Abstract
-
Objectives
The aim of this study was to evaluate the effects of endodontic treatment on levels of substance P (SP) and calcitonin gene-related peptide (CGRP) in the saliva of patients with symptomatic apical periodontitis.
-
Materials and Methods
Twelve patients with mandibular molars with symptomatic apical periodontitis were enrolled in this study. An initial saliva sample was collected just before administration of anesthesia for root canal treatment, which was performed at the first visit. A second saliva sample was collected at a control visit 1 week after treatment. Salivary SP and CGRP levels were evaluated quantitatively using biochemical assays. The data were analyzed using Pearson correlation analysis, the paired samples t-test, and the Mann-Whitney U test (p = 0.05).
-
Results
The postoperative salivary level of SP was significantly lower than the preoperative level (p = 0.005). However, the postoperative salivary level of CGRP was similar to the preoperative level (p = 0.932). Visual analog scale (VAS) scores of patients' subjective pain were found to be positively correlated with salivary levels of SP (r = 0.421; p = 0.040). No statistically significant correlations were observed between salivary levels of CGRP and VAS scores for patients' subjective percussion tenderness (p = 0.533) or VAS scores for patients' subjective pain (p = 0.459).
-
Conclusions
According to the results of the present study, salivary SP levels may be used as an objective indicator in the diagnosis and assessment of the degree of pain in endodontic diseases.
-
Trial Registration
-
Keywords: Endodontics; Substance P; Calcitonin gene-related peptide; Root canal treatment
INTRODUCTION
Pain associated with the dental pulp and periradicular tissue can induce neurogenic inflammation. The inflammatory response in these tissues has been shown to have a neurogenic origin [
1]. During such inflammatory reactions, the nervous component stimulates the vascular and immune systems through the release of neuropeptides such as substance P (SP), calcitonin gene-related peptide (CGRP), and neurokinin A [
2]. These neuropeptides have a variety of effects, including strong vasodilation and plasma extravasation; the activation of numerous types of inflammatory cells; and the release of growth factors and inflammatory mediators such as histamine, cytokines, and prostaglandins [
1,
2,
3].
SP is an 11-amino-acid peptide of the tachykinin family that is produced in neurons and released by nerve endings [
4,
5]. SP causes the proliferation of fibroblasts, macrophages, and B and T lymphocytes, as well as mast cell degranulation and capillary vasodilatation [
6,
7]. SP is considered a major mediator of neurogenic inflammation [
6]. CGRP, another mediator, increases the effects of SP on vascular permeability and the promotion of edema [
8].
Saliva is the main component of oral fluid and is secreted from a variety of glands, including the parotid, submandibular, sublingual, and minor salivary glands [
9]. Saliva contains proteins, glycoproteins, electrolytes, and small organic molecules originating from the blood, and it can be used for the detection of certain diseases [
10,
11]. SP and CGRP have been measured in the saliva of patients with migraine and cluster headaches [
12], and saliva has been used for the identification of biomarkers in oral diseases, including periodontal diseases [
13,
14,
15]. The salivary SP concentration has been demonstrated to be significantly lower in patients with burning mouth syndrome than in control patients [
13]. In another study, the levels of CGRP were reported to be non-significantly decreased in patients with burning mouth syndrome relative to healthy subjects [
14]. Non-significantly higher levels of SP and non-significantly lower levels of CGRP have also been observed in the saliva of patients with aggressive and chronic periodontitis [
15]. However, according to our search of the literature, no study has evaluated SP and CGRP levels in human saliva pre- and post-endodontic treatment.
Therefore, the aim of this study was to evaluate the effect of endodontic treatment on SP and CGRP levels in the saliva of patients with symptomatic apical periodontitis. The null hypothesis was that no significant difference would be found between preoperative and postoperative SP and CGRP levels in human saliva. The alternative hypothesis was that postoperative SP and CGRP levels in human saliva would be lower than preoperative SP and CGRP levels.
MATERIALS AND METHODS
We compared preoperative and postoperative SP and CGRP levels in human saliva. Since each participant served as his or her own control, this study did not include a dedicated control group.
Patient selection
The study was approved by the Research Ethics Committee of the Faculty of Dentistry at Ataturk University in Erzurum, Turkey (January 6, 2017; No. 2017/2). The clinical registration number is
TCTR20161228001, and the trial register is the Thai Clinical Trials Registry. Patients were referred to the Department of Endodontics at Ataturk University in Erzurum, Turkey between June 2017 and October 2017 and were assessed for eligibility. The inclusion criteria were the presence of a mandibular molar with symptomatic apical periodontitis (manifesting as preoperative spontaneous pain and preoperative percussion tenderness), the absence of systemic disease and of medication use within 3 days, the absence of periodontal disease, and the absence of previous endodontic treatment.
