Articles
- Page Path
- HOME > Restor Dent Endod > Volume 45(3); 2020 > Article
- Invited Review Article The effect of root canal irrigants on dentin: a focused review
-
Priti Pragati Rath1
 , Cynthia Kar Yung Yiu2
, Cynthia Kar Yung Yiu2 , Jukka Pekka Matinlinna3
, Jukka Pekka Matinlinna3 , Anil Kishen4,5
, Anil Kishen4,5 , Prasanna Neelakantan1
, Prasanna Neelakantan1
-
Restor Dent Endod 2020;45(3):e39.
DOI: https://doi.org/10.5395/rde.2020.45.e39
Published online: June 30, 2020
1Discipline of Endodontology, Division of Restorative Dental Sciences, Faculty of Dentistry, The University of Hong Kong, Hong Kong SAR.
2Discipline of Pediatric Dentistry, Division of Pediatric Dentistry and Orthodontics, Faculty of Dentistry, The University of Hong Kong, Hong Kong SAR.
3Dental Materials Science, Division of Applied Oral Sciences and Community Dental Care, Faculty of Dentistry, The University of Hong Kong, Hong Kong SAR.
4Faculty of Dentistry, The University of Toronto, Toronto, ON, Canada.
5Dentistry, Mount Sinai Hospital, Toronto, ON, Canada.
- Correspondence to Prasanna Neelakantan, MDS, PhD. Clinical Assistant Professor, Discipline of Endodontology, Division of Restorative Dental Sciences, Faculty of Dentistry, The University of Hong Kong, The Prince Philip Dental Hospital, 34 Hospital Road, Sai Ying Pun, Hong Kong SAR. prasanna@hku.hk
Copyright © 2020. The Korean Academy of Conservative Dentistry
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 5,127 Views
- 104 Download
- 45 Crossref
Abstract
- Despite the vast literature on the effects of root canal irrigants on the dentin characteristics, the precise effects of clinically relevant irrigation sequences remain unclear. In this review, we systematically dissect the role of different sequential irrigation approaches that are used in clinical endodontics. Using a systematic search strategy, we attempt to answer the question: ‘Which irrigating sequence has the most deleterious effects on dentin structure and properties?’ The effect of irrigants on the dentin composition and mechanical properties have been reviewed. A wide variety of concentrations, duration and techniques have been employed to characterize the effects of chemicals on dentin properties, thus making it impossible to draw guidelines or recommendations of irrigant sequences to be followed clinically. It was apparent that all the studied irrigation sequences potentially result in some deleterious effects on dentin such as decrease in the flexural strength, microhardness, modulus of elasticity and inorganic content and organic-inorganic ratio of the dentin. However, the literature still lacks comprehensive investigations to compare the deleterious effect of different irrigation sequences, using a wide variety of qualitative and quantitative methods. Such investigations are essential to make clinical recommendations and strategize efforts to minimize chemically-induced damage to dentin characteristics.
INTRODUCTION
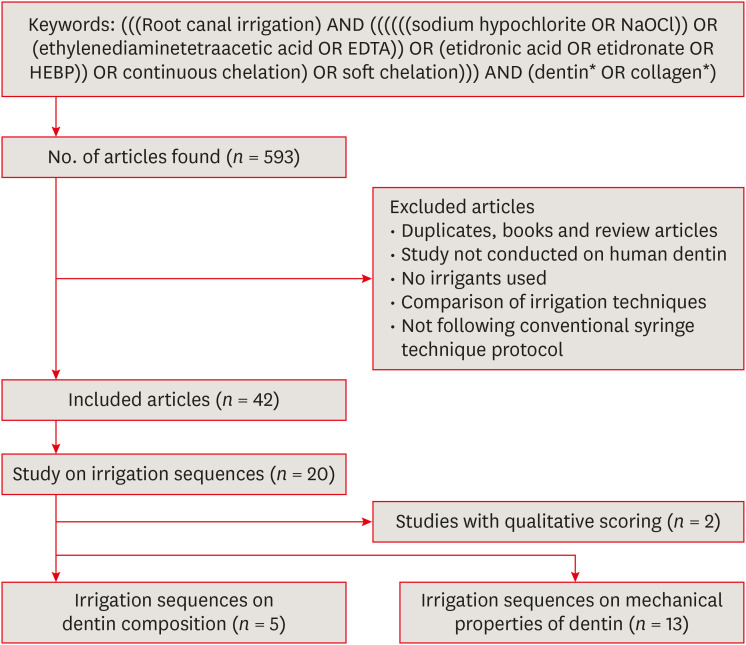
REVIEW
Search strategy and article selection for the review.
Effect of irrigation sequences on dentin composition
| No. | Author | Sample type | Groups | Outcome measure | Main findings | |
|---|---|---|---|---|---|---|
| Irrigation sequence | Duration (min)* | |||||
| 1 | Doğan et al. [33] | Root canal dentin | 17% EDTA/2.5% NaOCl | 15-? | Calcium/phosphate ratio | EDTA/NaOCl altered the Ca/P ratio and Mg level significantly when compared to the control group |
| 17% EDTA/saline | 15-? | |||||
| 2.5% NaOCl | ? | |||||
| Saline (control) | ? | |||||
| 2 | Sayin et al. [34] | Root canal dentin | 2.5% NaOCl | 1, 5 | Calcium loss | EDTA and EDTA/NaOCl treatment for 1 and 5 min showed significantly more calcium ion loss |
| 17% EDTA | 1, 5 | |||||
| 17% EDTA/2.5% NaOCl | 1-1, 5-5 | |||||
| Distilled water (control) | 1, 5 | |||||
| 3 | Wang et al. [35] | Root canal dentin | Control | Element distribution (EDS) | Reduced Ca and P values in NaOCl/EDTA/NaOCl group | |
| 3% NaOCl/8% EDTA | 5-2 | |||||
| 3% NaOCl/8% EDTA/3% NaOCl | 2-2-1 | |||||
| 5% NaOCl/17% EDTA | 2-2 | |||||
| 5% NaOCl/17% EDTA/5% NaOCl | 2-2-1 | |||||
| 5% NaOCl/17% EDTA/5% NaOCl | 5-5-5 | |||||
| 4 | Zhang et al. [36] | Dentin powder | Control–no treatment | - Apatite/collagen ratio (FTIR) | EDTA decreased the apatite/collagen ratio 5.25% NaOCl for more than 60 min showed a significant increase in apatite/collagen ratio and decrease in flexural strength | |
| 17% EDTA | 2 | - Flexural strength | ||||
| 1.3% NaOCl/17% EDTA | 10, 20, 30, 60, 120, 180, 240-2 | |||||
| 5.25% NaOCl/17% EDTA | 10, 20, 30, 60, 120, 180, 240-2 | |||||
| 5 | Zhang et al. [37] | Dentin powder | Control–no treatment | - Apatite/collagen ratio | - Significant increase in apatite/collagen ratio with time and concentration of NaOCl | |
