Articles
- Page Path
- HOME > Restor Dent Endod > Volume 45(2); 2020 > Article
- Research Article Influence of pain-relieving therapies on inflammation and the expression of proinflammatory neuropeptides after dental bleaching treatment
-
Livia Maria Alves Valentim da Silva1
 , Luciano Tavares Angelo Cintra1
, Luciano Tavares Angelo Cintra1 , Marjorie de Oliveira Gallinari1
, Marjorie de Oliveira Gallinari1 , Francine Benetti2
, Francine Benetti2 , Vanessa Rahal1
, Vanessa Rahal1 , Edilson Ervolino3
, Edilson Ervolino3 , Sibele de Alcântara1
, Sibele de Alcântara1 , André Luiz Fraga Briso1
, André Luiz Fraga Briso1
-
Restor Dent Endod 2020;45(2):e20.
DOI: https://doi.org/10.5395/rde.2020.45.e20
Published online: February 28, 2020
1Department of Restorative Dentistry, São Paulo State University (UNESP), School of Dentistry, Araçatuba, SP, Brazil.
2Department of Restorative Dentistry, Universidade Federal de Minas Gerais (UFMG), Belo Horizonte, MG, Brazil.
3Department of Basic Sciences, São Paulo State University (UNESP), School of Dentistry, Araçatuba, SP, Brazil.
- Correspondence to André Luiz Fraga Briso, DDS, MS, PhD. Assistant Professor, Department of Restorative Dentistry, São Paulo State University (UNESP) School of Dentistry, 1193 José Bonifácio, Araçatuba, SP 16015-050, Brazil. andre.briso@unesp.br
Copyright © 2020. The Korean Academy of Conservative Dentistry
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,694 Views
- 11 Download
- 12 Crossref
Abstract
-
Objectives To minimize the tooth sensitivity caused by in-office bleaching, many dentists use non-steroidal anti-inflammatory drugs and topical desensitizing gels containing potassium nitrate and sodium fluoride. This study aimed to evaluate the influence of these substances on inflammation and the expression of substance P and calcitonin gene-related peptide in pulp nerve fibers.
-
Materials and Methods Seventy-two rats were divided into 6 groups as follows: GI, control; GII, only dental bleaching; GIII, only ibuprofen; GIV, ibuprofen administered 30 minutes before and after the bleaching treatment and every 12 hours until the analysis; GV, only topical application of a desensitizing agent; and GVI, topical application of a desensitizing agent before dental bleaching. Placebo gel was applied to the upper left jaw and the bleaching agent was applied to the upper right jaw in all groups. Subsequently, the groups were divided into 3 subgroups based on the time of analysis: 0, 24, and 48 hours after bleaching (n = 8). The rats were euthanized and the maxillae were processed and evaluated by histopathological and immunohistochemical analyses. The data were analyzed using the Kruskal-Wallis test, followed by the Dunn test (p < 0.05).
-
Results In the bleaching groups, the inflammatory process and expression of neuropeptides decreased over time. The animals in which a desensitizing agent was applied showed better results within 24 hours.
-
Conclusions The use of a desensitizing agent had positive effects on inflammation and pain-related neuropeptide expression, minimizing the painful effects of dental bleaching treatment.
INTRODUCTION
MATERIALS AND METHODS
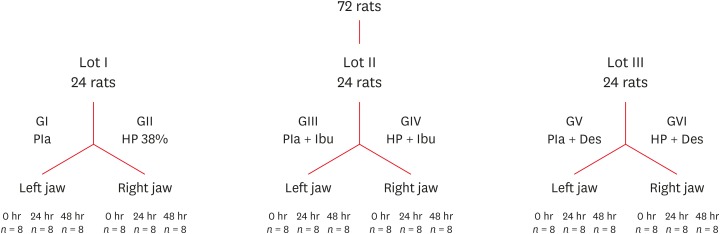
Flowchart detailing the division of the rats according to the experimental groups and analysis times.
RESULTS
Median scores from the histological analysis of each crown and radicular third for all groups
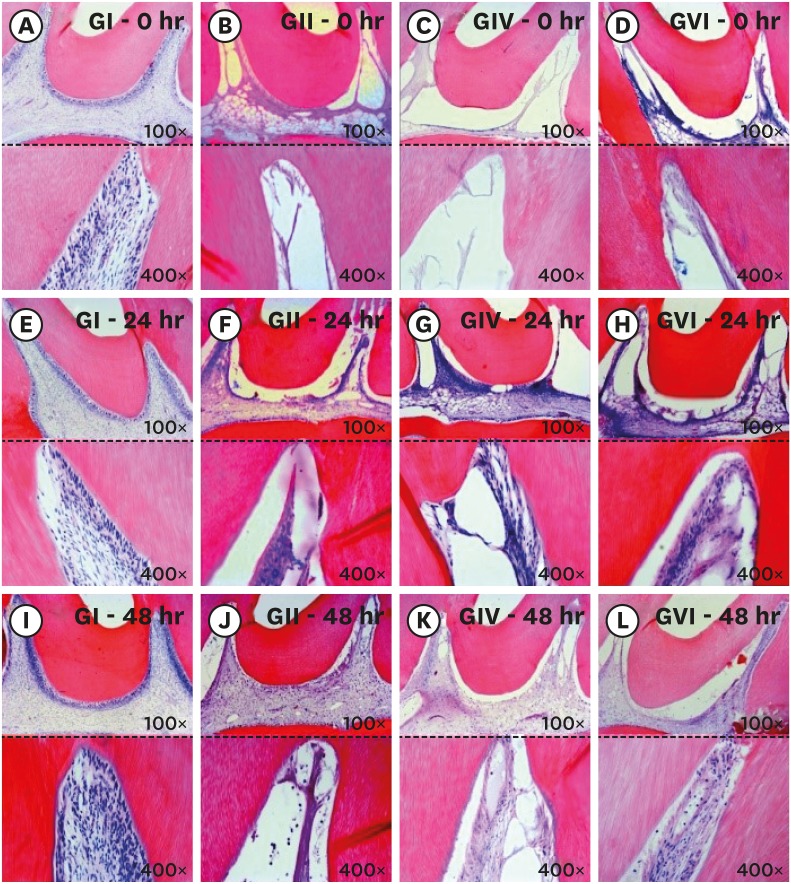
Longitudinal section of the coronary third of the pulp tissue of the study groups. The (A-D) correspond to photomicrographs immediately after bleaching: control group (A), 38% hydrogen peroxide without pain-relieving therapy (B), bleaching therapy associated with oral administration of ibuprofen (C), and bleaching therapy associated with topical application of Desensibilize KF 2% (D). The (E-H) correspond to photomicrographs at 24 hours after bleaching: control group (E), 38% hydrogen peroxide without pain-relieving therapy (F), bleaching therapy associated with oral administration of ibuprofen (G), and bleaching therapy associated with topical application of Desensibilize KF 2% (H). The (I-L) correspond to photomicrographs at 48 hours after bleaching: control group (I), 38% hydrogen peroxide without pain-relieving therapy (J), bleaching therapy associated with oral administration of ibuprofen (K), and bleaching therapy associated with topical application of Desensibilize KF 2%.