Patients' subjective preoperative spontaneous pain and preoperative percussion tenderness were recorded on a 10-cm visual analog scale (VAS) by the patients themselves. VAS values were measured using a ruler and recorded in millimeters. Pain of more than 60 mm on the scale was considered severe, and patients with severe pain and severe percussion tenderness were included in the study.
Patients who needed root canal treatment for only 1 tooth were included. Possible confounding factors (periodontal diseases, previously treated teeth, etc.) were accounted for via the inclusion criteria of the study. In the statistical analyses, the factors considered to be potential confounders were age, sex, analgesic intake, the vitality of the tooth, and the tooth number. The number of cases in the area during the study period was used to determine the sample size.
Saliva sampling
Patients refrained from eating, drinking, and brushing their teeth for 2 hours prior to sampling. Unstimulated whole saliva samples were obtained simply by asking patients to expectorate into polypropylene tubes (Eppendorf-Elkay, Shrewsbury, MA, USA). The desired volume (500 μL) of saliva was pipetted out in a polypropylene tube. To remove cell debris, saliva samples were centrifuged (EBA 20 Zentrifugen; Andreas Hettich GmbH, Tuttlingen, Germany) at 10,000 rpm for 6 minutes, and the clear, supernatant saliva was transferred to sterile polypropylene tubes and stored in a −80°C freezer (New Brunswick Scientific, New Brunswick, Canada) until the subsequent assays were performed.
Saliva samples were collected from each patient twice. The first sample was collected just before the administration of anesthesia (Ultracaine DS; Aventis Pharma, Istanbul, Turkey) for root canal treatment. Root canal treatments were performed at the first visit by a single operator, and patients were recalled after 1 week. The levels of spontaneous pain on the first, third, fifth, and seventh days were recorded on a VAS scale by the patients. The second saliva sample was collected at a control visit 1 week after root canal treatment. At this session, percussion tenderness was recorded on a VAS scale.
Determination of salivary SP and CGRP levels
All saliva samples collected before and after root canal treatment were analyzed at the same time, and laboratory personnel were unable to distinguish among the samples. The measurement of CGRP and SP levels in the saliva samples was carried out using enzyme-linked immune assay (ELISA) kits (Cayman, Ann Arbor, MI, USA). The frozen saliva samples were thawed until they reached room temperature. The amounts of sample used for each assay were determined according to the manufacturer's recommendation.
For the measurement of SP, 50 μL of each sample was pipetted into microplate wells (Cayman), which were pre-coated with mouse anti-immunoglobulin G antibody. Then, 50 μL of SP acetylcholinesterase tracer and ELISA antiserum were added to the wells. The wells were incubated overnight at 4°C and washed before adding the Ellman reagent to facilitate the development of the samples. The samples were allowed to develop for 90 minutes on an orbital shaker (Thermo Fisher Scientific, Waltham, MA, USA) in darkness. The absorbance levels of the developed plate were read with a microplate reader (Multiskan GO Microplate Spectrophotometer; Thermo Fisher Scientific) at 405 and 420 nm. The SP concentrations of the saliva samples were calculated according to the manufacturer's recommendations with the help of SP ELISA standards.
To calculate CGRP levels, similar steps were followed. First, 100 μL of each saliva sample was pipetted into the microplate wells, which were pre-coated with CGRP monoclonal antibody. The samples were incubated overnight at 4°C with the same amount of acetylcholinesterase tracer as was used in the measurement of SP concentration. Afterwards, the Ellman reagent was added to facilitate the development of the samples for spectrophotometric analysis, and those samples were incubated for 45 minutes. After the samples achieved the proper yellow color, the plates were read at 405 and 414 nm for the calculation of CGRP concentrations, per the provider's instructions.
Instead of including a control group of patients who did not have symptomatic apical periodontitis, 2 samples (before root canal treatment and 1 week after root canal treatment) were collected from each participant. Therefore, each participant served as his or her own control.
Statistical analysis
SPSS 20.0 (IBM Corp., Armonk, NY, USA) software was used for all statistical analyses. The Mann-Whitney U test was used to analyze the differences between the preoperative and postoperative salivary levels of SP and CGRP (p = 0.05), while the paired samples t-test was used to assess the differences between the preoperative and postoperative VAS scores of patients' subjective pain and percussion tenderness (p = 0.05). Pearson correlation analysis was used to determine any correlations among salivary levels of SP/CGRP and VAS scores of patients' subjective pain/percussion tenderness. Linear regression analysis was used to determine the confounding variables (age, sex, vitality, and tooth number) that affected the preoperative and postoperative salivary levels of CGRP and SP.