| 17% EDTA | 2 | - Collagen degradation (FTIR) | - Tunneling erosion pattern with subsurface erosion with 5.25% NaOCl/EDTA | |||
| 1.3% NaOCl | 10, 20, 30, 60, 120, 180, 240 | |||||
| 1.3% NaOCl/17% EDTA | 10, 20, 30, 60, 120, 180, 240-2 | |||||
| 5.25% NaOCl | 10, 20, 30, 60, 120, 180, 240 | |||||
| 5.25% NaOCl/17% EDTA | 10, 20, 30, 60, 120, 180, 240-2 | |||||
Effect of irrigation sequences on mechanical properties of dentin
| No. | Author | Dentin sample type | Groups | Outcome measure | Main findings | |
|---|---|---|---|---|---|---|
| Irrigation sequence | Duration* | |||||
| 1 | Eldeniz et al. [39] | Root canal dentin | 17% EDTA/5.25% NaOCl | 150-150 sec | Microhardness | EDTA/NaOCl significantly reduced the microhardness compared to the control |
| DI water (control) | ||||||
| 2 | Akcay et al. [40] | Root canal dentin | 7.5% EDTA | 1 min | Microhardness | Unclear if the changes were significant relative to an untreated dentin control |
| 7.5% EDTA/2.5% NaOCl | 1-1 min | |||||
| 3 | Kara Tuncer et al. [41] | Root canal dentin | 17% EDTA/2.5% NaOCl | 1-1 min | Microhardness | Unclear if the changes were significant relative to an untreated dentin control |
| 4 | Saghiri et al. [42] | Root canal dentin | 2.6% NaOCl | 5 min | Microhardness at different depths | EDTA/NaOCl (5 min) group had a significant decrease in microhardness at 100 µm |
| 17% EDTA/2.6% NaOCl | 5-5 min | |||||
| 17% EDTA/2.6% NaOCl | 1-5 min | |||||
| Saline (control) | 5 min | |||||
| 5 | Aranda-Garcia et al. [43] | Root canal dentin | Distilled water/2.5% NaOCl (control) | 3-? min | Microhardness | Compared to the control group, EDTA/NaOCl group showed greater reduction in microhardness |
| 17% EDTA/2.5% NaOCl | 3-? min | |||||
| 6 | Ulusoy et al. [44] | Root canal dentin | 17% EDTA/2.5% NaOCl | 5-5 min | Microhardness | Significant reduction in microhardness in EDTA/NaOCl group, compared to the control group |
| 5% NaOCl | 5 min | |||||
| 0.9% saline (control) | 5 min | |||||
| 7 | Baldasso et al. [45] | Root canal dentin | 17% EDTA/2.5% NaOCl | 2-5 min | Microhardness at different depths | Microhardness of EDTA/NaOCl group was significantly reduced compared to control groups at 500 and 1,000 µm |
| 2.5% NaOCl (solution control) | 5 min | |||||
| Distilled water (negative control) | 5 min | |||||
| 8 | Zaparolli et al. [46] | Dentin at furcation region of pulp chamber | 1% NaOCl | 10 min | Microhardness at furcation area | EDTA and NaOCl/EDTA group showed significantly lesser microhardness values compare to control and NaOCl group |
| 17% EDTA | 10 min | |||||
| 1% NaOCl /17% EDTA | 10-10 min | |||||
| Distilled water (control) | 10 min | |||||
| 9 | Dineshkumar et al. [47] | Root canal dentin | Distilled water (control) | Microhardness | No statistical difference was observed between the groups NaOCl/EDTA and NaOCl/HEBP | |
| 1.3% NaOCl/17% EDTA | 20-1 min | |||||
| 1.3% NaOCl/18% HEBP | 20-5 min | |||||
| 10 | Ghisi et al. [48] | Root canal dentin | 2% NaOCl | 30 min | Microhardness at different depths | No significant difference in microhardness values between different depths and between the groups |
| 5% NaOCl | 30 min | |||||
| 17% EDTA | 30 min | |||||
| 2% NaOCl/17% EDTA | 30-5 min | |||||
| 5% NaOCl/17% EDTA | 30-5 min | |||||
| 11 | Mai et al. [49] | Dentin beams | Control | Flexural strength | 60 min exposure of NaOCl significantly decreased the flexural strength compared to other groups | |
| 5.25 NaOCl/17% EDTA | 10-2 min | |||||
| 5.25 NaOCl/17% EDTA | 60-2 min | |||||
| 12 | Cecchin et al. [50] | Dentin beam (flexural strength), hourglass shaped root dentin sections (ultimate tensile strength), instrumented root canals (fracture resistance) | DI water/17% EDTA (control) | 30-1 min | - Flexural strength | Significant decrease in flexural strength, ultimate tensile strength and fracture resistance of NaOCl/ EDTA compared to control |
| 6% NaOCl/17% EDTA | 30-1 min | - Ultimate Tensile Strength | ||||
| - Fracture resistance | ||||||
| 13 | Marending et al. [51] | Dentin beams | 2.5% NaOCl/17% EDTA/2.5% NaOCl/DI water | 21-3-3-3 min | - Modulus of elasticity | NaOCl associated with EDTA reduced the flexural strength significantly compared to the other groups |
| 2.5% NaOCl/DI water/2.5% NaOCl/17% EDTA | 21-3-3-3 min | - Flexural strength | ||||
| 2.5% NaOCl/DI water/2.5% NaOCl/DI water | 21-3-3-3 min | |||||
| DI water/EDTA/DI water | 21-3-6 min | |||||
| DI water | 30 min | |||||
AUTHORS' PERSPECTIVES
CONCLUSIONS
-
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
-
Author Contributions:
Conceptualization: Neelakantan P, Kishen A.
Data curation: Rath PP.
Formal analysis: Rath PP, Neelakantan P.
Supervision: Neelakantan P, Kishen A, Yiu CKY, Matinlinna JP.
Writing - original draft: Rath PP, Kishen A, Neelakantan P, Yiu CKY, Matinlinna JP.
Writing - review & editing: Rath PP, Neelakantan P, Yiu CKY, Matinlinna JP.
