Articles
- Page Path
- HOME > Restor Dent Endod > Volume 44(1); 2019 > Article
- Research Article Evaluation of the effects of whitening mouth rinses combined with conventional tooth bleaching treatments
-
Jaqueline Costa Favaro1
 , Omar Geha1
, Omar Geha1 , Ricardo Danil Guiraldo1
, Ricardo Danil Guiraldo1 , Murilo Baena Lopes1
, Murilo Baena Lopes1 , Andreza Maria Fábio Aranha2
, Andreza Maria Fábio Aranha2 , Sandrine Bittencourt Berger1
, Sandrine Bittencourt Berger1
-
Restor Dent Endod 2019;44(1):e6.
DOI: https://doi.org/10.5395/rde.2019.44.e6
Published online: January 30, 2019
1Department of Restorative Dentistry, School of Dentistry, University of North Parana, Londrina, PR, Brazil.
2Department of Dental Sciences, School of Dentistry, University of Cuiabá, Cuiabá, MT, Brazil.
- Correspondence to Sandrine Bittencourt Berger, DDS, MS, PhD. Associate Professor, Department of Restorative Dentistry, School of Dentistry, University of North Parana, Rua Marselha, 183, Londrina, PR 86041-140, Brazil. berger.sandrine@gmail.com
Copyright © 2019. The Korean Academy of Conservative Dentistry
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,476 Views
- 13 Download
- 7 Crossref
Abstract
-
Objectives The aim of the present study was to evaluate the effect of whitening mouth rinses alone and in combination with conventional whitening treatments on color, microhardness, and surface roughness changes in enamel specimens.
-
Materials and Methods A total of 108 enamel specimens were collected from human third molars and divided into 9 groups (n = 12): 38% hydrogen peroxide (HP), 10% carbamide peroxide (CP), 38% HP + Listerine Whitening (LW), 10% CP + LW, 38% HP + Colgate Plax Whitening (CPW), 10% CP + CPW, LW, CPW, and the control group (CG). The initial color of the specimens was measured, followed by microhardness and roughness tests. Next, the samples were bleached, and their color, microhardness, and roughness were assessed. Data were analyzed through 2-way analysis of variance (ANOVA; microhardness and roughness) and 1-way ANOVA (color change), followed by the Tukey post hoc test. The Dunnett test was used to compare the roughness and microhardness data of the CG to those of the treated groups.
-
Results Statistically significant color change was observed in all groups compared to the CG. All groups, except the LW group, showed statistically significant decreases in microhardness. Roughness showed a statistically significant increase after the treatments, except for the 38% HP group.
-
Conclusions Whitening mouth rinses led to a whitening effect when they were used after conventional treatments; however, this process caused major changes on the surface of the enamel specimens.
INTRODUCTION
MATERIALS AND METHODS
Information about the materials used in the present study
Schematic illustration of the experimental design.
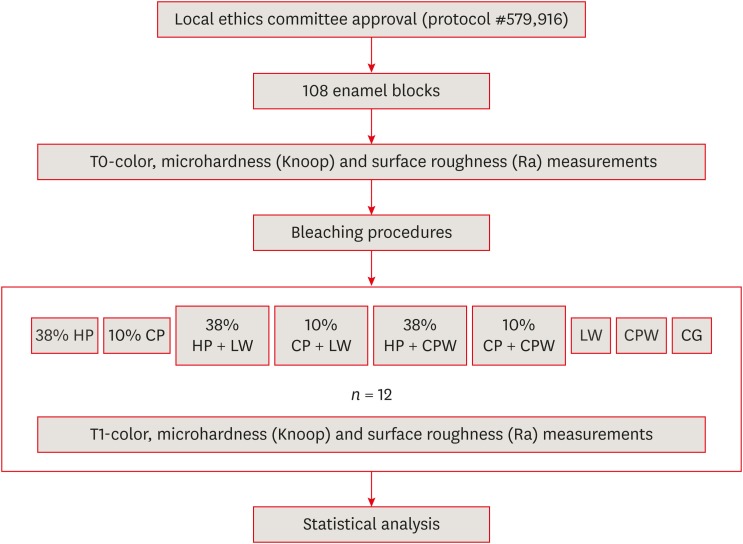
Application protocols of the bleaching agents in the experimental groups depending on treatment and application time
RESULTS
Color changes (ΔE) of the enamel specimens after various experimental bleaching protocols
Microhardness (KHN) values of the enamel specimens at baseline (T0), and after the bleaching treatments (T1), based on the bleaching protocols and evaluation time point (n = 12)
Surface roughness (Ra; in μm) values of the enamel specimens at baseline (T0) and after the bleaching treatments (T1) according to the experimental bleaching protocol and the evaluation time point (n = 12)
DISCUSSION
CONCLUSIONS
-
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
-
Author Contributions:
Conceptualization: Berger SB.