RESULTS
A total of 802 patients were referred to the clinic for root canal treatment. Of these patients, 308 declined to participate in the study, and 494 were assessed for eligibility. Of these individuals, 308 patients did not have mandibular molars requiring root canal treatment, and 174 were excluded because they did not meet the inclusion criteria (due to systemic disease [
n = 42], previously endodontically treated teeth [
n = 12], analgesic intake in the last 3 days [
n = 98], or periodontal disease [
n = 22]). No patients were lost to follow-up. Therefore, data from 12 patients were analyzed as shown in
Figure 1.
Figure 1 Flow diagram.
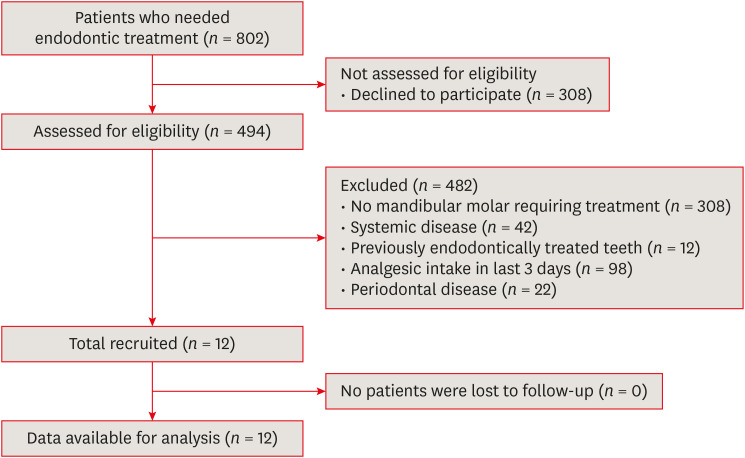
Eight men and 4 women were enrolled in this study. Nine teeth had vital pulp, while 3 teeth had nonvital pulp. The mean age of the patients was 26.33 ± 5.91 years (range, 18–39 years). The postoperative VAS scores of subjective percussion tenderness were significantly lower than the preoperative scores (
p < 0.001), and the VAS scores of subjective pain were also significantly lower than the preoperative scores (
p < 0.001) (
Table 1).
Table 1 Means and standard deviations of preoperative and postoperative VAS scores of pain and percussion tenderness (both in mm)
|
VAS Scores |
Preoperative |
Postoperative |
p value |
Effect size |
|
Patients' subjective pain |
83.25 ± 10.93 |
1.75 ± 3.1 |
< 0.001 |
8.150 |
|
Patients' subjective percussion tenderness |
73.25 ± 14.89 |
9.25 ± 18.06 |
< 0.001 |
2.370 |
The minimum and maximum preoperative salivary levels of SP were 9.58 and 183.24 pg/mL, respectively, while the minimum and maximum postoperative SP levels were 3.52 and 44.92 pg/mL, respectively. The minimum and maximum preoperative salivary levels of CGRP were 36.46 and 42.88 pg/mL, respectively, while the minimum and maximum postoperative CGRP levels were 36.79 and 39.54 pg/mL, respectively. The postoperative salivary level of SP was significantly lower than the preoperative salivary level (
p = 0.005). However, the postoperative salivary level of CGRP was similar to the preoperative level (
p = 0.932) (
Table 2).
Table 2 Means and standard deviations of preoperative and postoperative salivary levels of SP and CGRP (in pg/mL)
|
Neuropeptides |
Preoperative salivary level |
Postoperative salivary level |
p value |
Effect size |
|
SP |
50.91 ± 48.47 |
16.07 ± 13.47 |
0.005 |
0.835 |
|
CGRP |
38.17 ± 1.79 |
37.71 ± 0.83 |
0.932 |
0.295 |
The Pearson correlation analysis revealed that the VAS scores of subjective pain were positively correlated with salivary levels of SP (
r = 0.421;
p = 0.040) (
Table 3). No statistically significant correlations were observed between the salivary level of CGRP and the VAS score for subjective percussion tenderness (
p = 0.533) or the VAS score for subjective pain (
p = 0.459) (
Table 3).
Table 3 Correlation coefficients between parameters analyzed in this study
|
Variables |
SP |
CGRP |
Pain |
Percussion tenderness |
|
SP |
- |
0.176 |
0.421*
|
0.349 |
|
CGRP |
- |
- |
0.159 |
0.134 |
|
Pain |
- |
- |
- |
0.906*
|
|
Percussion tenderness |
- |
- |
- |
- |
Linear regression analysis was used to determine the confounding variables (age, sex, vitality, and tooth number) that affected the preoperative and postoperative salivary levels of CGRP and SP. The results of the regression analysis regarding the preoperative SP level showed that the
p-value of age as a potential confounder was 0.016 (
Table 4), while no variables were found to be significant confounders with regard to the postoperative SP level (
Table 5).