- 1. Marshall GW Jr. Dentin: microstructure and characterization. Quintessence Int 1993;24:606-617.PubMed
- 2. Tjäderhane L, Carrilho MR, Breschi L, Tay FR, Pashley DH. Dentin basic structure and composition—An overview. Endod Topics 2009;20:3-29.Article
- 3. Sloan AJ. Stem cell biology and tissue engineering in dental sciences. Amsterdam: Elsevier/Academic Press; 2015. p. Chapter 29.
- 4. Carda C, Peydró A. Ultrastructural patterns of human dentinal tubules, odontoblasts processes and nerve fibres. Tissue Cell 2006;38:141-150.ArticlePubMed
- 5. Nissan R, Segal H, Pashley D, Stevens R, Trowbridge H. Ability of bacterial endotoxin to diffuse through human dentin. J Endod 1995;21:62-64.ArticlePubMed
- 6. Love RM. Regional variation in root dentinal tubule infection by Streptococcus gordonii . J Endod 1996;22:290-293.ArticlePubMed
- 7. Wong DT, Cheung GS. Extension of bactericidal effect of sodium hypochlorite into dentinal tubules. J Endod 2014;40:825-829.ArticlePubMed
- 8. Berutti E, Marini R, Angeretti A. Penetration ability of different irrigants into dentinal tubules. J Endod 1997;23:725-727.ArticlePubMed
- 9. Ghorbanzadeh A, Aminsobhani M, Sohrabi K, Chiniforush N, Ghafari S, Shamshiri AR, Noroozi N. Penetration depth of sodium hypochlorite in dentinal tubules after conventional irrigation, passive ultrasonic agitation and Nd: YAG laser activated irrigation. J Lasers Med Sci 2016;7:105-111.ArticlePubMedPMC
- 10. Sulkala M, Tervahartiala T, Sorsa T, Larmas M, Salo T, Tjäderhane L. Matrix metalloproteinase-8 (MMP-8) is the major collagenase in human dentin. Arch Oral Biol 2007;52:121-127.ArticlePubMed
- 11. Martin-De Las Heras S, Valenzuela A, Overall CM. The matrix metalloproteinase gelatinase A in human dentine. Arch Oral Biol 2000;45:757-765.ArticlePubMed
- 12. Marashdeh MQ, Gitalis R, Lévesque C, Finer Y. Endodontic pathogens possess collagenolytic properties that degrade human dentine collagen matrix. Int Endod J 2019;52:416-423.ArticlePubMedPDF
- 13. Gu LS, Huang XQ, Griffin B, Bergeron BR, Pashley DH, Niu LN, Tay FR. Primum non nocere - The effects of sodium hypochlorite on dentin as used in endodontics. Acta Biomater 2017;61:144-156.ArticlePubMed
- 14. Siqueira JF Jr, Pérez AR, Marceliano-Alves MF, Provenzano JC, Silva SG, Pires FR, Vieira GC, Rôças IN, Alves FR. What happens to unprepared root canal walls: a correlative analysis using micro-computed tomography and histology/scanning electron microscopy. Int Endod J 2018;51:501-508.ArticlePubMedPDF
- 15. Peters OA, Arias A, Paqué F. A Micro–computed tomographic assessment of root canal preparation with a novel instrument, TRUShape, in mesial roots of mandibular molars. J Endod 2015;41:1545-1550.ArticlePubMed
- 16. Haapasalo M, Shen Y, Qian W, Gao Y. Irrigation in endodontics. Dent Clin North Am 2010;54:291-312.ArticlePubMed
- 17. Krause TA, Liewehr FR, Hahn CL. The antimicrobial effect of MTAD, sodium hypochlorite, doxycycline, and citric acid on Enterococcus faecalis. J Endod 2007;33:28-30.ArticlePubMed
- 18. Zehnder M. Root canal irrigants. J Endod 2006;32:389-398.ArticlePubMed
- 19. Sim TP, Knowles JC, Ng YL, Shelton J, Gulabivala K. Effect of sodium hypochlorite on mechanical properties of dentine and tooth surface strain. Int Endod J 2001;34:120-132.ArticlePubMedPDF
- 20. Slutzky-Goldberg I, Maree M, Liberman R, Heling I. Effect of sodium hypochlorite on dentin microhardness. J Endod 2004;30:880-882.ArticlePubMed
- 21. Uzunoglu E, Aktemur S, Uyanik MO, Durmaz V, Nagas E. Effect of ethylenediaminetetraacetic acid on root fracture with respect to concentration at different time exposures. J Endod 2012;38:1110-1113.ArticlePubMed
- 22. Gu XH, Mao CY, Kern M. Effect of different irrigation on smear layer removal after post space preparation. J Endod 2009;35:583-586.ArticlePubMed
- 23. Calt S, Serper A. Time-dependent effects of EDTA on dentin structures. J Endod 2002;28:17-19.ArticlePubMed
- 24. Grawehr M, Sener B, Waltimo T, Zehnder M. Interactions of ethylenediamine tetraacetic acid with sodium hypochlorite in aqueous solutions. Int Endod J 2003;36:411-417.ArticlePubMedPDF
- 25. Prado M, Gusman H, Gomes BP, Simão RA. Scanning electron microscopic investigation of the effectiveness of phosphoric acid in smear layer removal when compared with EDTA and citric acid. J Endod 2011;37:255-258.ArticlePubMed
- 26. Paqué F, Boessler C, Zehnder M. Accumulated hard tissue debris levels in mesial roots of mandibular molars after sequential irrigation steps. Int Endod J 2011;44:148-153.ArticlePubMed
- 27. Lottanti S, Gautschi H, Sener B, Zehnder M. Effects of ethylenediaminetetraacetic, etidronic and peracetic acid irrigation on human root dentine and the smear layer. Int Endod J 2009;42:335-343.ArticlePubMed
- 28. Ulusoy Öİ, Zeyrek S, Çelik B. Evaluation of smear layer removal and marginal adaptation of root canal sealer after final irrigation using ethylenediaminetetraacetic, peracetic, and etidronic acids with different concentrations. Microsc Res Tech 2017;80:687-692.PubMed
- 29. Ordinola-Zapata R, Bramante CM, Cavenago B, Graeff MS, Gomes de Moraes I, Marciano M, Duarte MA. Antimicrobial effect of endodontic solutions used as final irrigants on a dentine biofilm model. Int Endod J 2012;45:162-168.ArticlePubMed
- 30. Arias-Moliz MT, Ordinola-Zapata R, Baca P, Ruiz-Linares M, García García E, Hungaro Duarte MA, Monteiro Bramante C, Ferrer-Luque CM. Antimicrobial activity of chlorhexidine, peracetic acid and sodium hypochlorite/etidronate irrigant solutions against Enterococcus faecalis biofilms. Int Endod J 2015;48:1188-1193.ArticlePubMed