Data curation: Favaro JC, Geha O.
Formal analysis: Favaro JC, Geha O, Guiraldo RD, Lopes MB, Berger SB.
Funding acquisition: Lopes MB.
Investigation: Favaro JC, Geha O, Guiraldo RD, Aranha AMF.
Methodology: Favaro JC, Geha O, Guiraldo RD, Lopes MB.
Project administration: Berger SB.
Resources: Guiraldo RD.
Supervision: Berger SB.
Validation: Favaro JC, Geha O, Aranha AMF.
Visualization: Favaro JC, Geha O.
Writing - original draft: Favaro JC.
Writing - review & editing: Favaro JC, Geha O, Guiraldo RD, Lopes MB, Aranha AMF, Berger SB.
- 1. de Araújo LS, dos Santos PH, Anchieta RB, Catelan A, Fraga Briso AL, Fraga Zaze AC, Sundfeld RH. Mineral loss and color change of enamel after bleaching and staining solutions combination. J Biomed Opt 2013;18:108004.ArticlePubMed
- 2. Kishta-Derani M, Neiva G, Yaman P, Dennison J. In vitro evaluation of tooth-color change using four paint-on tooth whiteners. Oper Dent 2007;32:394-398.ArticlePubMedPDF
- 3. Haywood VB, Heymann HO. Nightguard vital bleaching. Quintessence Int 1989;20:173-176.PubMed
- 4. Demarco FF, Meireles SS, Masotti AS. Over-the-counter whitening agents: a concise review. Braz Oral Res 2009;23(Supplement 1):64-70.ArticlePubMed
- 5. Hilgenberg SP, Pinto SC, Farago PV, Santos FA, Wambier DS. Physical-chemical characteristics of whitening toothpaste and evaluation of its effects on enamel roughness. Braz Oral Res 2011;25:288-294.ArticlePubMed
- 6. Lima FG, Rotta TA, Penso S, Meireles SS, Demarco FF. In vitro evaluation of the whitening effect of mouth rinses containing hydrogen peroxide. Braz Oral Res 2012;26:269-274.ArticlePubMed
- 7. Torres CR, Perote LC, Gutierrez NC, Pucci CR, Borges AB. Efficacy of mouth rinses and toothpaste on tooth whitening. Oper Dent 2013;38:57-62.ArticlePubMedPDF
- 8. Jaime IM, França FM, Basting RT, Turssi CP, Amaral FL. Efficacy of hydrogen-peroxide-based mouthwash in altering enamel color. Am J Dent 2014;27:47-50.PubMed
- 9. Potgieter E, Osman Y, Grobler SR. The effect of three whitening oral rinses on enamel micro-hardness. SADJ 2014;69:152. 154-156.PubMed
- 10. Bistey T, Nagy IP, Simó A, Hegedus C. In vitro FT-IR study of the effects of hydrogen peroxide on superficial tooth enamel. J Dent 2007;35:325-330.ArticlePubMed
- 11. Soares DG, Ribeiro AP, Sacono NT, Loguércio AD, Hebling J, Costa CA. Mineral loss and morphological changes in dental enamel induced by a 16% carbamide peroxide bleaching gel. Braz Dent J 2013;24:517-521.ArticlePubMed
- 12. Cvikl B, Lussi A, Moritz A, Flury S. Enamel surface changes after exposure to bleaching gels containing carbamide peroxide or hydrogen peroxide. Oper Dent 2016;41:E39-E47.ArticlePubMedPDF