1. Immunohistochemical analysis of SP
Median scores from substance P immunostaining in each crown and radicular third for all bleaching groups
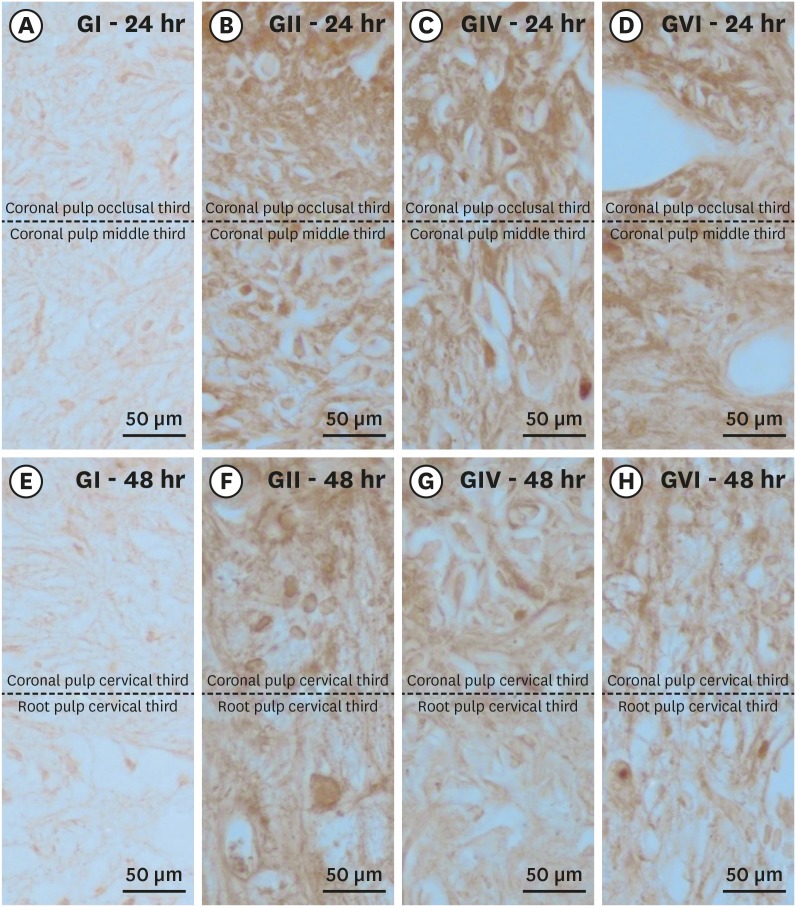
Histological sections showing immunostaining patterns for substance P. Photomicrographs showing the pattern of immunostaining for SP in the dental pulp in groups GI (A, E), GII (B, F), GIV (C, G) and GVI (D, H) after 24h (A–D) and 48h (E–H) local post treatment. It's possible observe the baseline level of SP expression in the control group and the significant increase in immunostaining for SP after bleaching therapy. Original magnification: ×1,000.