- 31. Neelakantan P, Sharma S, Shemesh H, Wesselink PR. Influence of irrigation sequence on the adhesion of root canal sealers to dentin: a fourier transform infrared spectroscopy and push-out bond strength analysis. J Endod 2015;41:1108-1111.ArticlePubMed
- 32. Dotto L, Sarkis Onofre R, Bacchi A, Rocha Pereira GK. Effect of root canal irrigants on the mechanical properties of endodontically treated teeth: a scoping review. J Endod 2020;46:596-604.e3.ArticlePubMed
- 33. Doğan H, Qalt S. Effects of chelating agents and sodium hypochlorite on mineral content of root dentin. J Endod 2001;27:578-580.ArticlePubMed
- 34. Sayin TC, Serper A, Cehreli ZC, Kalayci S. Calcium loss from root canal dentin following EDTA, EGTA, EDTAC, and tetracycline-HCl treatment with or without subsequent NaOCl irrigation. J Endod 2007;33:581-584.ArticlePubMed
- 35. Wang Z, Maezono H, Shen Y, Haapasalo M. Evaluation of root canal dentin erosion after different irrigation methods using energy-dispersive x-ray spectroscopy. J Endod 2016;42:1834-1839.ArticlePubMed
- 36. Zhang K, Kim YK, Cadenaro M, Bryan TE, Sidow SJ, Loushine RJ, Ling JQ, Pashley DH, Tay FR. Effects of different exposure times and concentrations of sodium hypochlorite/ethylenediaminetetraacetic acid on the structural integrity of mineralized dentin. J Endod 2010;36:105-109.PubMed
- 37. Zhang K, Tay FR, Kim YK, Mitchell JK, Kim JR, Carrilho M, Pashley DH, Ling JQ. The effect of initial irrigation with two different sodium hypochlorite concentrations on the erosion of instrumented radicular dentin. Dent Mater 2010;26:514-523.ArticlePubMed
- 38. Baldasso FE, Cardoso LR, Silva VD, Morgental RD, Kopper PM. Evaluation of the effect of four final irrigation protocols on root canal dentin components by polarized light microscopy and scanning electron microscopy. Microsc Res Tech 2017;80:1337-1343.ArticlePubMedPDF
- 39. Eldeniz AU, Erdemir A, Belli S. Effect of EDTA and citric acid solutions on the microhardness and the roughness of human root canal dentin. J Endod 2005;31:107-110.ArticlePubMed
- 40. Akcay I, Erdilek N, Sen BH. The efficacy of an experimental single solution versus alternate use of multiple irrigants on root dentin microhardness. J Clin Exp Dent 2013;5:e83-e88.ArticlePubMedPMC
- 41. Kara Tuncer A, Tuncer S, Siso SH. Effect of QMix irrigant on the microhardness of root canal dentine. Aust Dent J 2015;60:163-168.PubMed
- 42. Saghiri MA, Delvarani A, Mehrvarzfar P, Malganji G, Lotfi M, Dadresanfar B, Saghiri AM, Dadvand S. A study of the relation between erosion and microhardness of root canal dentin. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009;108:e29-e34.Article
- 43. Aranda-Garcia AJ, Kuga MC, Chavéz-Andrade GM, Kalatzis-Sousa NG, Hungaro Duarte MA, Faria G, Reis Só MV, Faria NB Jr. Effect of final irrigation protocols on microhardness and erosion of root canal dentin. Microsc Res Tech 2013;76:1079-1083.ArticlePubMed
- 44. Ulusoy Öİ, Görgül G. Effects of different irrigation solutions on root dentine microhardness, smear layer removal and erosion. Aust Endod J 2013;39:66-72.ArticlePubMed
- 45. Baldasso FE, Roleto L, da Silva VD, Morgental RD, Kopper PM. Effect of final irrigation protocols on microhardness reduction and erosion of root canal dentin. Br Oral Res 2017;31:e40.Article
- 46. Zaparolli D, Saquy PC, Cruz-Filho AM. Effect of sodium hypochlorite and EDTA irrigation, individually and in alternation, on dentin microhardness at the furcation area of mandibular molars. Braz Dent J 2012;23:654-658.ArticlePubMed
- 47. Dineshkumar MK, Vinothkumar TS, Arathi G, Shanthisree P, Kandaswamy D. Effect of ethylene diamine tetra-acetic acid, MTAD™, and HEBP as a final rinse on the microhardness of root dentin. J Conserv Dent 2012;15:170-173.ArticlePubMedPMC
- 48. Ghisi AC, Kopper PM, Baldasso FE, Stürmer CP, Rossi-Fedele G, Steier L, Figueiredo JA, Morgental RD, Vier-Pelisser FV. Effect of super-oxidized water, sodium hypochlorite and EDTA on dentin microhardness. Braz Dent J 2014;25:420-424.ArticlePubMed
- 49. Mai S, Kim YK, Arola DD, Gu LS, Kim JR, Pashley DH, Tay FR. Differential aggressiveness of ethylenediamine tetraacetic acid in causing canal wall erosion in the presence of sodium hypochlorite. J Dent 2010;38:201-206.ArticlePubMed
- 50. Cecchin D, Soares Giaretta V, Granella Cadorin B, Albino Souza M, Vidal CM, Paula Farina A. Effect of synthetic and natural-derived novel endodontic irrigant solutions on mechanical properties of human dentin. J Mater Sci Mater Med 2017;28:141.ArticlePubMedPDF
- 51. Marending M, Paqué F, Fischer J, Zehnder M. Impact of irrigant sequence on mechanical properties of human root dentin. J Endod 2007;33:1325-1328.ArticlePubMed
- 52. Qian W, Shen Y, Haapasalo M. Quantitative analysis of the effect of irrigant solution sequences on dentin erosion. J Endod 2011;37:1437-1441.ArticlePubMed
- 53. Estrela C, Estrela CR, Barbin EL, Spanó JC, Marchesan MA, Pécora JD. Mechanism of action of sodium hypochlorite. Braz Dent J 2002;13:113-117.ArticlePubMed
- 54. Gelse K, Pöschl E, Aigner T. Collagens--structure, function, and biosynthesis. Adv Drug Deliv Rev 2003;55:1531-1546.ArticlePubMed
- 55. Oyarzún A, Cordero AM, Whittle M. Immunohistochemical evaluation of the effects of sodium hypochlorite on dentin collagen and glycosaminoglycans. J Endod 2002;28:152-156.ArticlePubMed
