Van Meerbeek B, Hayakawa S. Chemical interaction mechanism of 10-MDP with zirconia. Sci Rep 2017;7:45563.ArticlePubMedPMCPDF
- 4. Xie H, Li Q, Zhang F, Lu Y, Tay FR, Qian M, Chen C. Comparison of resin bonding improvements to zirconia between one-bottle universal adhesives and tribochemical silica coating, which is better? Dent Mater 2016;32:403-411.ArticlePubMed
- 5. Kitayama S, Nikaido T, Takahashi R, Zhu L, Ikeda M, Foxton RM, Sadr A, Tagami J. Effect of primer treatment on bonding of resin cements to zirconia ceramic. Dent Mater 2010;26:426-432.ArticlePubMed
- 6. Koizumi H, Nakayama D, Komine F, Blatz MB, Matsumura H. Bonding of resin-based luting cements to zirconia with and without the use of ceramic priming agents. J Adhes Dent 2012;14:385-392.PubMed
- 7. Lim MJ, Yu MK, Lee KW. The effect of continuous application of MDP-containing primer and luting resin cement on bond strength to tribochemical silica-coated Y-TZP. Restor Dent Endod 2018;43:e19.ArticlePubMedPMCPDF
- 8. Cavalcanti AN, Foxton RM, Watson TF, Oliveira MT, Giannini M, Marchi GM. Bond strength of resin cements to a zirconia ceramic with different surface treatments. Oper Dent 2009;34:280-287.ArticlePubMedPDF
- 9. Guazzato M, Albakry M, Quach L, Swain MV. Influence of surface and heat treatments on the flexural strength of a glass-infiltrated alumina/zirconia-reinforced dental ceramic. Dent Mater 2005;21:454-463.ArticlePubMed
- 10. Wolfart M, Lehmann F, Wolfart S, Kern M. Durability of the resin bond strength to zirconia ceramic after using different surface conditioning methods. Dent Mater 2007;23:45-50.PubMed
- 11. Sriamporn T, Thamrongananskul N, Busabok C, Poolthong S, Uo M, Tagami J. Dental zirconia can be etched by hydrofluoric acid. Dent Mater J 2014;33:79-85.ArticlePubMed
- 12. Smielak B, Klimek L. Effect of hydrofluoric acid concentration and etching duration on select surface roughness parameters for zirconia. J Prosthet Dent 2015;113:596-602.ArticlePubMed
- 13. Cho JH, Kim SJ, Shim JS, Lee KW. Effect of zirconia surface treatment using nitric acid-hydrofluoric acid on the shear bond strengths of resin cements. J Adv Prosthodont 2017;9:77-84.ArticlePubMedPMCPDF
- 14. Dérand P, Dérand T. Bond strength of luting cements to zirconium oxide ceramics. Int J Prosthodont 2000;13:131-135.PubMed
- 15. Borges GA, Sophr AM, de Goes MF, Sobrinho LC, Chan DC. Effect of etching and airborne particle abrasion on the microstructure of different dental ceramics. J Prosthet Dent 2003;89:479-488.ArticlePubMed
- 16. Derand T, Molin M, Kvam K. Bond strength of composite luting cement to zirconia ceramic surfaces. Dent Mater 2005;21:1158-1162.ArticlePubMed
- 17. Lee MH, Son JS, Kim KH, Kwon TY. Improved resin-zirconia bonding by room temperature hydrofluoric acid etching. Materials (Basel) 2015;8:850-866.ArticlePubMedPMC
- 18. Liu D, Tsoi JK, Matinlinna JP, Wong HM. Effects of some chemical surface modifications on resin zirconia adhesion. J Mech Behav Biomed Mater 2015;46:23-30.ArticlePubMed
- 19. Ansari S, Jahedmanesh N, Cascione D, Zafarnia P, Shah KC, Wu BM, Moshaverinia A. Effects of an etching solution on the adhesive properties and surface microhardness of zirconia dental ceramics. J Prosthet Dent 2018;120:447-453.ArticlePubMed
- 20. Yang B, Scharnberg M, Wolfart S, Quaas AC, Ludwig K, Adelung R, Kern M. Influence of contamination on bonding to zirconia ceramic. J Biomed Mater Res B Appl Biomater 2007;81:283-290.ArticlePubMed