2. Immunohistochemical analysis of CGRP
Median scores from calcitonin gene-related peptide immunostaining in each crown and root third for all groups
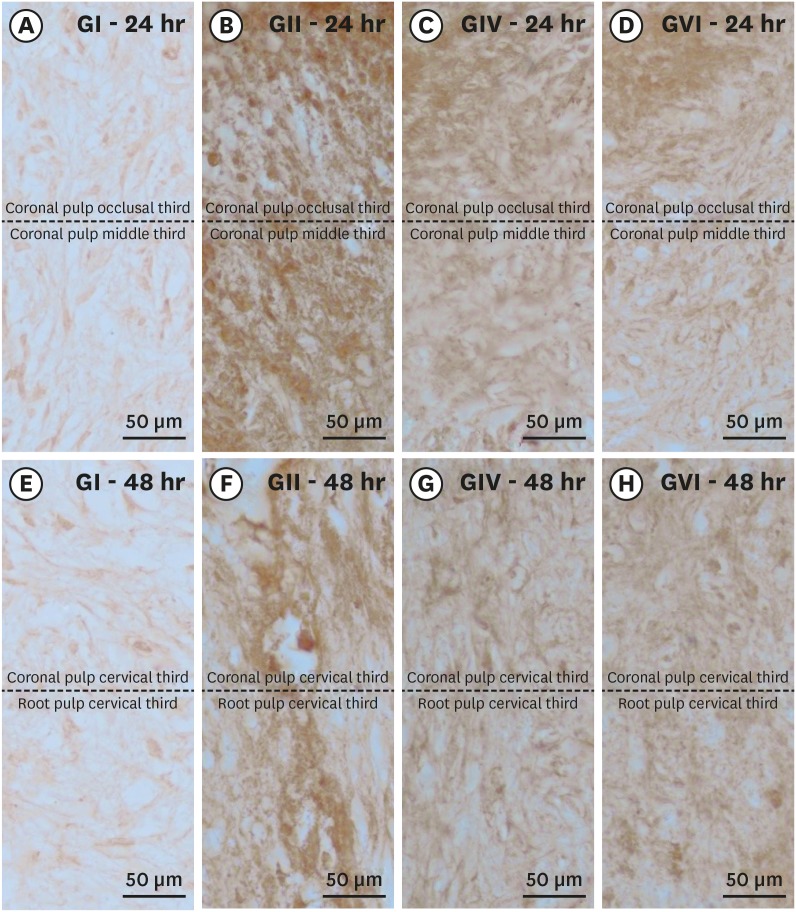
Histological sections showing the immunostaining pattern for calcitonin gene-related peptide. Photomicrographs showing the pattern of immunostaining for CGRP in the dental pulp in groups GI (A, E), GII (B, F), GIV (C, G) and GVI (D, H) after 24h (A–D) and 48h (E–H) after local treatment. It's possible observe the baseline level of CGRP expression in the control group and the significant increase in immunostaining for CGRP after bleaching therapy. Original magnification: ×1,000.
DISCUSSION
CONCLUSIONS
-
Funding: This research was supported by São Paulo Research Foundation (FAPESP), grant #2015/21682-8 and #2015/24090-4.
-
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
-
Author Contributions:
Conceptualization: Briso ALF, Cintra LTA.
Data curation: Cintra LTA, Gallinari MO.
Formal analysis: Ervolino E, Cintra LTA, Briso ALF.
Funding acquisition: Briso ALF.
Investigation: da Silva LMAV, de Alcântara S.
Methodology: da Silva LMAV, Gallinari MO, Rahal V, Ervolino E, Benetti F.
Project administration: Briso ALF.
Resources: Briso ALF.
Software: Briso ALF, Cintra LTA.
Supervision: Briso ALF, Cintra LTA.
Validation: Benetti F, Ervolino E.
Visualization: Briso ALF.
Writing - original draft: da Silva LMAV, Gallinari MO, Rahal V, de Alcântara S.
Writing - review & editing: Briso ALF, Cintra LTA.
- 1. Haywood VB, Heymann HO. Nightguard vital bleaching: how safe is it? Quintessence Int 1991;22:515-523.PubMed
- 2. Williams HA, Rueggeberg FA, Meister LW. Bleaching the natural dentition to match the color of existing restorations: case reports. Quintessence Int 1992;23:673-677.PubMed
- 3. Perdigão J. Dental whitening--revisiting the myths. Northwest Dent 2010;89:19-21.
- 4. Anderson DG, Chiego DJ Jr, Glickman GN, McCauley LK. A clinical assessment of the effects of 10% carbamide peroxide gel on human pulp tissue. J Endod 1999;25:247-250.ArticlePubMed
- 5. Kina JF, Huck C, Riehl H, Martinez TC, Sacono NT, Ribeiro AP, Costa CA. Response of human pulps after professionally applied vital tooth bleaching. Int Endod J 2010;43:572-580.ArticlePubMed
- 6. Costa CA, Riehl H, Kina JF, Sacono NT, Hebling J. Human pulp responses to in-office tooth bleaching. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010;109:e59-e64.Article
- 7. Soares DG, Basso FG, Hebling J, de Souza Costa CA. Concentrations of and application protocols for hydrogen peroxide bleaching gels: effects on pulp cell viability and whitening efficacy. J Dent 2014;42:185-198.ArticlePubMed
- 8. Caviedes-Bucheli J, Ariza-García G, Restrepo-Méndez S, Ríos-Osorio N, Lombana N, Muñoz HR. The effect of tooth bleaching on substance P expression in human dental pulp. J Endod 2008;34:1462-1465.ArticlePubMed
- 9. Cintra LT, Benetti F, da Silva Facundo AC, Ferreira LL, Gomes-Filho JE, Ervolino E, Rahal V, Briso AL. The number of bleaching sessions influences pulp tissue damage in rat teeth. J Endod 2013;39:1576-1580.ArticlePubMed
- 10. Benetti F, Gomes-Filho JE, Ferreira LL, Ervolino E, Briso AL, Sivieri-Araújo G, Dezan-Júnior E, Cintra LT. Hydrogen peroxide induces cell proliferation and apoptosis in pulp of rats after dental bleaching in vivo: effects of the dental bleaching in pulp. Arch Oral Biol 2017;81:103-109.PubMed
- 11. de Almeida LC, Costa CA, Riehl H, dos Santos PH, Sundfeld RH, Briso AL. Occurrence of sensitivity during at-home and in-office tooth bleaching therapies with or without use of light sources. Acta Odontol Latinoam 2012;25:3-8.PubMed
- 12. Almeida LC, Riehl H, Santos PH, Sundfeld ML, Briso AL. Clinical evaluation of the effectiveness of different bleaching therapies in vital teeth. Int J Periodontics Restorative Dent 2012;32:303-309.PubMed
- 13. Pashley DH. How can sensitive dentine become hypersensitive and can it be reversed? J Dent 2013;41(Supplement 4):S49-S55.ArticlePubMedPMC
- 14. Brain SD, Williams TJ, Tippins JR, Morris HR, MacIntyre I. Calcitonin gene-related peptide is a potent vasodilator. Nature 1985;313:54-56.ArticlePubMedPDF
- 15. Casasco A, Calligaro A, Casasco M, Springall DR, Polak JM, Poggi P, Marchetti C. Peptidergic nerves in human dental pulp. An immunocytochemical study. Histochemistry 1990;95:115-121.ArticlePubMedPDF
- 16. Lundberg JM, Pernow J, Tatemoto K, Dahlöf C. Pre- and postjunctional effects of NPY on sympathetic control of rat femoral artery. Acta Physiol Scand 1985;123:511-513.ArticlePubMed
- 17. Haywood VB. Treating sensitivity during tooth whitening. Compend Contin Educ Dent 2005;26:11-20.