- 56. Mohammadi Z. Sodium hypochlorite in endodontics: an update review. Int Dent J 2008;58:329-341.ArticlePubMed
- 57. Hülsmann M. Effects of mechanical instrumentation and chemical irrigation on the root canal dentin and surrounding tissues. Endod Topics 2013;29:55-86.Article
- 58. Pascon FM, Kantovitz KR, Sacramento PA, Nobre-dos-Santos M, Puppin-Rontani RM. Effect of sodium hypochlorite on dentine mechanical properties. A review. J Dent 2009;37:903-908.ArticlePubMed
- 59. Violich DR, Chandler NP. The smear layer in endodontics - a review. Int Endod J 2010;43:2-15.ArticlePubMed
- 60. Correr GM, Alonso RC, Grando MF, Borges AF, Puppin-Rontani RM. Effect of sodium hypochlorite on primary dentin--a scanning electron microscopy (SEM) evaluation. J Dent 2006;34:454-459.ArticlePubMed
- 61. Zou L, Shen Y, Li W, Haapasalo M. Penetration of sodium hypochlorite into dentin. J Endod 2010;36:793-796.ArticlePubMed
- 62. Di Renzo M, Ellis TH, Sacher E, Stangel I. A photoacoustic FTIRS study of the chemical modifications of human dentin surfaces: II. Deproteination. Biomaterials 2001;22:793-797.ArticlePubMed
REFERENCES
Tables & Figures
REFERENCES
Citations

- OZONIOTERAPIA NA ENDODONTIA: ANÁLISE CRÍTICA DO POTENCIAL E APLICAÇÕES DO O3 GASOSO, AQUOSO, OLEOSO
Cíntia Bueno de Paula, Fernanda dos Santos Lacerda, Eduarda Calisto de Almeida, Sandra Regina Fernandes Albuquerque
Revista Contemporânea.2026; 6(2): e10259. CrossRef - Optimised clinical protocol for root canal obturation using single cone and hydraulic cement sealer
Sweta Surana Bhandari, William M. Palin, Sarah A. Kuehne, Josette Camilleri
Dental Materials.2026;[Epub] CrossRef - Effect of 980 nm diode laser irradiation in comparison with conventional irrigation on smear layer removal from radicular dentin—an in vitro experimental study
Syeda Abeerah Tanveer, Robia Ghafoor, Adil Omerson
BDJ Open.2026;[Epub] CrossRef - A Comparative Evaluation of the Antimicrobial Properties of 50% Grape Seed Extract, N-acetyl Cysteine and 5.25% Sodium Hypochlorite against Enterococcus faecalis (ATCC 19433) – An In vitro Study
Nikita Vishweshwar Kurtkoti, Madhura Vivek Pawar, Vaishnavi Ketan Mathawala, Shraddha Mahadeo Shirsat
Advances in Human Biology.2025; 15(2): 237. CrossRef - Comparative Evaluation of Pulp Dissolution and Smear Layer Removal Properties of Various Herbal Extracts: An in vitro Study
Suleman Abbas Khan, Harshal Gaidhane, Saumya Navit, Meenakshi Upadhyay, Sujeet Shriram Pal, Nishi Grover
Advances in Human Biology.2025; 15(4): 496. CrossRef - Exploring a new Portland cement-free calcium silicate cement —Part 1: Synthesis of dicalcium and tricalcium silicate
Tomomi ITOH, Kohei SHINTANI, Takashi HORIGUCHI, Norihiro SASAMOTO, Katsushi OKUYAMA, Yukimichi TAMAKI, Takeshi SUWABE, Satoshi YOKOSE, Satoshi KAWANO
Dental Materials Journal.2025; 44(2): 140. CrossRef - Effect of phthalocyanine, methylene blue and toluidine blue photosensitizers on the adhesive interface of fiber posts: a confocal laser microscopy study
Tuba Gök, Gamze Er Karaoglu, Hulde Korucu
Lasers in Medical Science.2025;[Epub] CrossRef - Antimicrobial effect of 2.5% sodium hypochlorite irradiated with the 445 Nm diode laser against bacterial biofilms in root canal - in vitro pilot study
Ivan Katalinić, Antonija Pranjić, Ana Budimir, Lucija Kanižaj, Ivona Bago, Valentina Rajić
Lasers in Medical Science.2025;[Epub] CrossRef - When oral health affects overall health: biofilms, dental infections, and emerging antimicrobial strategies
Ahmed Adel Abdelaziz, Ahmed S. Doghish, Akram N. Salah, Reda M. Mansour, Yasser M. Moustafa, Sherif S. Abdel Mageed, Hebatallah Ahmed Mohamed Moustafa, Walaa A. El-Dakroury, Sama A. Doghish, Osama A. Mohammed, Mustafa Ahmed Abdel-Reheim, Shaimaa O. Abbass
Infection.2025; 53(5): 1603. CrossRef - Enhanced Bond Strength and Adhesive Interface of Resin‐Based Sealer to Root Dentine Using a Novel Single Multifunctional Endodontic Irrigant Solution
Paulo Oliveira Silva, Julia Godoi Lopes, Iago Ramirez, Helena Cristina de Assis, Vinícius Leite Rosa‐e‐Silva, Gustavo Alexandre de Castro‐Vasconcelos, Antonio Miranda da Cruz‐Filho, Renato Roperto, Manoel Damião de Sousa‐Neto, Benedetta Ghezzi, Fabiane Ca
Australian Endodontic Journal.2025; 51(2): 423. CrossRef - Enhanced Cleaning, Enhanced Healing: A Systematic Review of Advances in Endodontic Irrigation
Shubhi Gupta, Karunakaran Venkataraman Jeyaraman, M. Deepthi, Rohan Shinkre, Neha Singh, Sagar Shah
Journal of Pharmacy and Bioallied Sciences.2025; 17(Suppl 2): S1845. CrossRef - ВПЛИВ ХІМІЧНИХ ІРИГАНТІВ НА СТАН БІОПЛІВКИ КОРЕНЕВОГО КАНАЛУ ПРИ ЛІКУВАННІ ПЕРІОДОНТИТІВ
Р. І. Новосядлий, М. М. Рожко
Art of Medicine.2025; : 33. CrossRef - Dual- or single rinse? The tubular sealer penetration of endodontic chelating agents
Beliz Ozel, Tuba Ayhan, Figen Kaptan, Fikrettin Sahin, Meriç Karapınar-Kazandağ, Ajinkya M. Pawar
PLOS ONE.2024; 19(6): e0303377. CrossRef - Mechanical properties of simulated dentin caries treated with metal cations and l-ascorbic acid 2-phosphate
Mohammad Ali Saghiri, Julia Vakhnovetsky, Amir Abdolmaleki, Elham Samadi, Fatereh Samadi, Salvatore Napoli, Michael Conte, Steven M. Morgano
Odontology.2024; 112(2): 489. CrossRef - The advancement in irrigation solution within the field of endodontics, A Review
Fatima Fahad , Raghad A Al-Hashimi , Munther J Hussain
