Articles
- Page Path
- HOME > Restor Dent Endod > Volume 43(3); 2018 > Article
- Research Article Effects of a bleaching agent on properties of commercial glass-ionomer cements
-
Fernanda Lúcia Lago de Camargo1
 , Ailla Carla Lancellotti2
, Ailla Carla Lancellotti2 , Adriano Fonseca de Lima3
, Adriano Fonseca de Lima3 , Vinícius Rangel Geraldo Martins4
, Vinícius Rangel Geraldo Martins4 , Luciano de Souza Gonçalves5
, Luciano de Souza Gonçalves5
-
Restor Dent Endod 2018;43(3):e32.
DOI: https://doi.org/10.5395/rde.2018.43.e32
Published online: July 5, 2018
1Department of Restorative Dentistry, Integrated School of Carajás School of Dentistry, Redenção, PA, Brazil.
2Department of Restorative Dentistry, Piracicaba Dental School, Piracicaba, SP, Brazil.
3Department of Restorative Dentistry, Paulista University School of Dentistry, São Paulo, SP, Brazil.
4Department of Restorative Dentistry, Uberaba University School of Dentistry, Uberaba, MG, Brazil.
5Department of Conservative Dentistry, Federal University of Rio Grande do Sul School of Dentistry, Porto Alegre, RS, Brazil.
- Correspondence to Luciano de Souza Gonçalves, DDS, MSc, PhD. Adjunct Professor, Department of Conservative Dentistry, Federal University of Rio Grande do Sul School of Dentistry, Rua Ramiro Barcelos, 2492, Porto Alegre, RS 90035003, Brazil. goncalves1976@yahoo.com.br
Copyright © 2018. The Korean Academy of Conservative Dentistry
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,437 Views
- 5 Download
- 10 Crossref
Abstract
-
Objectives This study evaluated the effects of a bleaching agent on the composition, mechanical properties, and surface topography of 6 conventional glass-ionomer cements (GICs) and one resin-modified GIC.
-
Materials and Methods For 3 days, the specimens were subjected to three 20-minute applications of a 37% H2O2-based bleaching agent and evaluated for water uptake (WTK), weight loss (WL), compressive strength (CS), and Knoop hardness number (KHN). Changes in surface topography and chemical element distribution were also analyzed by energy-dispersive X-ray spectroscopy and scanning electron microscopy. For statistical evaluation, the Kruskal-Wallis and Wilcoxon paired tests (α = 0.05) were used to evaluate WTK and WL. CS specimens were subjected to 2-way analysis of variance (ANOVA) and the Tukey post hoc test (α = 0.05), and KH was evaluated by one-way ANOVA, the Holm-Sidak post hoc test (α = 0.05), and the t-test for independent samples (α = 0.05).
-
Results The bleaching agent increased the WTK of Maxxion R, but did not affect the WL of any GICs. It had various effects on the CS, KHN, surface topography, and the chemical element distribution of the GICs.
-
Conclusions The bleaching agent with 37% H2O2 affected the mechanical and surface properties of GICs. The extent of the changes seemed to be dependent on exposure time and cement composition.
INTRODUCTION
MATERIALS AND METHODS
Glass-ionomer cements (GICs) evaluated in the present study
RESULTS
Median, interquartile range, and percentages of water uptake (WTK) and weight loss (WL)
Compressive strength (MPa) of glass-ionomer cement (GIC) restoratives used in this study
Knoop hardness number of glass-ionomer cement (GIC) restoratives used in this study after different bleaching sessions
1. EDS analysis
Distribution of the chemical elements of the composition of the glass-ionomer cements (GICs) in relative percentage by weight (wt%).
2. Surface evaluation
Scanning electron microscopic images of glass-ionomer cements (×2,000): (A) Ketac Cem, (B) Ketac Molar, (C) Maxxion R, (D) Vitremer, (E) Vitro Fil, (F) Vitro Molar, and (G) Vidrion R. In the first column are shown the untreated specimens, followed by the treated groups, including the first (′), second (′′), and third (′′′) sessions, after 24, 48, and 72 hours, respectively.