Table 4 Results of logistic regression analysis with regard to preoperative SP levels
|
Potential confounders |
Standardized coefficient |
Unstandardized coefficient |
Standard error |
p value |
Effect on preoperative SP |
|
95% CI low |
95% CI high |
|
Age |
−0.699 |
−5.729 |
1.807 |
0.016 |
−10.002 |
−1.455 |
|
Sex |
−0.226 |
−22.250 |
27.567 |
0.446 |
−87.436 |
42.935 |
|
Vitality |
−0.143 |
−15.353 |
24.432 |
0.550 |
−73.124 |
42.419 |
|
Tooth number |
0.668 |
6.075 |
2.588 |
0.051 |
−0.045 |
12.194 |
Table 5 Results of logistic regression analysis with regard to postoperative SP levels
|
Potential confounders |
Standardized coefficient |
Unstandardized coefficient |
Standard error |
p value |
Effect on postoperative SP |
|
95% CI low |
95% CI high |
|
Age |
−0.440 |
−1.004 |
0.779 |
0.239 |
−2.846 |
0.839 |
|
Sex |
−0.194 |
−5.295 |
11.882 |
0.669 |
−33.393 |
22.802 |
|
Vitality |
−0.207 |
−6.158 |
10.531 |
0.577 |
−31.059 |
18.744 |
|
Tooth number |
0.459 |
1.160 |
1.115 |
0.333 |
−1.478 |
3.797 |
The results of the regression analysis regarding both preoperative and postoperative CGRP levels also showed that none of the variables were significant confounders (
Tables 6 and
7). The effect size for SP was determined to be 5.755, while the effect size for CGRP was 0.643. The observed power of the present study with regard to salivary levels of SP was 0.631, while the observed power with regard to salivary CGRP levels was 0.120.
Table 6 Results of logistic regression analysis with regard to preoperative CGRP levels
|
Potential confounders |
Standardized coefficient |
Unstandardized coefficient |
Standard error |
p value |
Effect on preoperative CGRP |
|
95% CI low |
95% CI high |
|
Age |
−0.443 |
−0.134 |
0.100 |
0.221 |
−0.370 |
0.102 |
|
Sex |
−0.180 |
−0.654 |
1.522 |
0.680 |
−4.254 |
2.945 |
|
Vitality |
0.266 |
1.055 |
1.349 |
0.460 |
−2.136 |
4.245 |
|
Tooth number |
0.594 |
0.200 |
0.143 |
0.205 |
−0.138 |
0.537 |
Table 7 Results of logistic regression analysis with regard to postoperative CGRP levels
|
Potential confounders |
Standardized coefficient |
Unstandardized coefficient |
Standard error |
p value |
Effect on postoperative CGRP |
|
95% CI low |
95% CI high |
|
Age |
0.136 |
0.019 |
0.055 |
0.736 |
−0.111 |
0.150 |
|
Sex |
−0.438 |
−0.746 |
0.840 |
0.404 |
−2.731 |
1.240 |
|
Vitality |
0.143 |
0.265 |
0.744 |
0.732 |
−1.495 |
2.025 |
|
Tooth number |
0.280 |
0.044 |
0.079 |
0.594 |
−0.142 |
0.230 |
DISCUSSION
We hypothesized that no significant difference would exist between preoperative and postoperative SP and CGRP levels in human saliva. This null hypothesis was rejected. The results showed that the postoperative salivary level of SP was lower than the preoperative level of that substance; however, no significant difference was observed between preoperative and postoperative CGRP levels.
The decrease in salivary SP levels after root canal treatment may be associated with a reduction in peripheral neuropeptide release. SP, released from sensory nervous fibers, is a neurotransmitter that plays an important role in the transmission and production of moderate to intense pain signals [
16]. The increased levels of preoperative SP in the saliva of patients with symptomatic apical periodontitis may indicate an association with pulpal inflammation and pain. In this context, the findings of the present study revealed that the VAS score of subjective pain was positively correlated with the salivary level of SP. Evidence supports an immunoregulatory influence of salivary neuropeptides. One report suggested that the salivary glands may be a key organ in the neuroimmunoregulatory network because they play a role in regulating the mucosal immune/inflammatory response and in regeneration and healing [
17]. After the stimulation of sensory nerves (mainly C and Aδ fibers), SP and other neuropeptides are released from peripheral nerve endings. This process results in vasodilatation, increased microvascular permeability, and plasma extravasation, leading to neurogenic inflammation [
18,
19]. SP also plays an important role in neurogenic inflammation; a mouse study showed that when the preprotachykinin A gene, which encodes SP, was disrupted, the mice did not exhibit neurogenic inflammation [
20].