Journal of Baghdad College of Dentistry.2024; 36(1): 54. CrossRef - Comparative evaluation of effect of modified triple antibiotic paste and calcium hydroxide as intracanal medicament on microhardness of root dentin: An in vitro study
Aparna Palekar, Piyush Mantri, Minal Awinashe, Basawaraj Biradar, Mukund Singh
Endodontology.2024;[Epub] CrossRef - Effect of herbal irrigants on surface roughness of intraradicular dentin using quantitative method of 3D surface texture analysis
Sabah M. Sobhy, Heba Abdelfatah, Hanaa M. Elgamily, Nesreen Y. Mohammed
Scientific Reports.2024;[Epub] CrossRef - Effect of different root canal irrigants on surface roughness and microhardness of Biodentine combined with triple antibiotic paste: An in vitro study
Rahul Halkai, S. Syed Ishaq, Kiran R. Halkai, Syeda Uzma Mahveen
Journal of Conservative Dentistry and Endodontics.2024; 27(5): 508. CrossRef - Bacteria debridement efficacy of two sonic root canal irrigant activation systems
Chang Zeng, Pei Hu, Colin P. Egan, Brian E. Bergeron, Franklin Tay, Jingzhi Ma
Journal of Dentistry.2024; 140: 104770. CrossRef - Effects of endodontic irrigation solutions on structural, chemical, and mechanical properties of coronal dentin: A scoping review
Joana A. Marques, Rui I. Falacho, João Miguel Santos, João Carlos Ramos, Paulo J. Palma
Journal of Esthetic and Restorative Dentistry.2024; 36(4): 606. CrossRef - Effect of ultrasonic and Er,Cr:YSGG laser-activated irrigation protocol on dual-species root canal biofilm removal: An in vitro study
Venkata Divya Durga Datla, Lakshman Varma Uppalapati, Hema Prakash Kumari Pilli, Jyothi Mandava, Sirisha Kantheti, Sri Naagaja Krishnaveni Komireddy, Vedamani Chandolu
Journal of Conservative Dentistry and Endodontics.2024; 27(6): 613. CrossRef - Comparative Evaluation of Dental Pulp Tissue Dissolution Ability of Sapindus mukorossi and Sodium Hypochlorite
Sriram Kaliamoorthy, Sreeram Rayar, Shanmugapriya SundarRaj, Sugantha Priya Sayeeram, V.V. Premkumar, Sapna C Muddappa, Venkatraman Muthukumaran, Kanmani Raju, Agila Samidorai
Cureus.2024;[Epub] CrossRef - Effect of Different Irrigating Solutions on Root Canal Dentin Microhardness—A Systematic Review with Meta-Analysis
Sunidhi Agarwal, Lora Mishra, Naomi Ranjan Singh, Rini Behera, Manoj Kumar, Ravishankar Nagaraja, Krzysztof Sokolowski, Barbara Lapinska
Journal of Functional Biomaterials.2024; 15(5): 132. CrossRef - Evaluation of Effect of Herbal Irrigant on Microhardness of Root Dentin: An in vitro Study
Anuya Ravindra Koparde, Anupam Sandeep Sharma, Aniket Jadhav, Aishwarya Handa, Abhijit Bajirao Jadhav, Madhura A. Jadhav
Journal of the International Clinical Dental Research Organization.2024; 16(2): 170. CrossRef - Impact of calcium hydroxide and 2-hydroxyisocaproic acid on the microhardness of root dentine: an in vitro study
Nandini T. Niranjan, Protim Ghosh Dastidar, Raghavendra Penukonda, Galvin Sim Siang Lin, Roopa Babannavar, Arun Jaysheel, Harshada Pattar
Odontology.2024; 112(3): 711. CrossRef - Endodontic irrigants from a comprehensive perspective
Rayana Duarte Khoury, Lara Steffany de Carvalho, Mauro Felipe Rios do Nascimento, Fadi Alhussain, Amjad Abu Hasna
World Journal of Clinical Cases.2024; 12(21): 4460. CrossRef - Exploring Periostracum as an Alternative Root Canal Irrigant: Insights From Zebrafish Embryo Experiments
Annie Sylvea Valan, Jogikalmat Krithikadatta, Ajay Guru
Cureus.2024;[Epub] CrossRef - A Systematic Review of the Comparative Efficacy of Lactobacillus Probiotics and Sodium Hypochlorite as Intracanal Irrigants Against Enterococcus faecalis
Mrinalini Mrinalini, Alpa Gupta, Dax Abraham, Arun Kumar Duraisamy, Rajat Sharma
Cureus.2024;[Epub] CrossRef - Effect of sodium hypochlorite and ethylenediaminotetraacetic acid activated by laser and ultrasonic energy on surface morphology and chemical composition of intracanal dentin
Adriana Katunarić, Sandra Flinčec Grgac, Dragana Gabrić, Božidar Pavelić, Ivona Bago
Microscopy Research and Technique.2024; 87(4): 818. CrossRef - Impact of antimicrobial photodynamic therapy on the bond-strength and penetration of endodontic sealers: A systematic review
Khalid H Almadi
Photodiagnosis and Photodynamic Therapy.2023; 41: 103249. CrossRef - In Vitro Assessment of SWEEPS and Antimicrobial Photodynamic Therapy Alone or in Combination for Eradicating Enterococcus faecalis Biofilm in Root Canals
Ali Shahi Ardakani, Shima Afrasiabi, Pegah Sarraf, Stefano Benedicenti, Luca Solimei, Nasim Chiniforush
Pharmaceutics.2023; 15(11): 2628. CrossRef - Effects of traditional and novel proteolytic agents on tissue dissolution and dentine microhardness
Shwetha Elizabeth Jacob, Niharika Prasad, Sreya Dutta, Vasavi Kumblekar, Srikant Natarajan, Kukkila Jayaprakash, Manuel Sebastian Thomas
Australian Endodontic Journal.2023; 49(2): 287. CrossRef - Push-Out Bond Strength of EndoSeal Mineral Trioxide Aggregate and AH Plus Sealers after Using Three Different Irrigation Protocols
Shimaa Rifaat, Ahmed Rahoma, Fatimah Alkhalifa, Ghofran AlQuraini, Zahraa Alsalman, Zahraa Alwesaibi, Noha Taymour
European Journal of Dentistry.2023; 17(01): 076. CrossRef - Can natural irrigants replace sodium hypochlorite? A systematic review
Anand Venkatraman Susila, Shamini Sai, Nikita Sharma, Arthi Balasubramaniam, Aruna Kumari Veronica, Sureshbabu Nivedhitha
Clinical Oral Investigations.2023; 27(5): 1831. CrossRef - Advances in the Role of Sodium Hypochlorite Irrigant in Chemical Preparation of Root Canal Treatment