- 18. Armênio RV, Fitarelli F, Armênio MF, Demarco FF, Reis A, Loguercio AD. The effect of fluoride gel use on bleaching sensitivity: a double-blind randomized controlled clinical trial. J Am Dent Assoc 2008;139:592-597.PubMed
- 19. Basting RT, Amaral FL, França FM, Flório FM. Clinical comparative study of the effectiveness of and tooth sensitivity to 10% and 20% carbamide peroxide home-use and 35% and 38% hydrogen peroxide in-office bleaching materials containing desensitizing agents. Oper Dent 2012;37:464-473.ArticlePubMedPDF
- 20. Palé M, Mayoral JR, Llopis J, Vallès M, Basilio J, Roig M. Evaluation of the effectiveness of an in-office bleaching system and the effect of potassium nitrate as a desensitizing agent. Odontology 2014;102:203-210.ArticlePubMedPDF
- 21. Paula E, Kossatz S, Fernandes D, Loguercio A, Reis A. The effect of perioperative ibuprofen use on tooth sensitivity caused by in-office bleaching. Oper Dent 2013;38:601-608.ArticlePubMedPDF
- 22. Charakorn P, Cabanilla LL, Wagner WC, Foong WC, Shaheen J, Pregitzer R, Schneider D. The effect of preoperative ibuprofen on tooth sensitivity caused by in-office bleaching. Oper Dent 2009;34:131-135.ArticlePubMedPDF
- 23. Schäfers M, Marziniak M, Sorkin LS, Yaksh TL, Sommer C. Cyclooxygenase inhibition in nerve-injury- and TNF-induced hyperalgesia in the rat. Exp Neurol 2004;185:160-168.ArticlePubMed
- 24. Benetti F, Briso ALF, Carminatti M, de Araújo Lopes JM, Barbosa JG, Ervolino E, Gomes-Filho JE, Cintra LTA. The presence of osteocalcin, osteopontin and reactive oxygen species-positive cells in pulp tissue after dental bleaching. Int Endod J 2019;52:665-675.ArticlePubMedPDF
- 25. Lillie RD. Histopathologic technic and practical histochemistry. 2nd ed. New York: Blakinston; 1954. p. 501.
- 26. de Almeida LC, Soares DG, Gallinari MO, de Souza Costa CA, Dos Santos PH, Briso AL. Color alteration, hydrogen peroxide diffusion, and cytotoxicity caused by in-office bleaching protocols. Clin Oral Investig 2015;19:673-680.ArticlePubMedPDF
- 27. Cintra LT, Benetti F, Ferreira LL, Rahal V, Ervolino E, Jacinto RC, Gomes Filho JE, Briso AL. Evaluation of an experimental rat model for comparative studies of bleaching agents. J Appl Oral Sci 2016;24:95-104.ArticlePubMedPMC
- 28. Nelson-Filho P, Lucisano MP, Da Silva RA, Da Silva RS, Serra MC, Gerlach RF, Neto FC, Carneiro ZA, Zamarioli A, Morse L, Battaglino R. Systemically alendronate was incorporated into dental tissues but did not cause morphological or mechanical changes in rats teeth. Microsc Res Tech 2012;75:1265-1271.ArticlePubMed
- 29. Ferreira VG, Nabeshima CK, Marques MM, Paris AF, Gioso MA, dos Reis RS, Machado ME. Tooth bleaching induces changes in the vascular permeability of rat incisor pulps. Am J Dent 2013;26:298-300.PubMed
- 30. In: Cintra LTA, Benetti F, Rahal V, Facundo ACS, Ferreira LL, Gomes-Filho JE, Ervolino E, Briso ALF, editors. Influence of dental bleaching sessions on the pulp of rats. IADR/AADR/CADR 91st General Session and Exhibition; 2013 Mar 20–23; Seattle, WA. Alexandria: International Association for Dental Research (IADR); 2013.