SP is released via the activation of transient receptor potential vanilloid 1 (TRPV1), which is a member of the transient receptor potential gene family of ion channel subunits [
21]. TRPV1 is highly expressed in the dorsal root ganglia, trigeminal ganglia, small sensory C fibers, and some Aδ fibers. It is also detectable in some organs of the nervous system, such as the brain and spinal cord [
22]. The significant difference observed between the preoperative and postoperative salivary levels of SP suggests that the release of SP may occur via the activation of TRPV1 at the level of the trigeminal ganglia or above. Animal experiments have shown that the salivary glands are supplied with SP-containing fibers; the parotid gland is innervated by the auriculotemporal nerve, and the submandibular and sublingual glands are innervated by the chorda tympani [
23,
24]. Activation of TRPV1 at the trigeminal ganglia may cause the release of SP from branches of the trigeminal nerve. The auriculotemporal nerve is a branch of the trigeminal nerve. Activation of TRPV1 at higher levels than the trigeminal ganglia, such as the dorsal root ganglia or part of the brain, may cause the release of SP from additional nerve fibers. SP may enter the saliva after being released from nerve terminals in and around the salivary glands [
25]. Neurogenic inflammation and changes in the salivary level of SP may occur in this manner.
The activation of TRPV1 may also lead to increased levels of SP in the gingival crevicular fluid, because the gingiva is innervated by branches of the trigeminal nerve. SP has been reported to be present in significantly greater amounts in the gingival crevicular fluid of painful teeth compared with healthy teeth [
26]. In the same study, it was suggested that the activation of TRPV1 is 1-sided [
26]. In the present study, the increased level of SP in the saliva of patients with symptomatic apical periodontitis may occur due to the 1-sided activation and release of TRPV1 and SP, respectively. Further investigations in which saliva is obtained directly from the salivary glands are needed. Saliva contains a large proportion of gingival crevicular fluid [
27], which may be another reason for the high salivary SP level observed in the present study.
Another result of the present study was the lack of a significant difference observed between preoperative and postoperative CGRP levels. CGRP is co-localized with SP, and these 2 neuropeptides may be released simultaneously in response to noxious stimuli such as pain or inflammation [
28]. The result of the present study does not contrast with the co-localization of the 2 peptides in the same neurons. Indeed, it is clear that SP and CGRP are partly located in different subpopulations of fibers [
12]. The carboxypeptidase enzyme has been detected in both the saliva and the gingival crevicular fluid [
29,
30], and selective inactivation of CGRP but not SP by this enzyme may occur. This mechanism for the accelerated degradation of CGRP in the saliva of patients with symptomatic apical periodontitis may explain the absence of a significant difference between preoperative and postoperative levels of CGRP.
According to our search of the literature, no study has evaluated SP and CGRP levels in human saliva pre- and post-endodontic treatment. Therefore, this is the first study to evaluate the effect of endodontic treatment on SP and CGRP levels in human saliva, and a direct comparison with other studies is not possible. In a previous study, SP levels in the saliva of patients with dental pain were compared to those of patients without pain, and the salivary concentrations of SP were reported to be significantly higher in patients with dental pain than in patients without pain [
31]. Although SP levels in human saliva before and after endodontic treatment were not evaluated in that study, its results are consistent with the results of the present study. In another study, the expression of CGRP and SP was quantified, and the expression of these neuropeptides was reported to be significantly higher in inflamed human dental pulp than in healthy pulp [
32]. Although that study examined pulp tissue, its results are consistent with the SP results but not with the CGRP results of our study. In yet another study, amounts of SP and CGRP in gingival crevicular fluid were investigated, and it was concluded that, while SP is present in significantly greater amounts in the gingival crevicular fluid of painful teeth than in that of healthy teeth, the amounts of CGRP were not significantly different [
26]. The results of that study align with the findings of the present study.
Results of the regression analysis with regard to preoperative SP level showed a significant difference in terms of age (
p = 0.016). This result can be attributed to decreased SP expression in the nerves of older patients [
33]. In contrast, the results of the regression analysis for postoperative SP level showed no significant difference based on age. This may be due to the reduction in SP expression that had already taken place postoperatively.
The results of the regression analysis showed no significant difference in terms of pulpal status. Thus, it can be claimed that the main source of SP in saliva is not the pulp. However, this claim should be supported with further studies with larger sample sizes.
It has been reported that the quantity of neurochemical markers in tissues affected by periodontal disease suggests a possible role for neuropeptides in the pathogenesis of periodontitis [
34,
35]. To avoid any impact of periodontal disease on our results, patients with periodontal disease were excluded from the present study.