- 13. Özkan P, Kansu G, Özak ST, Kurtulmuş-Yilmaz S, Kansu P. Effect of bleaching agents and whitening dentifrices on the surface roughness of human teeth enamel. Acta Odontol Scand 2013;71:488-497.PubMed
- 14. Berger SB, Cavalli V, Ambrosano GM, Giannini M. Changes in surface morphology and mineralization level of human enamel following in-office bleaching with 35% hydrogen peroxide and light irradiation. Gen Dent 2010;58:e74-e79.PubMed
- 15. Yasa B, Arslan H, Akcay M, Kavrik F, Hatirli H, Ozkan B. Comparison of bleaching efficacy of two bleaching agents on teeth discoloured by different antibiotic combinations used in revascularization. Clin Oral Investig 2015;19:1437-1442.ArticlePubMedPDF
- 16. Horuztepe SA, Baseren M. Effect of resin infiltration on the color and microhardness of bleached white-spot lesions in bovine enamel (an in vitro study). J Esthet Restor Dent 2017;29:378-385.ArticlePubMedPDF
- 17. Karadas M, Hatipoglu O. Efficacy of mouthwashes containing hydrogen peroxide on tooth whitening. Sci World J 2015;2015:961403.ArticlePubMedPMCPDF
- 18. Melo CF, Manfroi FB, Spohr AM. Microhardness and roughness of enamel bleached with 10% carbamide peroxide and brushed with different toothpastes: an in situ study. J Int Oral Health 2014;6:18-24.
- 19. Bolay S, Cakir FY, Gurgan S. Effects of toothbrushing with fluoride abrasive and whitening dentifrices on both unbleached and bleached human enamel surface in terms of roughness and hardness: an in vitro study. J Contemp Dent Pract 2012;13:584-589.ArticlePubMed
- 20. Joiner A. Review of the effects of peroxide on enamel and dentine properties. J Dent 2007;35:889-896.ArticlePubMed
- 21. Matis BA, Cochran MA, Eckert G. Review of the effectiveness of various tooth whitening systems. Oper Dent 2009;34:230-235.ArticlePubMedPDF
- 22. Faraoni-Romano JJ, Da Silveira AG, Turssi CP, Serra MC. Bleaching agents with varying concentrations of carbamide and/or hydrogen peroxides: effect on dental microhardness and roughness. J Esthet Restor Dent 2008;20:395-402.ArticlePubMed
REFERENCES
Tables & Figures
REFERENCES
Citations

- Which Whitening Mouthwash With Different Ingredients Is More Effective on Color and Bond Strength of Enamel?
Elif Varli Tekingur, Fatih Bedir, Muhammet Karadas, Rahime Zeynep Erdem
Journal of Esthetic and Restorative Dentistry.2025; 37(4): 960. CrossRef - Do Different Tooth Bleaching–Remineralizing Regimens Affect the Bleaching Effectiveness and Enamel Microhardness In Vitro?