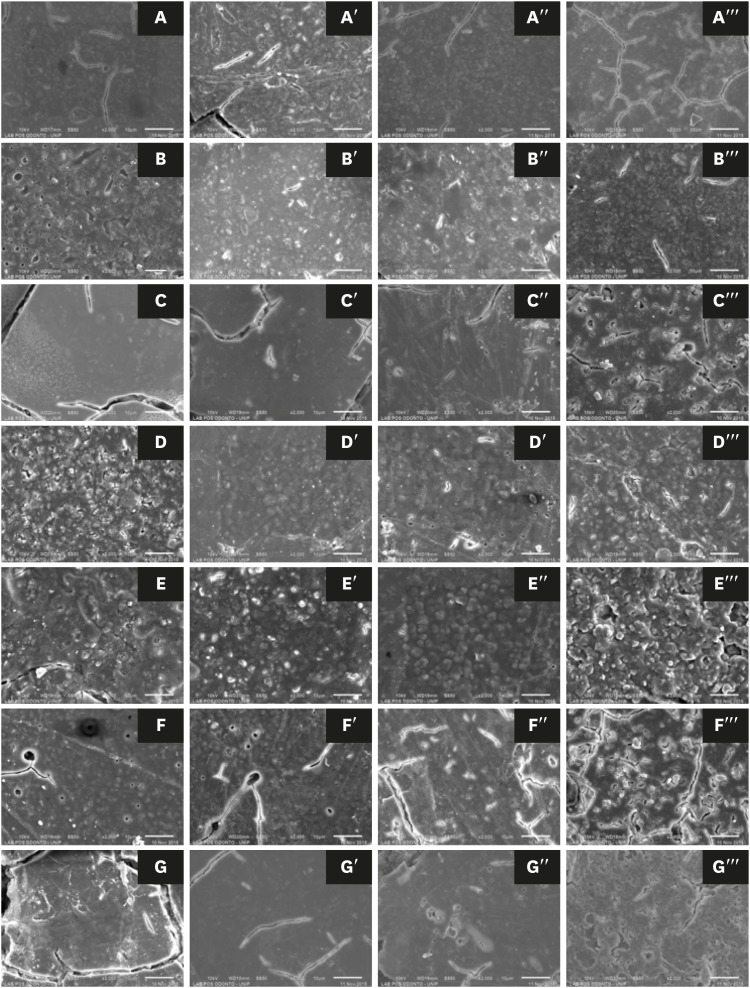
DISCUSSION
CONCLUSIONS
-
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
-
Author Contributions:
Conceptualization: Gonçalves LS.
Data curation: Camargo FLL, Gonçalves LS.
Formal analysis: Camargo FLL, Lancellotti AC, Lima AF, Geraldo-Martins VR, Gonçalves LS.
Funding acquisition: Gonçalves LS.
Investigation: Camargo FLL, Lancellotti AC, Lima AF.
Methodology: Camargo FLL, Lancellotti AC, Lima AF.
Project administration: Gonçalves LS.
Resources: Gonçalves LS.
Supervision: Gonçalves LS.
Validation: Lima AF, Geraldo-Martins VR, Gonçalves LS.
Visualization: Lima AF, Geraldo-Martins VR, Gonçalves LS.
Writing - original draft: Camargo FLL.
Writing - review & editing: Lancellotti AC, Lima AF, Geraldo-Martins VR, Gonçalves LS.