According to our search of the literature, no study has yet evaluated the effect of caries on the salivary levels of SP or CGRP. In addition, the incidence of caries is high. Therefore, in the present study, the presence of caries was not an exclusion criterion.
To gain a better understanding of the effects of TRPV1 and carboxypeptidase on the salivary levels of SP and CGRP, studies are needed to evaluate this receptor and enzyme. One limitation of the present study was that TRPV1 and carboxypeptidase levels were not evaluated. It is possible to collect saliva via electrical drainage, which allows collection directly from the salivary glands [
12]. This method may also allow the exclusion of gingival crevicular fluid; therefore, not having used this method is another limitation of the present study. Additionally, pain is subjective, and the patients being treated expect that their pain will decrease [
36]. Together, these may cause bias, which is another limitation of the present study.
The molar amount of neuropeptides produced over time (in pmol/min) can increase with salivary stimulation. However, the concentration of neuropeptides (in pmol/L) is diluted by the increased volume of saliva and is also affected by the mode of stimulation [
37]. Thus, in the present study, resting (or unstimulated) saliva was collected to prevent the effect of the stimulation mode on the levels of SP and CGRP in saliva. Salivary flow rate can vary from person to person. Some systemic diseases, such as autoimmune diseases (Sjögren syndrome, AIDS, lupus erythematosus), hormonal diseases (diabetes mellitus), neurological diseases (Parkinson disease), and psychogenic diseases (depression), can be associated with hyposalivation [
38]. In addition to this effect of systemic diseases, medications such as antidepressants, diuretics, and antihistamines can affect salivary secretion [
38]. Therefore, patients who had systemic disease or who were using drugs or medications within 3 days were not included in the present study. However, according to our literature search, no study has evaluated the effect of salivary flow rate on the levels of neuropeptides in saliva. Further studies of the effect of salivary flow rate are required.
As this was a pilot study, we first evaluated 12 patients with mandibular molars with symptomatic apical periodontitis. While the sample size used in the present study is similar to those of previous pilot studies [
39,
40], it remains a limitation of the present study. However, despite the small sample size, a clear significant difference was observed.
Many studies have evaluated the levels of neuropeptides such as SP and CGRP in the saliva in various pain conditions [
12,
13,
14,
31,
41]. It The salivary level of SP in patients with tension‐type headaches during active headache periods has been reported to be significantly higher than that in healthy controls [
41]. Salivary SP concentrations have also been shown to be higher in patients with dental pain than in those without such pain [
31]. In light of the results of these studies and of the present study, we conclude that the levels of neuropeptides in saliva may be diagnostic parameters that help detect the type or the location of pain in the future.
CONCLUSIONS
In this pilot study, we evaluated the effect of endodontic treatment on SP and CGRP levels in the saliva of patients with symptomatic apical periodontitis. According to the results, we conclude that salivary SP level may be used as an objective indicator in the diagnosis and determination of the degree of pain in endodontic diseases.
-
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
-
Author Contributions:
Conceptualization: Arslan H, Köseoğlu S.
Data curation: Arslan H, Doğanay Yıldız E.
Formal analysis: Arslan H.
Methodology: Arslan H, Doğanay Yıldız E, Köseoğlu S.
Writing - original draft: Arslan H, Doğanay Yıldız E, Köseoğlu S.
Writing - review & editing: Arslan H, Doğanay Yıldız E, Köseoğlu S.