Hamideh Sadat Mohammadipour, Parnian Shokrollahi, Sima Gholami, Hosein Bagheri, Fatemeh Namdar, Salehe Sekandari, Cesar Rogério Pucci
International Journal of Dentistry.2024;[Epub] CrossRef - Effect of hydrogen peroxide versus charcoal-based whitening mouthwashes on color, surface roughness, and color stability of enamel
Mayada S. Sultan
BMC Oral Health.2024;[Epub] CrossRef - Effects of online marketplace-sourced over-the-counter tooth whitening products on the colour, microhardness, and surface topography of enamel: an in vitro study
Radhika Agarwal, Nikki Vasani, Urmila Sachin Mense, Niharika Prasad, Aditya Shetty, Srikant Natarajan, Arindam Dutta, Manuel S. Thomas
BDJ Open.2024;[Epub] CrossRef - Effect of Whitening Mouthwashes on Color Change and Enamel Mineralization: An In Vitro Study
Rosa Josefina Roncal Espinoza, José Alberto Castañeda Vía, Alexandra Mena-Serrano, Lidia Yileng Tay
World Journal of Dentistry.2023; 14(9): 739. CrossRef - Effectiveness and Adverse Effects of Over-the-Counter Whitening Products on Dental Tissues
Maiara Rodrigues de Freitas, Marynara Mathias de Carvalho, Priscila Christiane Suzy Liporoni, Ana Clara Borges Fort, Rodrigo de Morais e Moura, Rayssa Ferreira Zanatta
Frontiers in Dental Medicine.2021;[Epub] CrossRef - Renklendirilmiş kompozit rezinin renk değişimine ve yüzey pürüzlülüğüne beyazlatıcı ağız gargarasının etkisi
Şeref Nur MUTLU, Makbule Tuğba TUNCDEMIR
Selcuk Dental Journal.2020; 7(3): 435. CrossRef
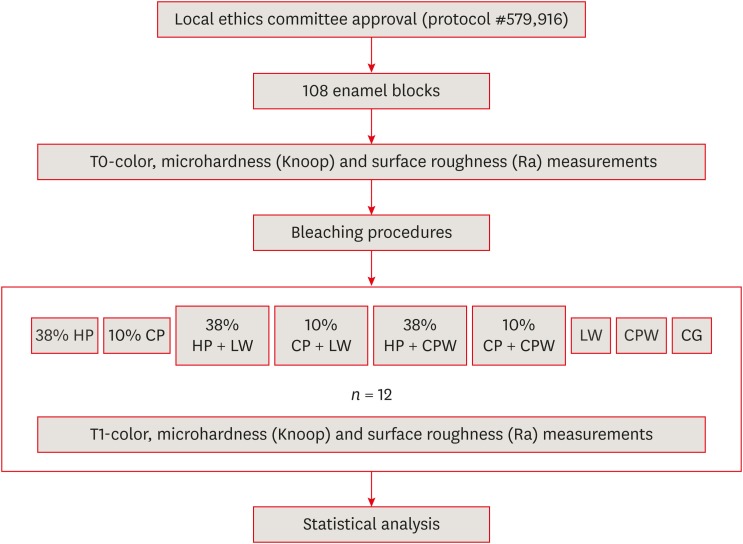
Figure 1
Information about the materials used in the present study
| Product | Manufacturer/batch | Composition | Classification | pH |
|---|---|---|---|---|
| Opalescence Boost (38% HP) | Ultradent Products Inc., South Jordan, UT, USA/DO 19U | Gel: Hydrogen peroxide | Whitening gel | 6.88 |
| Activator: Potassium hydroxide, 1.1% fluoride, and 3% potassium nitrate | ||||
| Opalescence 10% PF (10% CP) | Ultradent Products Inc., South Jordan, UT, USA/D014W | Carbamide peroxide, potassium nitrate, 0.11% fluoride ion, carbopol, glycerin, flavor | Whitening gel | 6.5 |
| Listerine Whitening (LW) | Johnson & Johnson do Brasil Indústria e Comércio de Produtos para Saúde Ltda, São Paulo City, São Paulo, Brazil/0285C | Water, alcohol (8%), hydrogen peroxide, sodium phosphate, poloxamer 407, sodium lauryl sulfate, sodium citrate, mint flavor, menthol, eucalyptol, sodium saccharin, sucralose | Mouth rinse | 5.38 |
| Colgate Plax Whitening (CPW) | Colgate-Palmolive Ind. Ltda. São Paulo City, São Paulo, Brazil/5260BR121C | Water, ethyl alcohol, hydrogen peroxide, sorbitol, poloxamer 338, polysorbate 20, methyl salicylate, menthol, sodium saccharin, CL 42090 | Mouth rinse | 3.42 |