Chen Cai, Xuan Chen, Yang Li, Qianzhou Jiang, Yeliz Guven
BioMed Research International.2023;[Epub] CrossRef - A laboratory investigation on the effect of biguanide‐ and pyridine‐derived antiseptics on the adhesion of resin composites to dentin
Arzu Yağmur Uçar, Türkay Kölüş, D. Alperen Bozkurt, Prasanna Neelakantan, Islam A. A. Ali, Sema Belli
Australian Endodontic Journal.2023; 49(3): 599. CrossRef - Minimally invasive management of vital teeth requiring root canal therapy
E. Karatas, M. Hadis, W. M. Palin, M. R. Milward, S. A. Kuehne, J. Camilleri
Scientific Reports.2023;[Epub] CrossRef - Disinfection of radicular dentin using Riboflavin, Rose Bengal, Curcumin, and Porfimer sodium on extrusion bond strength of fiber post to radicular dentin
Sami A Alturaiki, Ahmed A. Bamanie, Mohammed A. Albulowey, Abdullah A. Al Daafas, Abdullah Almalki, Ali Alqerban
Photodiagnosis and Photodynamic Therapy.2022; 37: 102625. CrossRef - Present status and future directions: Minimally invasive root canal preparation and periradicular surgery
Prasanna Neelakantan, Vijetha Vishwanath, Silvio Taschieri, Stefano Corbella
International Endodontic Journal.2022; 55(S4): 845. CrossRef - Ex Vivo Effect of Novel Lipophosphonoxins on Root Canal Biofilm Produced by Enterococcus faecalis: Pilot Study
Yuliya Morozova, Iva Voborná, Radovan Žižka, Kateřina Bogdanová, Renata Večeřová, Dominik Rejman, Milan Kolář, Duy Dinh Do Pham, Pavel Holík, Roman Moštěk, Matej Rosa, Lenka Pospíšilová
Life.2022; 12(1): 129. CrossRef - Irrigating Solutions and Activation Methods Used in Clinical Endodontics: A Systematic Review
Riccardo Tonini, Matteo Salvadori, Elisabetta Audino, Salvatore Sauro, Maria Luisa Garo, Stefano Salgarello
Frontiers in Oral Health.2022;[Epub] CrossRef - Evaluation of Effects of Various Irrigating Solutions on Chemical Structure of Root Canal Dentin Using FTIR, SEM, and EDS: An In Vitro Study
Indu Padmakumar, Dharam Hinduja, Abdul Mujeeb, Raghu Kachenahalli Narasimhaiah, Ashwini Kumar Saraswathi, Mubashir Baig Mirza, Ali Robaian, Syed Nahid Basheer, Mohmed Isaqali Karobari, Giuseppe Alessandro Scardina
Journal of Functional Biomaterials.2022; 13(4): 197. CrossRef - Final irrigation protocols affect radicular dentin DMP1-CT expression, microhardness, and biochemical composition
Cristina Retana-Lobo, Tatiana Ramírez-Mora, Fabian Murillo-Gómez, Juliane Maria Guerreiro-Tanomaru, Mario Tanomaru-Filho, Jessie Reyes-Carmona
Clinical Oral Investigations.2022; 26(8): 5491. CrossRef - Comparative Evaluation of Antimicrobial Efficacy of Herbal Formulations of Septilin and Triphala with Conventional 2% Chlorhexidine on Root Canal and Oral Commensal Bacteria using Kirby Bauer Method
Shadab Ahmed, Kamil Shahnawaz, Tapan Kumar Mandal, Mamnoon Ghafir, Shiva Shankar Gummaluri, Gaurav Vishal
Contemporary Clinical Dentistry.2022; 13(4): 383. CrossRef - Adjunctive procedure with solvent mixtures in non-surgical endodontic retreatment: does it affect root dentin hardness?
Inês Ferreira, Ana Cristina Braga, Maria Ascensão Lopes, Irene Pina-Vaz
Odontology.2021; 109(4): 812. CrossRef
Figure 1
Effect of irrigation sequences on dentin composition
| No. | Author | Sample type | Groups | Outcome measure | Main findings | |
|---|---|---|---|---|---|---|
| Irrigation sequence | Duration (min)* | |||||
| 1 | Doğan et al. [ | Root canal dentin | 17% EDTA/2.5% NaOCl | 15-? | Calcium/phosphate ratio | EDTA/NaOCl altered the Ca/P ratio and Mg level significantly when compared to the control group |
| 17% EDTA/saline | 15-? | |||||
| 2.5% NaOCl | ? | |||||
| Saline (control) | ? | |||||
| 2 | Sayin et al. [ | Root canal dentin | 2.5% NaOCl | 1, 5 | Calcium loss | EDTA and EDTA/NaOCl treatment for 1 and 5 min showed significantly more calcium ion loss |
| 17% EDTA | 1, 5 | |||||
| 17% EDTA/2.5% NaOCl | 1-1, 5-5 | |||||
| Distilled water (control) | 1, 5 | |||||
| 3 | Wang et al. [ | Root canal dentin | Control | Element distribution (EDS) | Reduced Ca and P values in NaOCl/EDTA/NaOCl group | |
| 3% NaOCl/8% EDTA | 5-2 | |||||
| 3% NaOCl/8% EDTA/3% NaOCl | 2-2-1 | |||||
| 5% NaOCl/17% EDTA | 2-2 | |||||
| 5% NaOCl/17% EDTA/5% NaOCl | 2-2-1 | |||||
| 5% NaOCl/17% EDTA/5% NaOCl | 5-5-5 | |||||
| 4 | Zhang et al. [ | Dentin powder | Control–no treatment | - Apatite/collagen ratio (FTIR) | EDTA decreased the apatite/collagen ratio 5.25% NaOCl for more than 60 min showed a significant increase in apatite/collagen ratio and decrease in flexural strength | |
| 17% EDTA | 2 | - Flexural strength | ||||
| 1.3% NaOCl/17% EDTA | 10, 20, 30, 60, 120, 180, 240-2 | |||||
| 5.25% NaOCl/17% EDTA | 10, 20, 30, 60, 120, 180, 240-2 | |||||
| 5 | Zhang et al. [ | Dentin powder | Control–no treatment | - Apatite/collagen ratio | - Significant increase in apatite/collagen ratio with time and concentration of NaOCl | |
| 17% EDTA | 2 | - Collagen degradation (FTIR) | - Tunneling erosion pattern with subsurface erosion with 5.25% NaOCl/EDTA | |||
| 1.3% NaOCl | 10, 20, 30, 60, 120, 180, 240 | |||||
| 1.3% NaOCl/17% EDTA | 10, 20, 30, 60, 120, 180, 240-2 | |||||
| 5.25% NaOCl | 10, 20, 30, 60, 120, 180, 240 | |||||
| 5.25% NaOCl/17% EDTA | 10, 20, 30, 60, 120, 180, 240-2 | |||||
EDTA, ethylenediaminetetraacetic acid; NaOCl, sodium hypochlorite; Ca, calcium; P, phosphorus; EDS, energy dispersive spectroscopy; FTIR, Fourier-transform infrared spectroscopy.