REFERENCES
- 1. Stashenko P, Teles R, D'Souza R. Periapical inflammatory responses and their modulation. Crit Rev Oral Biol Med 1998;9:498-521.ArticlePubMedPDF
- 2. Caviedes-Bucheli J, Muñoz HR, Azuero-Holguín MM, Ulate E. Neuropeptides in dental pulp: the silent protagonists. J Endod 2008;34:773-788.ArticlePubMed
- 3. Siqueira JF Jr, Rôças IN, Favieri A, Machado AG, Gahyva SM, Oliveira JC, Abad EC. Incidence of postoperative pain after intracanal procedures based on an antimicrobial strategy. J Endod 2002;28:457-460.ArticlePubMed
- 4. Steinhoff M, Ständer S, Seeliger S, Ansel JC, Schmelz M, Luger T. Modern aspects of cutaneous neurogenic inflammation. Arch Dermatol 2003;139:1479-1488.ArticlePubMed
- 5. Caviedes-Bucheli J, Rojas P, Escalona M, Estrada A, Sandoval C, Rivero C, Lombana N, Muñoz HR. The effect of different vasoconstrictors and local anesthetic solutions on substance P expression in human dental pulp. J Endod 2009;35:631-633.ArticlePubMed
- 6. Roosterman D, Goerge T, Schneider SW, Bunnett NW, Steinhoff M. Neuronal control of skin function: the skin as a neuroimmunoendocrine organ. Physiol Rev 2006;86:1309-1379.ArticlePubMed
- 7. Peters EM, Ericson ME, Hosoi J, Seiffert K, Hordinsky MK, Ansel JC, Paus R, Scholzen TE. Neuropeptide control mechanisms in cutaneous biology: physiological and clinical significance. J Invest Dermatol 2006;126:1937-1947.ArticlePubMed
- 8. N'Diaye A, Gannesen A, Borrel V, Maillot O, Enault J, Racine PJ, Plakunov V, Chevalier S, Lesouhaitier O, Feuilloley MG. Substance P and calcitonin gene-related peptide: key regulators of cutaneous microbiota homeostasis. Front Endocrinol (Lausanne) 2017;8:15.PubMedPMC
- 9. Fischer HP, Eich W, Russell IJ. A possible role for saliva as a diagnostic fluid in patients with chronic pain. Semin Arthritis Rheum 1998;27:348-359.ArticlePubMed
- 10. Lee YH, Wong DT. Saliva: an emerging biofluid for early detection of diseases. Am J Dent 2009;22:241-248.PubMedPMC
- 11. Li Y, Denny P, Ho CM, Montemagno C, Shi W, Qi F, Wu B, Wolinsky L, Wong DT. The Oral Fluid MEMS/NEMS Chip (OFMNC): diagnostic and translational applications. Adv Dent Res 2005;18:3-5.ArticlePubMedPDF
- 12. Nicolodi M, Del Bianco E. Sensory neuropeptides (substance P, calcitonin gene-related peptide) and vasoactive intestinal polypeptide in human saliva: their pattern in migraine and cluster headache. Cephalalgia 1990;10:39-50.ArticlePubMedPDF
- 13. Borelli V, Marchioli A, Di Taranto R, Romano M, Chiandussi S, Di Lenarda R, Biasotto M, Zabucchi G. Neuropeptides in saliva of subjects with burning mouth syndrome: a pilot study. Oral Dis 2010;16:365-374.ArticlePubMed
- 14. Zidverc-Trajkovic J, Stanimirovic D, Obrenovic R, Tajti J, Vécsei L, Gardi J, Németh J, Mijajlovic M, Sternic N, Jankovic L. Calcitonin gene-related peptide levels in saliva of patients with burning mouth syndrome. J Oral Pathol Med 2009;38:29-33.ArticlePubMed
- 15. Haririan H, Andrukhov O, Böttcher M, Pablik E, Wimmer G, Moritz A, Rausch-Fan X. Salivary neuropeptides, stress, and periodontitis. J Periodontol 2018;89:9-18.ArticlePubMed
- 16. Cao YQ, Mantyh PW, Carlson EJ, Gillespie AM, Epstein CJ, Basbaum AI. Primary afferent tachykinins are required to experience moderate to intense pain. Nature 1998;392:390-394.ArticlePubMedPDF
- 17. Sabbadini E, Berczi I. The submandibular gland: a key organ in the neuro-immuno-regulatory network? Neuroimmunomodulation 1995;2:184-202.ArticlePubMedPDF
- 18. Lembeck F, Holzer P. Substance P as neurogenic mediator of antidromic vasodilation and neurogenic plasma extravasation. Naunyn Schmiedebergs Arch Pharmacol 1979;310:175-183.ArticlePubMedPDF
- 19. Mashaghi A, Marmalidou A, Tehrani M, Grace PM, Pothoulakis C, Dana R. Neuropeptide substance P and the immune response. Cell Mol Life Sci 2016;73:4249-4264.ArticlePubMedPMCPDF
- 20. De Felipe C, Herrero JF, O'Brien JA, Palmer JA, Doyle CA, Smith AJ, Laird JM, Belmonte C, Cervero F, Hunt SP. Altered nociception, analgesia and aggression in mice lacking the receptor for substance P. Nature 1998;392:394-397.ArticlePubMedPDF
- 21. Bessac BF, Jordt SE. Breathtaking TRP channels: TRPA1 and TRPV1 in airway chemosensation and reflex control. Physiology (Bethesda) 2008;23:360-370.ArticlePubMedPMC