| Control group | - | Distilled water | - | 7.0 |
Application protocols of the bleaching agents in the experimental groups depending on treatment and application time
| Group | Bleaching agent | Application protocol* |
|---|---|---|
| 38% HP | Opalescence Boost | 3 sessions (3 applications, 15 min each), 1-week interval between sessions |
| 10% CP | Opalescence 10% PF | 6 hr/day, 15 days |
| 38% HP + LW | Opalescence Boost + Listerine Whitening | 3 sessions 38% HP + LW: 1 min × 2 times/day, 12 weeks* |
| 10% CP + LW | Opalescence 10% PF + Listerine Whitening | 10% CP, 6 hr/day, 15 days; LW, 1 min × 2 times/day, 12 weeks |
| 38% HP + CPW | Opalescence Boost + Colgate Plax Whitening | 3 sessions with 38% HP; CPW, 2 min × 2 times/day, 12 weeks |
| 10% CP + CPW | Opalescence 10% PF + Colgate Plax Whitening | 10% CP, 6 hr/day, 15 days; CPW, 2 min × 2 times/day, 12 weeks |
| LW | Listerine Whitening | 1 min × 2 times/day, 12 weeks |
| CPW | Colgate Plax Whitening | 2 min × 2 times/day, 12 weeks |
| CG | Distilled water | Immersion for 12 weeks |
HP, hydrogen peroxide (38% HP; Opalescence Boost, Ultradent Products Inc., South Jordan, UT, USA); CP, carbamide peroxide (10% CP; Opalescence 10% PF, Ultradent Products Inc.); LW, Listerine Whitening (Johnson & Johnson do Brasil Indústria e Comércio de Produtos para Saúde Ltda, São Paulo, SP, Brazil); CPW, Colgate Plax Whitening (Colgate-Palmolive Ind. Ltda., São Paulo, SP, Brazil); CG, control group.
*According to the manufacturer's instructions.
Color changes (ΔE) of the enamel specimens after various experimental bleaching protocols
| Treatment | ΔL | Δa | Δb | ΔE |
|---|---|---|---|---|
| 38% HP | 6.74 ±; 2.35 | −1.44 ±; 0.82 | −5.98 ±; 2.38 | 9.51 ±; 2.20bc* |
| 10% CP | 8.86 ±; 3.67 | −1.72 ±; 0.95 | −8.95 ±; 4.27 | 13.15 ±; 4.5ab* |
| 38% HP + LW | 4.66 ±; 3.28 | −2.13 ±; 1.42 | −10.10 ±; 5.25 | 12.02 ±; 4.70b* |
| 10% CP + LW | 3.11 ±; 2.98 | −2.05 ±; 1.03 | −7.86 ±; 5.87 | 9.75 ±; 4.70bc* |
| 38% HP + CPW | 5.02 ±; 6.67 | −3.03 ±; 1,86 | −15.17 ±; 3.49 | 17.84 ±; 4.30a* |
| 10% CP + CPW | 7.75 ±; 4.91 | −1.81 ±; 1.40 | −10.72 ±; 4.03 | 14.20 ±; 4.40ab* |
| LW | 3.48 ±; 3.66 | −1.40 ±; 0.82 | −1.73 ±; 3.50 | 6.08 ±; 2.50cd* |
| CPW | 3.48 ±; 3.66 | −1.40 ±; 0.82 | −1,73 ±; 3.50 | 3.66 ±; 2.00d* |
| CG | 0.88 ±; 0.59 | 0.25 ±; 0.52 | 0.44 ±; 0.58 | 1.30 ±; 0.59 |
Data are presented as means ± standard deviations. Values with different superscript letters were statistically different according to the Tukey test (p < 0.05).