- 31. Gallinari MO, Cintra LT, Benetti F, Rahal V, Ervolino E, Briso AL. Pulp response of rats submitted to bleaching and the use of different anti-inflammatory drugs. PLoS One 2019;14:e0210338.ArticlePubMedPMC
- 32. Tay LY, Kose C, Loguercio AD, Reis A. Assessing the effect of a desensitizing agent used before in-office tooth bleaching. J Am Dent Assoc 2009;140:1245-1251.ArticlePubMed
- 33. Caviedes-Bucheli J, Lombana N, Azuero-Holguín MM, Munoz HR. Quantification of neuropeptides (calcitonin gene-related peptide, substance P, neurokinin A, neuropeptide Y and vasoactive intestinal polypeptide) expressed in healthy and inflamed human dental pulp. Int Endod J 2006;39:394-400.ArticlePubMed
- 34. Awawdeh LA, Lundy FT, Linden GJ, Shaw C, Kennedy JG, Lamey PJ. Quantitative analysis of substance P, neurokinin A and calcitonin gene-related peptide in gingival crevicular fluid associated with painful human teeth. Eur J Oral Sci 2002;110:185-191.ArticlePubMedPDF
- 35. Caviedes-Bucheli J, Muñoz HR, Azuero-Holguín MM, Ulate E. Neuropeptides in dental pulp: the silent protagonists. J Endod 2008;34:773-788.ArticlePubMed
- 36. Rahal V, Gallinari MO, Perdigão J, Cintra LT, dos Santos PH, Briso AL. Quantitative sensory testing of the effect of desensitizing treatment after dental bleaching. Acta Odontol Latinoam 2015;28:263-270.PubMed
- 37. Cintra LT, Benetti F, Ferreira LL, Gomes-Filho JE, Ervolino E, Gallinari MO, Rahal V, Briso AL. Penetration capacity, color alteration and biological response of two in-office bleaching protocols. Braz Dent J 2016;27:169-175.ArticlePubMed
REFERENCES
Tables & Figures
REFERENCES
Citations

- Effectiveness of Analgesics in Dental Whitening Pain Management: A Systematic Review and Meta-Analysis
Gabriella Alves Julião Costa, Caio Ferreira Freire Caetano, Ravy Jucá Farias, Diana Araújo Cunha, Dayrine Silveira de Paula, Edson Luiz Cetira Filho, Paulo Goberlânio de Barros Silva
Expert Opinion on Pharmacotherapy.2025; 26(5): 639. CrossRef - The Use of Ozone Therapy in Combination with a Desensitizing Agent for Dentinal Tubules Occlusion: An In Vitro Study
Banna Alnufaiy
The Open Dentistry Journal.2025;[Epub] CrossRef - The role of neurogenic inflammation in pulp repair and the techniques used for its assessment (narrative review)
Muna Sh. Ahmed, Anas F. Mahdee
Frontiers in Dental Medicine.2025;[Epub] CrossRef - Influence of dental bleaching on the pulp tissue: A systematic review of in vivo studies