- 22. Gunthorpe MJ, Benham CD, Randall A, Davis JB. The diversity in the vanilloid (TRPV) receptor family of ion channels. Trends Pharmacol Sci 2002;23:183-191.ArticlePubMed
- 23. Ekström J, Brodin E, Ekman R, Håkanson R, Sundler F. Vasoactive intestinal peptide and substance P in salivary glands of the rat following denervation or duct ligation. Regul Pept 1984;10:1-10.ArticlePubMed
- 24. de Paula F, Teshima TH, Hsieh R, Souza MM, Nico MM, Lourenco SV. Overview of human salivary glands: highlights of morphology and developing processes. Anat Rec (Hoboken) 2017;300:1180-1188.ArticlePubMedPDF
- 25. Konttinen YT, Hukkanen M, Kemppinen P, Segerberg M, Sorsa T, Malmström M, Rose S, Itescu S, Polak JM. Peptide-containing nerves in labial salivary glands in Sjögren's syndrome. Arthritis Rheum 1992;35:815-820.ArticlePubMed
- 26. Awawdeh LA, Lundy FT, Linden GJ, Shaw C, Kennedy JG, Lamey PJ. Quantitative analysis of substance P, neurokinin A and calcitonin gene-related peptide in gingival crevicular fluid associated with painful human teeth. Eur J Oral Sci 2002;110:185-191.ArticlePubMedPDF
- 27. Taylor JJ, Preshaw PM. Gingival crevicular fluid and saliva. Periodontol 2000 2016;70:7-10.ArticlePubMed
- 28. Schlereth T, Schukraft J, Krämer-Best HH, Geber C, Ackermann T, Birklein F. Interaction of calcitonin gene related peptide (CGRP) and substance P (SP) in human skin. Neuropeptides 2016;59:57-62.ArticlePubMed
- 29. Albo F, Antonangeli R, Cavazza A, Marini M, Roda LG, Rossi P. Neuropeptide enzyme hydrolysis in allergic human saliva. Peptides 2002;23:185-192.ArticlePubMed
- 30. Lundy FT, Salmon AL, Lamey PJ, Shaw C, Linden GJ. Carboxypeptidase-mediated metabolism of calcitonin gene-related peptide in human gingival crevicular fluid--a rôle in periodontal inflammation? J Clin Periodontol 2000;27:499-505.ArticlePubMedPDF
- 31. Ahmad M, Williams J, Al-Abbousi R, Wheater M. Substance P concentration in saliva of patients who report dental pain. J Adv Oral Res 2014;5:1-5.ArticlePDF
- 32. Caviedes-Bucheli J, Lombana N, Azuero-Holguín MM, Munoz HR. Quantification of neuropeptides (calcitonin gene-related peptide, substance P, neurokinin A, neuropeptide Y and vasoactive intestinal polypeptide) expressed in healthy and inflamed human dental pulp. Int Endod J 2006;39:394-400.ArticlePubMed
- 33. Marco B, Alessandro R, Philippe F, Fabio B, Paolo R, Giulio F. The effect of aging on nerve morphology and substance P expression in mouse and human corneas. Invest Ophthalmol Vis Sci 2018;59:5329-5335.ArticlePubMed
- 34. Luthman J, Friskopp J, Dahllöf G, Ahlström U, Sjöström L, Johansson O. Immunohistochemical study of neurochemical markers in gingiva obtained from periodontitis-affected sites. J Periodontal Res 1989;24:267-278.ArticlePubMed
- 35. Bartold PM, Kylstra A, Lawson R. Substance P: an immunohistochemical and biochemical study in human gingival tissues. A role for neurogenic inflammation? J Periodontol 1994;65:1113-1121.ArticlePubMedPDF
- 36. Bender IB. Pulpal pain diagnosis--a review. J Endod 2000;26:175-179.ArticlePubMed
- 37. Dawidson I, Blom M, Lundeberg T, Theodorsson E, Angmar-Månsson B. Neuropeptides in the saliva of healthy subjects. Life Sci 1997;60:269-278.PubMed
- 38. Tschoppe P, Wolgin M, Pischon N, Kielbassa AM. Etiologic factors of hyposalivation and consequences for oral health. Quintessence Int 2010;41:321-333.PubMed
- 39. Alobaid AS, Cortes LM, Lo J, Nguyen TT, Albert J, Abu-Melha AS, Lin LM, Gibbs JL. Radiographic and clinical outcomes of the treatment of immature permanent teeth by revascularization or apexification: a pilot retrospective cohort study. J Endod 2014;40:1063-1070.ArticlePubMedPMC
- 40. Yazdanfar I, Gutknecht N, Franzen R. Effects of diode laser on direct pulp capping treatment: a pilot study. Lasers Med Sci 2015;30:1237-1243.ArticlePubMedPDF
- 41. Marukawa H, Shimomura T, Takahashi K. Salivary substance P, 5-hydroxytryptamine, and gamma-aminobutyric acid levels in migraine and tension-type headache. Headache 1996;36:100-104.ArticlePubMed
 , Ezgi Doğanay Yıldız2
, Ezgi Doğanay Yıldız2 , Serhat Köseoğlu3
, Serhat Köseoğlu3


 KACD
KACD

 ePub Link
ePub Link Cite
Cite