- 21. Kim MJ, Kim YK, Kim KH, Kwon TY. Shear bond strengths of various luting cements to zirconia ceramic: surface chemical aspects. J Dent 2011;39:795-803.ArticlePubMed
- 22. Qian M, Lu Z, Chen C, Zhang H, Xie H. Alkaline nanoparticle coatings improve resin bonding of 10-methacryloyloxydecyldihydrogenphosphate-conditioned zirconia. Int J Nanomedicine 2016;11:5057-5066.ArticlePubMedPMCPDF
REFERENCES
Tables & Figures
REFERENCES
Citations

- Etchability of zirconia ceramics and its effect on adhesion: A systematic review and meta-analysis
Anina Sieber, Luiza Freitas Brum Souza, Tan Fırat Eyüboğlu, Mutlu Özcan
International Journal of Adhesion and Adhesives.2026; 148: 104303. CrossRef - Evaluation of Different Surface Roughening Techniques on Clear Aligner Attachments Bonded to Monolithic Zirconia: In Vitro Study
Nehal F Albelasy, Ahmad M Hafez, Abdullah S Alhunayni
The Journal of Contemporary Dental Practice.2025; 25(12): 1104. CrossRef - Effect of Acid Surface Treatments on the Shear Bond Strength of Metal Bracket to Zirconia Ceramics
Punchanit Wongrachit, Bancha Samruajbenjakun, Boonlert Kukiattrakoon, Tanapat Jearanai, Supontep Teerakanok, Pannapat Chanmanee
Ceramics.2024; 7(2): 689. CrossRef - Exploring Zirconia Adhesion: Pre and Postsintering Physical Surface Treatment, Chemical Treatment, and Cement Interactions
Flávia Gonçalves, Mirko Dennys Ayala-Perez, Francisco Carlos dos Santos Reis, Walter Gomes Miranda-Júnior, Letícia Cristina Cidreira Boaro, Heng Bo Jiang
BioMed Research International.2024;[Epub] CrossRef - Evaluation of zirconia surfaces and shear bond strength after acid–etching with ultrasonic vibration
Xiaozhen Zhang, Hepeng Nie, Jiaxin Lv, Shanshan Yuan, Juan Wang, Kunzhan Cai, Jin Wu, Qingqing Zhang, Chunbo Tang
Materials Research Express.2024; 11(2): 025401. CrossRef - Effects of Surface-Etching Systems on the Shear Bond Strength of Dual-Polymerized Resin Cement and Zirconia
Sang-Hyun Kim, Kyung Chul Oh, Hong-Seok Moon
Materials.2024; 17(13): 3096. CrossRef - Zirconia bond strength durability following artificial aging: A systematic review and meta-analysis of in vitro studies
Athanasios E. Rigos, Katia Sarafidou, Eleana Kontonasaki
Japanese Dental Science Review.2023; 59: 138. CrossRef - Y-TZP Physicochemical Properties Conditioned with ZrO2 and SiO2 Nanofilms and Bond Strength to Dual Resin Cement
Ricardo Faria Ribeiro, Danilo Flamini Oliveira, Camila Bussola Tovani, Ana Paula Ramos, Ana Flavia Sanches Borges, Adriana Claudia Lapria Faria, Rossana Pereira de Almeida, Renata Cristina Silveira Rodrigues
Materials.2022; 15(22): 7905. CrossRef - Effect of the nanofilm-coated zirconia ceramic on resin cement bond strength
Viviane Maria Gonçalves de Figueiredo, Alecsandro de Moura Silva, Marcos Massi, Argemiro Soares da Silva Sobrinho, José Renato Cavalcanti de Queiroz, João Paulo Barros Machado, Renata Falchete do Prado, Lafayette Nogueira Junior
Journal of Dental Research, Dental Clinics, Dental Prospects.2022; 16(3): 170. CrossRef - Change of phase transformation and bond strength of Y-TZP with various hydrofluoric acid etching
Mi-Kyung Yu, Eun-Jin Oh, Myung-Jin Lim, Kwang-Won Lee
Restorative Dentistry & Endodontics.2021;[Epub] CrossRef - Changes in Bond Strength and Topography for Y-TZP Etched with Hydrofluoric Acid Depending on Concentration and Temperature Conditions
Hyo-Eun Kim, Myung-Jin Lim, Mi-Kyung Yu, Kwang-Won Lee
Medicina.2020; 56(11): 568. CrossRef - Do different sintering conditions influence bond strength between the resin cements and a currently used esthetic zirconia?