HP, hydrogen peroxide (38% HP; Opalescence Boost, Ultradent Products Inc., South Jordan, UT, USA); CP, carbamide peroxide (10% CP; Opalescence 10% PF, Ultradent Products Inc.); LW, Listerine Whitening (Johnson & Johnson do Brasil Indústria e Comércio de Produtos para Saúde Ltda, São Paulo, SP, Brazil); CPW, Colgate Plax Whitening (Colgate-Palmolive Ind. Ltda., São Paulo, SP, Brazil); CG, control group.
*Asterisk means that the value is statistically significantly different from the corresponding value in the control group according to the Dunnett test (p < 0.05).
Microhardness (KHN) values of the enamel specimens at baseline (T0), and after the bleaching treatments (T1), based on the bleaching protocols and evaluation time point (n = 12)
| Treatment | Evaluation time point | Hardness loss (%) | |
|---|---|---|---|
| T0 | T1 | ||
| 38% HP | 328.33 ±; 7.16Aa | 252.64 ±; 23.18Bb* | 23.05 |
| 10% CP | 324.65 ±; 11.56Aa | 234.57 ±; 7.68Bbc* | 27.75 |
| 38% HP + LW | 320.59 ±; 27.68Aa | 208.75 ±; 25.01Bc* | 12.43 |
| 10% CP + LW | 314.69 ±; 26.51Aa | 226.75 ±; 34.57Bbc* | 27.94 |
| 38% HP + CPW | 315.94 ±; 19.69Aa | 129.53 ±; 21.48Bd* | 59.00 |
| 10% CP + CPW | 328.72 ±; 13.86Aa | 148.66 ±; 36.66Bd* | 54.78 |
| LW | 318.52 ±; 29.15Aa | 298.42 ±; 21.56Aa* | 6.31 |
| CPW | 325.57 ±; 15.06Aa | 242.28 ±; 36.46Bbc* | 25.58 |
| CG | 326.46 ±; 12.06 | ||
Data are presented as means ± standard deviations. Values with different uppercase superscript letters in each row were statistically significantly different for each treatment between the time points before (T0) and after (T1) treatment based on the Tukey test (p < 0.05). Values with different lowercase superscript letters in each column were statistically significantly different at each time point between the experimental treatment groups based on the Tukey test (p < 0.05).
HP, hydrogen peroxide (38% HP; Opalescence Boost, Ultradent Products Inc., South Jordan, UT, USA); CP, carbamide peroxide (10% CP; Opalescence 10% PF, Ultradent Products Inc.); LW, Listerine Whitening (Johnson & Johnson do Brasil Indústria e Comércio de Produtos para Saúde Ltda, São Paulo, SP, Brazil); CPW, Colgate Plax Whitening (Colgate-Palmolive Ind. Ltda., São Paulo, SP, Brazil); CG, control group.
*Asterisk means that the value is statistically significantly different from the corresponding value in the control group according to the Dunnett test (p < 0.05).
Surface roughness (Ra; in μm) values of the enamel specimens at baseline (T0) and after the bleaching treatments (T1) according to the experimental bleaching protocol and the evaluation time point (n = 12)
| Treatment | Evaluation time point | Roughness change (%) | |
|---|---|---|---|
| T0 | T1 | ||
| 38% HP | 0.037 ±; 0.014Aa | 0.047 ±; 0.024Ac | 127.03 |
| 10% CP | 0.030 ±; 0.008Ba | 0.050 ±; 0.012Ac | 166.67 |
| 38% HP + LW | 0.029 ±; 0.008Ba | 0.183 ±; 0.033Aab* | 631.03 |
| 10% CP + LW | 0.027 ±; 0.010Ba | 0.163 ±; 0.037Aab* | 603.70 |
| 38% HP + CPW | 0.031 ±; 0.013Ba | 0.208 ±; 0.115Aa* | 670.97 |
| 10% CP + CPW | 0.044 ±; 0.033Ba | 0.201 ±; 0.019Aab* | 456.82 |
| LW | 0.025 ±; 0.012Ba | 0.133 ±; 0.027Ab* | 532.00 |
| CPW | 0.033 ±; 0.021Ba | 0.194 ±; 0.023Aab* | 587.88 |
| CG | 0.031 ±; 0.0130 | ||
Data are presented as means ± standard deviations. Values with different uppercase superscript letters in each row were statistically significantly different for each treatment between the time points before (T0) and after (T1) treatment based on the Tukey test (p < 0.05). Values with different lowercase superscript letters in each column were statistically significantly different at each time point between the experimental treatment groups based on the Tukey test (p < 0.05).