- 1. Wilson AD, Kent BE. The glass-ionomer cement, a new translucent dental filling material. J Chem Technol Biotechnol 1971;21:313.Article
- 2. Baroudi K, Mahmoud RS, Tarakji B. Fluoride release of glass ionomer restorations after bleaching with two different bleaching materials. Eur J Dent 2013;7:196-200.ArticlePubMedPMC
- 3. Markovic DL, Petrovic BB, Peric TO. Fluoride content and recharge ability of five glassionomer dental materials. BMC Oral Health 2008;8:21.ArticlePubMedPMCPDF
- 4. Wiegand A, Buchalla W, Attin T. Review on fluoride-releasing restorative materials--fluoride release and uptake characteristics, antibacterial activity and influence on caries formation. Dent Mater 2007;23:343-362.ArticlePubMed
- 5. Arita K, Yamamoto A, Shinonaga Y, Harada K, Abe Y, Nakagawa K, Sugiyama S. Hydroxyapatite particle characteristics influence the enhancement of the mechanical and chemical properties of conventional restorative glass ionomer cement. Dent Mater J 2011;30:672-683.ArticlePubMed
- 6. Benetti AR, Jacobsen J, Lehnhoff B, Momsen NC, Okhrimenko DV, Telling MT, Kardjilov N, Strobl M, Seydel T, Manke I, Bordallo HN. How mobile are protons in the structure of dental glass ionomer cements? Sci Rep 2015;5:8972.ArticlePubMedPMCPDF
- 7. Bresciani E, Barata TJ, Fagundes TC, Adachi A, Terrin MM, Navarro MF. Compressive and diametral tensile strength of glass ionomer cements. J Appl Oral Sci 2004;12:344-348.ArticlePubMed
- 8. Carvalho FG, Sampaio CS, Fucio SB, Carlo HL, Correr-Sobrinho L, Puppin-Rontani RM. Effect of chemical and mechanical degradation on surface roughness of three glass ionomers and a nanofilled resin composite. Oper Dent 2012;37:509-517.ArticlePubMedPDF
- 9. Khoroushi M, Keshani F. A review of glass-ionomers: From conventional glass-ionomer to bioactive glass-ionomer. Dent Res J (Isfahan) 2013;10:411-420.PubMedPMC
- 10. Baroudi K, Mahmoud RS, Tarakji B, Altamimi MA. Effect of vital bleaching on disintegration tendency of glass ionomer restorations. J Clin Diagn Res 2014;8:214-217.
- 11. de Paula AB, de Fúcio SB, Alonso RC, Ambrosano GM, Puppin-Rontani RM. Influence of chemical degradation on the surface properties of nano restorative materials. Oper Dent 2014;39:E109-E117.ArticlePubMedPDF
- 12. El-Murr J, Ruel D, St-Georges AJ. Effects of external bleaching on restorative materials: a review. J Can Dent Assoc 2011;77:b59.PubMed
- 13. Markovic L, Jordan RA, Glasser MC, Arnold WH, Nebel J, Tillmann W, Ostermann T, Zimmer S. Effects of bleaching agents on surface roughness of filling materials. Dent Mater J 2014;33:59-63.ArticlePubMed
- 14. Turker SB, Biskin T. Effect of three bleaching agents on the surface properties of three different esthetic restorative materials. J Prosthet Dent 2003;89:466-473.ArticlePubMed