Fatma Ayse Sanal, Hamiyet Kilinc
Journal of Adhesion Science and Technology.2020; 34(16): 1809. CrossRef
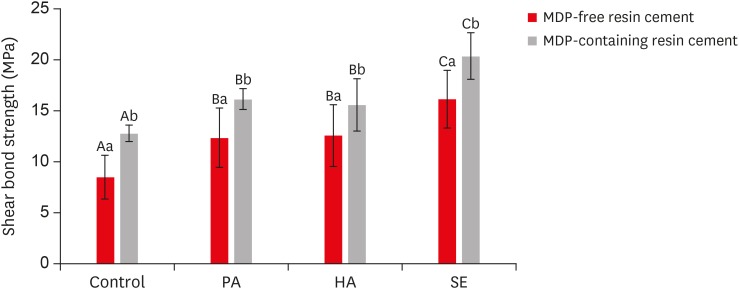
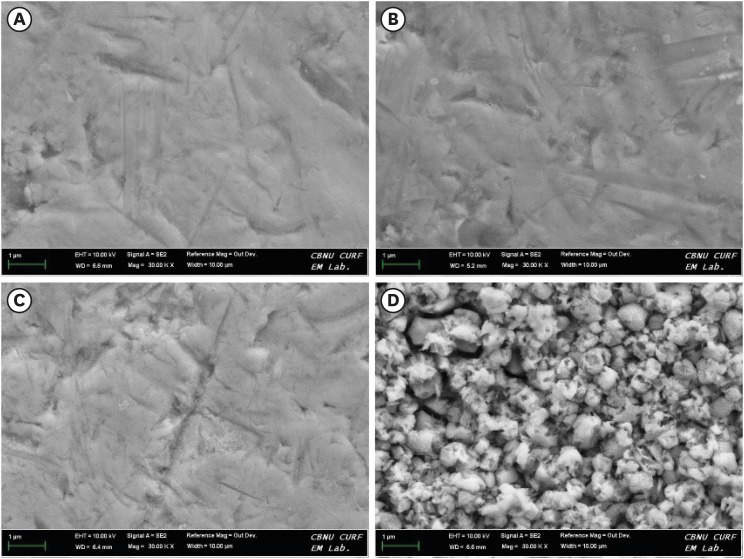
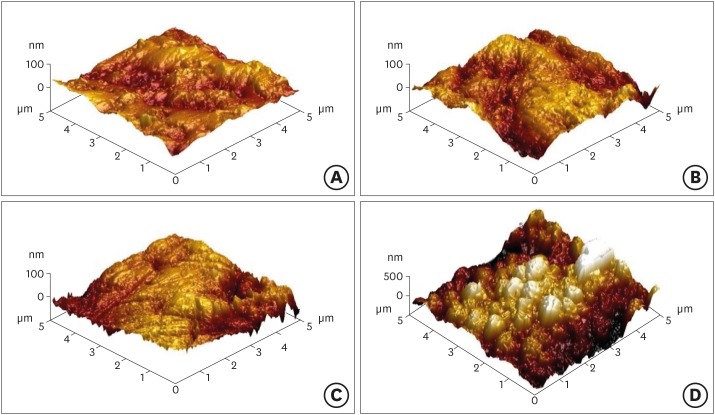
Figure 1
Figure 2
Figure 3
Materials used, manufacturers, and major components
| Material | Trade name | Manufacturer | Main components |
|---|---|---|---|
| PA etching | Etch-37 | Bisco Inc., Schaumburg, IL, USA | 37% PA gel |
| w/benzalkonium chloride | |||
| HA etching | Porcelain Etchant | Bisco Inc., Schaumburg, IL, USA | 4% buffered HA gel |
| SE solution etching | Smart Etching | Yesbiogold Inc., Seoul, Korea | 40% HA (vol %) |
| 59% PA (vol %) | |||
| 1% HCl (vol %) | |||
| Zirconia primer | Z-prime Plus | Bisco Inc., Schaumburg, IL, USA | BPDM, HEMA, ethanol |
| MDP-containing resin cement | G-CEM LinkAce | GC Inc., Tokyo, Japan | Paste A: Fluoro-alumino-silicate glass, UDMA, dimethacrylate, silicon dioxide |
| Paste B: PA ester monomer (MDP), silicon dioxide, UDMA, dimethacrylate | |||
| MDP-free resin cement | Duo-link Universal | Bisco Inc., Schaumburg, IL, USA | Base: Bis-GMA, TEGDMA, UDMA, glass filler |
| Catalyst: Bis-GMA, TEGDMA, glass filler |
PA, phosphoric acid; HA, hydrofluoric acid; SE, smart etching; HCl, hydrochloric acid; BPDM, biphenyl dimethacrylate; HEMA, hydroxyethyl methacrylate; UDMA, urethane dimethacrylate; MDP, methacryloyloxydecyl dihydrogen phosphate; Bis-GMA, bisphenyl A glycidyl methacrylate; TEGDMA, triethylene glycol dimethacrylate.