*? represent unknown.
Effect of irrigation sequences on mechanical properties of dentin
| No. | Author | Dentin sample type | Groups | Outcome measure | Main findings | |
|---|---|---|---|---|---|---|
| Irrigation sequence | Duration* | |||||
| 1 | Eldeniz et al. [ | Root canal dentin | 17% EDTA/5.25% NaOCl | 150-150 sec | Microhardness | EDTA/NaOCl significantly reduced the microhardness compared to the control |
| DI water (control) | ||||||
| 2 | Akcay et al. [ | Root canal dentin | 7.5% EDTA | 1 min | Microhardness | Unclear if the changes were significant relative to an untreated dentin control |
| 7.5% EDTA/2.5% NaOCl | 1-1 min | |||||
| 3 | Kara Tuncer et al. [ | Root canal dentin | 17% EDTA/2.5% NaOCl | 1-1 min | Microhardness | Unclear if the changes were significant relative to an untreated dentin control |
| 4 | Saghiri et al. [ | Root canal dentin | 2.6% NaOCl | 5 min | Microhardness at different depths | EDTA/NaOCl (5 min) group had a significant decrease in microhardness at 100 µm |
| 17% EDTA/2.6% NaOCl | 5-5 min | |||||
| 17% EDTA/2.6% NaOCl | 1-5 min | |||||
| Saline (control) | 5 min | |||||
| 5 | Aranda-Garcia et al. [ | Root canal dentin | Distilled water/2.5% NaOCl (control) | 3-? min | Microhardness | Compared to the control group, EDTA/NaOCl group showed greater reduction in microhardness |
| 17% EDTA/2.5% NaOCl | 3-? min | |||||
| 6 | Ulusoy et al. [ | Root canal dentin | 17% EDTA/2.5% NaOCl | 5-5 min | Microhardness | Significant reduction in microhardness in EDTA/NaOCl group, compared to the control group |
| 5% NaOCl | 5 min | |||||
| 0.9% saline (control) | 5 min | |||||
| 7 | Baldasso et al. [ | Root canal dentin | 17% EDTA/2.5% NaOCl | 2-5 min | Microhardness at different depths | Microhardness of EDTA/NaOCl group was significantly reduced compared to control groups at 500 and 1,000 µm |
| 2.5% NaOCl (solution control) | 5 min | |||||
| Distilled water (negative control) | 5 min | |||||
| 8 | Zaparolli et al. [ | Dentin at furcation region of pulp chamber | 1% NaOCl | 10 min | Microhardness at furcation area | EDTA and NaOCl/EDTA group showed significantly lesser microhardness values compare to control and NaOCl group |
| 17% EDTA | 10 min | |||||
| 1% NaOCl /17% EDTA | 10-10 min | |||||
| Distilled water (control) | 10 min | |||||
| 9 | Dineshkumar et al. [ | Root canal dentin | Distilled water (control) | Microhardness | No statistical difference was observed between the groups NaOCl/EDTA and NaOCl/HEBP | |
| 1.3% NaOCl/17% EDTA | 20-1 min | |||||
| 1.3% NaOCl/18% HEBP | 20-5 min | |||||
| 10 | Ghisi et al. [ | Root canal dentin | 2% NaOCl | 30 min | Microhardness at different depths | No significant difference in microhardness values between different depths and between the groups |
| 5% NaOCl | 30 min | |||||
| 17% EDTA | 30 min | |||||
| 2% NaOCl/17% EDTA | 30-5 min | |||||
| 5% NaOCl/17% EDTA | 30-5 min | |||||
| 11 | Mai et al. [ | Dentin beams | Control | Flexural strength | 60 min exposure of NaOCl significantly decreased the flexural strength compared to other groups | |
| 5.25 NaOCl/17% EDTA | 10-2 min | |||||
| 5.25 NaOCl/17% EDTA | 60-2 min | |||||
| 12 | Cecchin et al. [ | Dentin beam (flexural strength), hourglass shaped root dentin sections (ultimate tensile strength), instrumented root canals (fracture resistance) | DI water/17% EDTA (control) | 30-1 min | - Flexural strength | Significant decrease in flexural strength, ultimate tensile strength and fracture resistance of NaOCl/ EDTA compared to control |
| 6% NaOCl/17% EDTA | 30-1 min | - Ultimate Tensile Strength | ||||
| - Fracture resistance | ||||||
| 13 | Marending et al. [ | Dentin beams | 2.5% NaOCl/17% EDTA/2.5% NaOCl/DI water | 21-3-3-3 min | - Modulus of elasticity | NaOCl associated with EDTA reduced the flexural strength significantly compared to the other groups |
| 2.5% NaOCl/DI water/2.5% NaOCl/17% EDTA | 21-3-3-3 min | - Flexural strength | ||||
| 2.5% NaOCl/DI water/2.5% NaOCl/DI water | 21-3-3-3 min | |||||
| DI water/EDTA/DI water | 21-3-6 min | |||||
| DI water | 30 min | |||||
EDTA, ethylenediaminetetraacetic acid; NaOCl, sodium hypochlorite; DI, deionized; HEBP, 1-hydroxyethylidene-1, 1-bis-phosphonate.
*? represent unknown.
EDTA, ethylenediaminetetraacetic acid; NaOCl, sodium hypochlorite; Ca, calcium; P, phosphorus; EDS, energy dispersive spectroscopy; FTIR, Fourier-transform infrared spectroscopy.
*? represent unknown.
EDTA, ethylenediaminetetraacetic acid; NaOCl, sodium hypochlorite; DI, deionized; HEBP, 1-hydroxyethylidene-1, 1-bis-phosphonate.
*? represent unknown.

 KACD
KACD
 ePub Link
ePub Link Cite
Cite