- 15. Samiei M, Janani M, Vahdati A, Alemzadeh Y, Bahari M. Scanning electron microscopy and energy-dispersive X-ray microanalysis of set CEM cement after application of different bleaching agents. Iran Endod J 2017;12:191-195.PubMedPMC
- 16. Yu H, Li Q, Lin Y, Buchalla W, Wang Y. Influence of carbamide peroxide on the flexural strength of tooth-colored restorative materials: an in vitro study at different environmental temperatures. Oper Dent 2010;35:300-307.ArticlePubMedPDF
- 17. Yu H, Li Q, Wang YN, Cheng H. Effects of temperature and in-office bleaching agents on surface and subsurface properties of aesthetic restorative materials. J Dent 2013;41:1290-1296.ArticlePubMed
- 18. Zavanelli AC, Mazaro VQ, Silva CR, Zavanelli RA, Mancuso DN. Surface roughness analysis of four restorative materials exposed to 10% and 15% carbamide peroxide. Int J Prosthodont 2011;24:155-157.PubMed
- 19. Crim GA. Prerestorative bleaching: effect on microleakage of Class V cavities. Quintessence Int 1992;23:823-825.PubMed
- 20. Mair L, Joiner A. The measurement of degradation and wear of three glass ionomers following peroxide bleaching. J Dent 2004;32(Supplementary 1):41-45.Article
- 21. Briso AL, Lima AP, Gonçalves RS, Gallinari MO, dos Santos PH. Transenamel and transdentinal penetration of hydrogen peroxide applied to cracked or microabrasioned enamel. Oper Dent 2014;39:166-173.ArticlePubMedPDF
- 22. Gonçalves LS, Moraes RR, Ogliari FA, Boaro L, Braga RR, Consani S. Improved polymerization efficiency of methacrylate-based cements containing an iodonium salt. Dent Mater 2013;29:1251-1255.ArticlePubMed
- 23. Fonseca RB, Branco CA, Quagliatto PS, Gonçalves LS, Soares CJ, Carlo HL, Correr-Sobrinho L. Influence of powder/liquid ratio on the radiodensity and diametral tensile strength of glass ionomer cements. J Appl Oral Sci 2010;18:577-584.PubMedPMC
- 24. Lim HN, Kim SH, Yu B, Lee YK. Influence of HEMA content on the mechanical and bonding properties of experimental HEMA-added glass ionomer cements. J Appl Oral Sci 2009;17:340-349.ArticlePubMedPMC
- 25. Topaloglu-Ak A, Cogulu D, Ersin NK, Sen BH. Microhardness and surface roughness of glass ionomer cements after APF and TiF4 applications. J Clin Pediatr Dent 2012;37:45-51.ArticlePubMedPDF
- 26. Sidhu SK, Nicholson JW. A review of glass-ionomer cements for clinical dentistry. J Funct Biomater 2016;7:16.ArticlePubMedPMC
- 27. Della Bona A, Rosa V, Cecchetti D. Influence of shade and irradiation time on the hardness of composite resins. Braz Dent J 2007;18:231-234.ArticlePubMed
- 28. Cehreli ZC, Yazici R, García-Godoy F. Effect of home-use bleaching gels on fluoride releasing restorative materials. Oper Dent 2003;28:605-609.PubMed
- 29. Cacciafesta V, Sfondrini MF, Tagliani P, Klersy C. In-vitro fluoride release rates from 9 orthodontic bonding adhesives. Am J Orthod Dentofacial Orthop 2007;132:656-662.ArticlePubMed