Shear bond strength values of each experimental group according to the use of methacryloyloxydecyl dihydrogen phosphate (MDP)-free or MDP-containing resin cement after etching the yttria-stabilized tetragonal zirconia polycrystal (Y-TZP) surface under various conditions
| Etching condition | Resin cement, Mean ± SD (MPa) | |
|---|---|---|
| MDP-free resin cement | MDP-containing resin cement | |
| Control | 8.50 ± 2.16Aa | 12.80 ± 0.81Ab |
| 37% PA at 20°C–25°C | 12.37 ± 2.92Ba | 16.17 ± 0.99Bb |
| 4% HA at 20°C–25°C | 12.60 ± 3.03Ba | 15.60 ± 2.55Bb |
| HA-based SE at 70°C–80°C | 16.15 ± 2.82Ca | 20.39 ± 2.29Cb |
SD, standard deviation; PA, phosphoric acid; HA, hydrofluoric acid; SE, smart etching.
Identical uppercase letters indicate no statistically significant differences (p > 0.05).
PA, phosphoric acid; HA, hydrofluoric acid; SE, smart etching; HCl, hydrochloric acid; BPDM, biphenyl dimethacrylate; HEMA, hydroxyethyl methacrylate; UDMA, urethane dimethacrylate; MDP, methacryloyloxydecyl dihydrogen phosphate; Bis-GMA, bisphenyl A glycidyl methacrylate; TEGDMA, triethylene glycol dimethacrylate.
SD, standard deviation; PA, phosphoric acid; HA, hydrofluoric acid; SE, smart etching.
Identical uppercase letters indicate no statistically significant differences (

 KACD
KACD
 ePub Link
ePub Link Cite
Cite