HP, hydrogen peroxide (38% HP; Opalescence Boost, Ultradent Products Inc., South Jordan, UT, USA); CP, carbamide peroxide (10% CP; Opalescence 10% PF, Ultradent Products Inc.); LW, Listerine Whitening (Johnson & Johnson do Brasil Indústria e Comércio de Produtos para Saúde Ltda, São Paulo, SP, Brazil); CPW, Colgate Plax Whitening (Colgate-Palmolive Ind. Ltda., São Paulo, SP, Brazil); CG, control group.
*Asterisk means that the value is statistically significantly different from the corresponding value in the control group according to the Dunnett test (p < 0.05).
HP, hydrogen peroxide (38% HP; Opalescence Boost, Ultradent Products Inc., South Jordan, UT, USA); CP, carbamide peroxide (10% CP; Opalescence 10% PF, Ultradent Products Inc.); LW, Listerine Whitening (Johnson & Johnson do Brasil Indústria e Comércio de Produtos para Saúde Ltda, São Paulo, SP, Brazil); CPW, Colgate Plax Whitening (Colgate-Palmolive Ind. Ltda., São Paulo, SP, Brazil); CG, control group.
*According to the manufacturer's instructions.
Data are presented as means ± standard deviations. Values with different superscript letters were statistically different according to the Tukey test (
HP, hydrogen peroxide (38% HP; Opalescence Boost, Ultradent Products Inc., South Jordan, UT, USA); CP, carbamide peroxide (10% CP; Opalescence 10% PF, Ultradent Products Inc.); LW, Listerine Whitening (Johnson & Johnson do Brasil Indústria e Comércio de Produtos para Saúde Ltda, São Paulo, SP, Brazil); CPW, Colgate Plax Whitening (Colgate-Palmolive Ind. Ltda., São Paulo, SP, Brazil); CG, control group.
*Asterisk means that the value is statistically significantly different from the corresponding value in the control group according to the Dunnett test (
Data are presented as means ± standard deviations. Values with different uppercase superscript letters in each row were statistically significantly different for each treatment between the time points before (T0) and after (T1) treatment based on the Tukey test (
HP, hydrogen peroxide (38% HP; Opalescence Boost, Ultradent Products Inc., South Jordan, UT, USA); CP, carbamide peroxide (10% CP; Opalescence 10% PF, Ultradent Products Inc.); LW, Listerine Whitening (Johnson & Johnson do Brasil Indústria e Comércio de Produtos para Saúde Ltda, São Paulo, SP, Brazil); CPW, Colgate Plax Whitening (Colgate-Palmolive Ind. Ltda., São Paulo, SP, Brazil); CG, control group.
*Asterisk means that the value is statistically significantly different from the corresponding value in the control group according to the Dunnett test (
Data are presented as means ± standard deviations. Values with different uppercase superscript letters in each row were statistically significantly different for each treatment between the time points before (T0) and after (T1) treatment based on the Tukey test (
HP, hydrogen peroxide (38% HP; Opalescence Boost, Ultradent Products Inc., South Jordan, UT, USA); CP, carbamide peroxide (10% CP; Opalescence 10% PF, Ultradent Products Inc.); LW, Listerine Whitening (Johnson & Johnson do Brasil Indústria e Comércio de Produtos para Saúde Ltda, São Paulo, SP, Brazil); CPW, Colgate Plax Whitening (Colgate-Palmolive Ind. Ltda., São Paulo, SP, Brazil); CG, control group.
*Asterisk means that the value is statistically significantly different from the corresponding value in the control group according to the Dunnett test (

 KACD
KACD
 ePub Link
ePub Link Cite
Cite