Mariana Viana Donato, Alexandre Henrique dos Reis‐Prado, Lucas Guimarães Abreu, Lara Cancella de Arantes, Juliana Goto, Hebertt Gonzaga dos Santos Chaves, Luciano Tavares Angelo Cintra, André Luiz Fraga Briso, Isabella Faria da Cunha Peixoto, Francine Ben
International Endodontic Journal.2024; 57(6): 630. CrossRef - Role of induced nitric oxide synthases in orofacial nociception/discomfort after dental tooth bleaching with hydrogen peroxide
Marcílio Rodrigues Pinto, Kirlya Isabel da Silva Medeiros, Letícia Menezes Maia, Antonio Alexandre Coelho, Ana Paula Negreiros Nunes Alves, Caio Ferreira Freire Caetano, Karine Cestaro Mesquita, Paulo Goberlânio de Barros Silva, Fabricio Bitu Sousa
Archives of Oral Biology.2024; 161: 105937. CrossRef - Can different agents reduce the damage caused by bleaching gel to pulp tissue? A systematic review of basic research
Letícia Aparecida Silva Batista, Alexandre Henrique dos Reis-Prado, Hebertt Gonzaga dos Santos Chaves, Lara Cancella de Arantes, Luís Fernando Santos Alves Morgan, Carolina Bosso André, Thaís Yumi Suzuki, Francine Benetti
Restorative Dentistry & Endodontics.2023;[Epub] CrossRef - The Effects of Different Drugs with Anti-Inflamatory Potential in Prevention of Pulp Damage During the Teeth Bleaching
Miona Glisic, Andjela Milojevic, Milica Milinkovic, Marina Rankovic
Experimental and Applied Biomedical Research (EABR).2023;[Epub] CrossRef - Bleaching gel volume influences hydrogen peroxide diffusion, inflammation, and the presence of nitric oxide in the pulp tissue: in vitro and in vivo model
Sibele de ALCÂNTARA, Francine BENETTI, Lívia Maria Alves Valentim da SILVA, Nathália Evelyn da Silva MACHADO, Isabela Joane Prado SILVA, Lara Maria Bueno ESTEVES, Edilson ERVOLINO, Luciano Tavares Angelo CINTRA, André Luiz Fraga BRISO
Journal of Applied Oral Science.2023;[Epub] CrossRef - Design of a thermosensitive ibuprofen-loaded nanogel as smart material applied as anti-inflammatory in tooth bleaching: An in vivo study
Samara K.S.C.F. Moura, Milena L.V. dos Santos, Lucas A. do Nascimento, Mariana F.A. da Silva, Glória M. de França, Lucas M. da Costa, Aldo C. Medeiros, Raimundo F. Araújo-Júnior, Aurigena A. de Araújo, Cláudia N. Oliveira, André L. Dorini, Rejane A. de Ca
Journal of Drug Delivery Science and Technology.2022; 68: 103123. CrossRef - Topical application of Otosporin® before in-office bleaching: a split mouth, triple-blind, multicenter randomized clinical trial
Michael Willian Favoreto, Laína Vochikovski, Renata Maria Oleniki Terra, Veridiana Silva Campos, Mariana Evangelista Santos, Sônia Saeger Meireles, Alessandra Reis, Alessandro D. Loguercio
Clinical Oral Investigations.2022; 26(3): 2555. CrossRef - A novel tooth bleaching gel based on peroxymonosulfate/polyphosphates advanced oxidation process: Effective whitening avoiding pulp damage and sensitivity
Su Yang, Baiyan Sui, Xin Liu, Jiao Sun, Jun Wang
Chemical Engineering Journal.2022; 429: 132525. CrossRef - Effectiveness of Violet LED alone or in association with bleaching gel during dental photobleaching: A Systematic Review
Bianca Rossi, Susana Morimoto, Tamara Kerber Tedesco, Sandra Ribeiro Cunha, Anna Carolina Ratto Tempestini Horliana, Karen Müller Ramalho
Photodiagnosis and Photodynamic Therapy.2022; 38: 102813. CrossRef
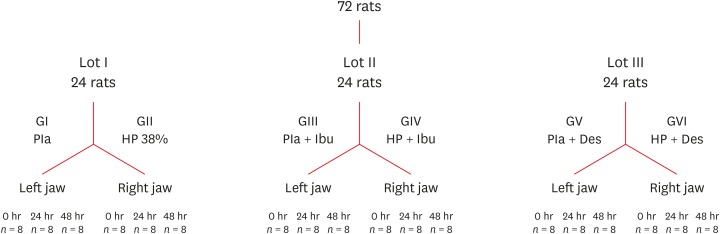
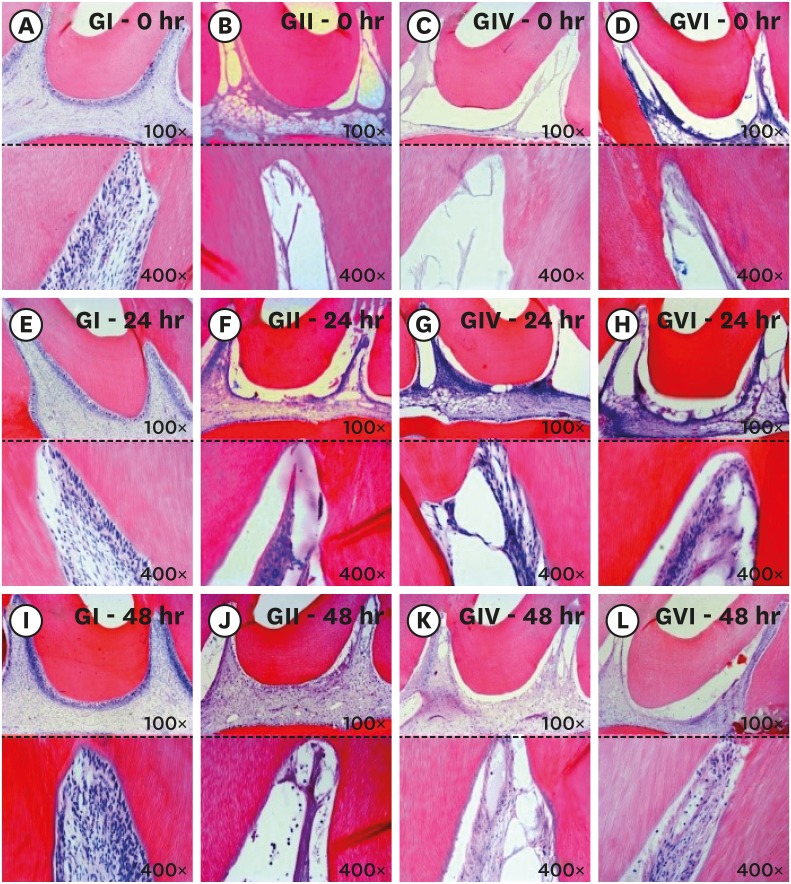
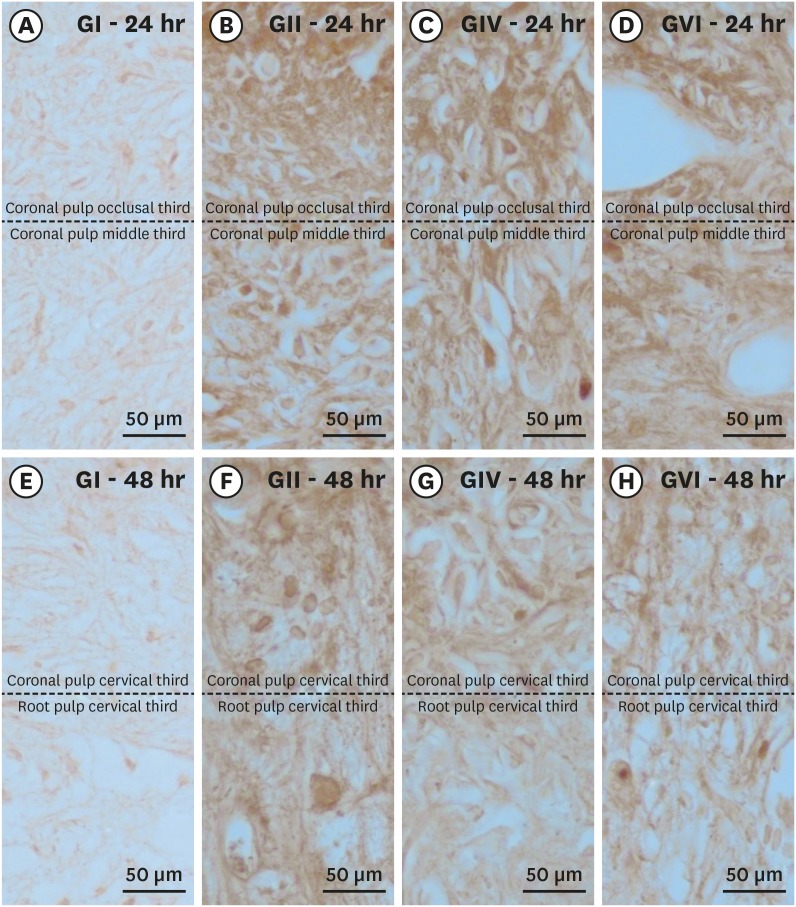
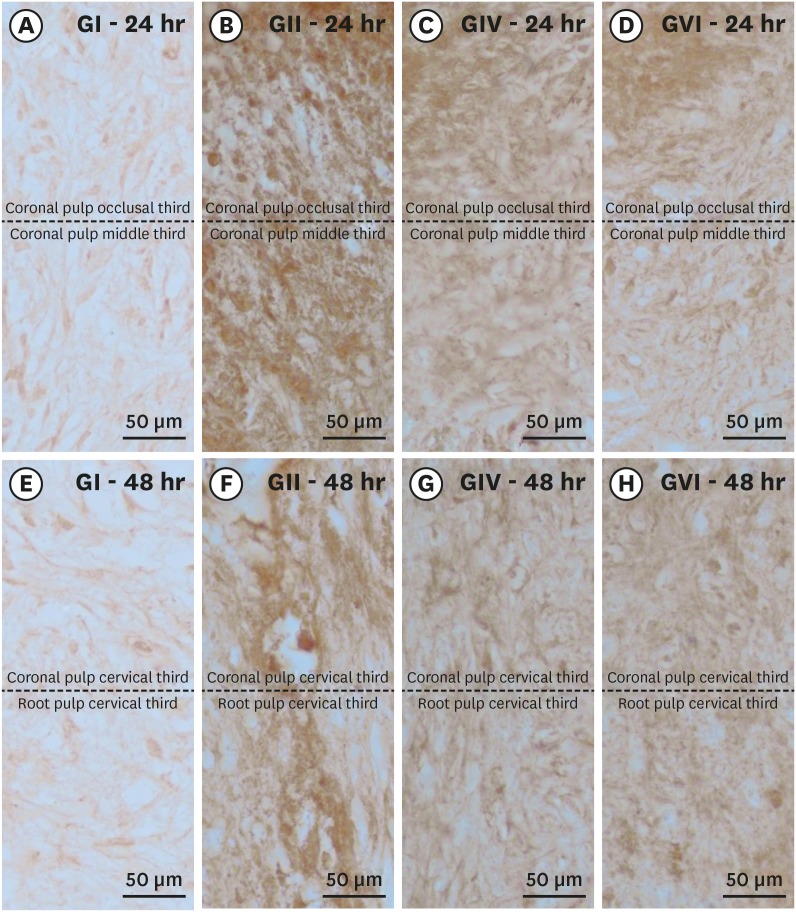
Figure 1
Figure 2
Figure 3
Figure 4
Median scores from the histological analysis of each crown and radicular third for all groups
| Third | GI | GII | GIII | GIV | GV | GVI | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 hr | 24 hr | 48 hr | 0 hr | 24 hr | 48 hr | 0 hr | 24 hr | 48 hr | 0 hr | 24 hr | 48 hr | 0 hr | 24 hr | 48 hr | 0 hr | 24 hr | 48 hr | ||
| Crown | |||||||||||||||||||
| Occlusal | 1Ab | 1Ab | 1Ab | 5Aa | 4Ba | 3Ba | 1Ab | 1Ab | 1Ab | 5Aa | 3Ba | 3Bab | 1Ab | 1Ab | 1Ab | 5Aab | 3Bab | 3Ba | |
| Medial | 1Ab | 1Ab | 1Ab | 5Aa | 3ABa | 2Ba | 1Ab | 1Ab | 1Ab | 5Aa | 3Ba | 2Ba | 1Ab | 1Ab | 1Ab | 5Aa | 2Ba | 2Ba | |
| Cervical | 1Ab | 1Ab | 1Ab | 5Aa | 2ABa | 2Ba | 1Ab | 1Ab | 1Ab | 4Aa | 2Ba | 1Ba | 1Ab | 1Ab | 1Ab | 5Aa | 1Bab | 1Ba | |
| Root | |||||||||||||||||||
| Coronal | 1Ab | 1Aa | 1Aa | 3Aa | 1ABa | 1Ba | 1Ab | 1Aa | 1Aa | 2Aa | 1Ba | 1Ba | 1Ab | 1Aa | 1Aa | 2Aa | 1Ba | 1Ba | |
| Medial | 1Ab | 1Aa | 1Aa | 2Aa | 1Aa | 1Aa | 1Ab | 1Aa | 1Aa | 1Aa | 1Aa | 1Aa | 1Ab | 1Aa | 1Aa | 1Aa | 1Aa | 1Aa | |
| Apical | 1Aa | 1Aa | 1Aa | 1Aa | 1Aa | 1Aa | 1Aa | 1Aa | 1Aa | 1Aa | 1Aa | 1Aa | 1Aa | 1Aa | 1Aa | 1Aa | 1Aa | 1Aa | |
Six groups were divided as follows: GI, control; GII, boost 38%; GIII, control + ibuprofen; GIV, boost 38% + ibuprofen; GV, control + desensibilize; and GVI, boost 38% + desensibilize.
Means followed by different letters represent statistically significant differences (p < 0.05). Different lowercase letters indicate statistically significant differences between columns in the same rows, and uppercase letters indicate statistically significant differences between rows within the same column.
Median scores from substance P immunostaining in each crown and radicular third for all bleaching groups
| Third | GI | GII | GIII | GIV | GV | GVI | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 hr | 24 hr | 48 hr | 0 hr | 24 hr | 48 hr | 0 hr | 24 hr | 48 hr | 0 hr | 24 hr | 48 hr | 0 hr | 24 hr | 48 hr | 0 hr | 24 hr | 48 hr | ||
| Crown | |||||||||||||||||||
| Occlusal | 2Ab | 2Ab | 2Ab | 5Aa | 4Ba | 4Ba | 2Ab | 2Ab | 2Ab | 5Aa | 4ABa | 4Ba | 2Ab | 2Ab | 2Ab | 5Aa | 4Aba | 3Bab | |
| Medial | 2Ab | 2Ab | 2Ab | 5Aa | 4ABa | 4Ba | 2Ab | 2Ab | 2Ab | 5Aa | 4ABa | 3Ba | 2Ab | 2Ab | 2Ab | 5Aa | 3Bab | 3Bab | |
| Cervical | 2Ab | 2Ab | 2Ab | 5Aa | 4ABa | 3Ba | 2Ab | 2Ab | 2Ab | 5Aa | 3ABab | 3Bab | 2Ab | 2Ab | 2Ab | 5Aa | 3ABab | 2Bab | |
| Root | |||||||||||||||||||
| Coronal | 2Ab | 2Ab | 2Aa | 5Aa | 3ABa | 2Ba | 2Ab | 2Ab | 2Aa | 4Aa | 3ABab | 2Ba | 2Ab | 2Ab | 2Aa | 4Aab | 3ABab | 2Ba | |
| Medial | 2Ab | 2Ab | 2Aa | 4Aa | 3ABa | 2Ba | 2Ab | 2Ab | 2Aa | 4Aa | 2Bab | 2Ba | 2Ab | 2Ab | 2Aa | 4Aab | 2Bb | 2Ba | |
| Apical | 2Ab | 2Aa | 2Aa | 3Ab | 2Aa | 2Aa | 2Ab | 2Aa | 2Aa | 3Aa | 2Ba | 2Ba | 2Ab | 2Aa | 2Aa | 3Aab | 2Ba | 2Ba | |
Six groups were divided as follows: GI, control; GII, boost 38%; GIII, control + ibuprofen; GIV, boost 38% + ibuprofen; GV, control + desensibilize; and GVI, boost 38% + desensibilize.
Means followed by different letters represent statistically significant differences (p < 0.05). Different lowercase letters indicate statistically significant differences between columns in the same rows, and uppercase letters indicate statistically significant differences between rows within the same column.
Median scores from calcitonin gene-related peptide immunostaining in each crown and root third for all groups
| Third | GI | GII | GIII | GIV | GV | GVI | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 hr | 24 hr | 48 hr | 0 hr | 24 hr | 48 hr | 0 hr | 24 hr | 48 hr | 0 hr | 24 hr | 48 hr | 0 hr | 24 hr | 48 hr | 0 hr | 24 hr | 48 hr | ||
| Crown | |||||||||||||||||||
| Occlusal | 2Ab | 2Ab | 2Ab | 5Aa | 4Ba | 4Ba | 2Ab | 2Ab | 2Ab | 5Aa | 4Aa | 4Ba | 2Ab | 2Ab | 2Ab | 5Aa | 3Ba | 3Bb | |
| Medial | 2Ab | 2Ab | 2Ab | 5Aa | 4ABa | 4Ba | 2Ab | 2Ab | 2Ab | 5Aa | 4Bab | 4Ba | 2Ab | 2Ab | 2Ab | 5Aa | 3Bab | 3Bb | |
| Cervical | 2Ab | 2Ab | 2Ab | 5Aa | 4ABa | 3Ba | 2Ab | 2Ab | 2Ab | 5Aa | 4Aab | 3Ba | 2Ab | 2Ab | 2Ab | 5Aa | 3ABab | 2Bb | |
| Root | |||||||||||||||||||
| Coronal | 2Ab | 2Ab | 2Aa | 5Aa | 3ABa | 2Ba | 2Ab | 2Ab | 2Aa | 5Aa | 3Ba | 2Ba | 2Ab | 2Ab | 2Aa | 4Aab | 3Bab | 2Ba | |
| Medial | 2Ab | 2Ab | 2Aa | 4Aa | 3ABa | 2Ba | 2Ab | 2Ab | 2Aa | 4Aa | 3Bab | 2Ba | 2Ab | 2Ab | 2Aa | 4Aab | 2Bab | 2Ba | |
| Apical | 2Ab | 2Aa | 2Aa | 4Aa | 2Ba | 2Ba | 2Ab | 2Aa | 2Aa | 3Aab | 2Ba | 2Ba | 2Ab | 2Aa | 2Aa | 3Aab | 2Ba | 2Ba | |
Six groups were divided as follows: GI, control; GII, boost 38%; GIII, control + ibuprofen; GIV, boost 38% + ibuprofen; GV, control + desensibilize; and GVI, boost 38% + desensibilize.
Means followed by different letters represent statistically significant differences (p < 0.05). Different lowercase letters indicate statistically significant differences between columns in the same rows, and uppercase letters indicate statistically significant differences between rows within the same column.
Six groups were divided as follows: GI, control; GII, boost 38%; GIII, control + ibuprofen; GIV, boost 38% + ibuprofen; GV, control + desensibilize; and GVI, boost 38% + desensibilize.
Means followed by different letters represent statistically significant differences (
Six groups were divided as follows: GI, control; GII, boost 38%; GIII, control + ibuprofen; GIV, boost 38% + ibuprofen; GV, control + desensibilize; and GVI, boost 38% + desensibilize.
Means followed by different letters represent statistically significant differences (
Six groups were divided as follows: GI, control; GII, boost 38%; GIII, control + ibuprofen; GIV, boost 38% + ibuprofen; GV, control + desensibilize; and GVI, boost 38% + desensibilize.
Means followed by different letters represent statistically significant differences (

 KACD
KACD
 ePub Link
ePub Link Cite
Cite