- 30. Xu X, Burgess JO. Compressive strength, fluoride release and recharge of fluoride-releasing materials. Biomaterials 2003;24:2451-2461.ArticlePubMed
- 31. Lee KH, Kim HI, Kim KH, Kwon YH. Mineral loss from bovine enamel by a 30% hydrogen peroxide solution. J Oral Rehabil 2006;33:229-233.ArticlePubMed
REFERENCES
Tables & Figures
REFERENCES
Citations

- Multidisciplinary conservative management of a severely discolored nonvital tooth
Álvaro Ferrando Cascales, Francesc Abella Sans, Rubén Agustín-Panadero, José Amengual Lorenzo
The Journal of Prosthetic Dentistry.2025; 133(4): 941. CrossRef - The effects of bleaching products on the color stability of ion-releasing restoratives
Jian Sheng Lee, Noor Azlin Yahya, Azwatee Abdul Aziz, Adrian U-Jin Yap
BMC Oral Health.2025;[Epub] CrossRef - Physical-mechanical, chemical and biological properties of graphene-reinforced glass ionomer cements
Tatiane Ramos dos Santos Jordão, Laura Soares Viana Fernandes, Karla Lorene de França Leite, Adílis Alexandria, Emmanuel João Nogueira Leal Silva, Lucianne Cople Maia, Tatiana Kelly da Silva Fidalgo
Restorative Dentistry & Endodontics.2024;[Epub] CrossRef - An In Vitro Exploration of Interaction Mechanisms of Intracoronal Bleaching on the Compressive Strength of Conventional and Calcium Silicate–Based Self‐Adhesive Resins and Their Bonding to Composite Resin Restorative Material
Fereshteh Shafiei, Paria Dehghanian, Shadi Tivay, Yasamin Ghahramani, Luca Testarelli
International Journal of Dentistry.2024;[Epub] CrossRef - Éclaircissement dentaire
V. Pilliol, B. Ballester, T. Baudinet, G. Aboudharama, E. Terrer
EMC - Odontologie.2023; 39(2): 1. CrossRef - The Effect of Home and In-Office Bleaching on Microhardness and Color of Different CAD/CAM Ceramic Materials
Ruwaida Z. Alshali, Mohammed A. Alqahtani
Materials.2022; 15(17): 5948. CrossRef - Éclaircissement dentaire
V. Pilliol, B. Ballester, T. Baudinet, G. Aboudharama, E. Terrer
EMC - Médecine buccale.2022; 15(4): 1. CrossRef - Éclaircissement dentaire
V. Pilliol, B. Ballester, T. Baudinet, G. Aboudharam, E. Terrer
EMC - Orthopédie dentofaciale.2022; 34(1): 1. CrossRef - Comparative Evaluation of Two Glass Polyalkenoate Cements: An In Vivo Pilot Study Using a Sheep Model
Leyla Hasandoost, Daniella Marx, Paul Zalzal, Oleg Safir, Mark Hurtig, Cina Mehrvar, Stephen D. Waldman, Marcello Papini, Mark R. Towler
Journal of Functional Biomaterials.2021; 12(3): 44. CrossRef - The Effect of Simulated Field Storage Conditions on Dental Restorative Materials for Military Field Use
David J Lemon, Wen Chen, Trevor Smith, April A Ford, Steven X Moffett, Jeffrey T Hoyle, Nicholas J Hamlin, Yoon Y Hwang
Military Medicine.2020; 185(5-6): e831. CrossRef
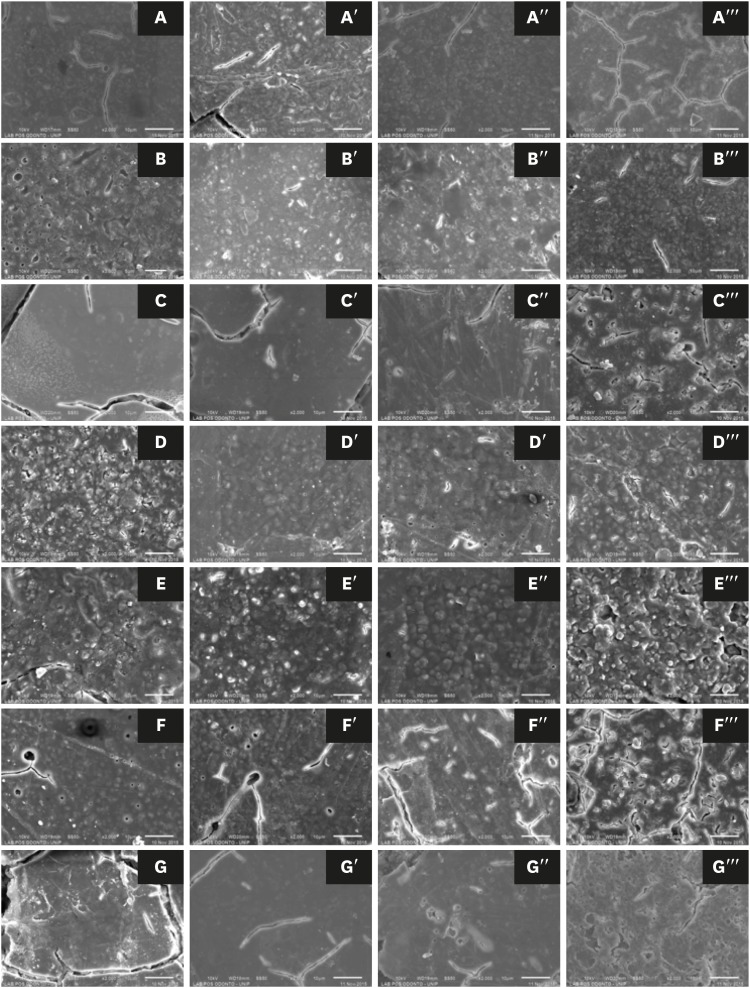
Figure 1
Glass-ionomer cements (GICs) evaluated in the present study
| GIC/batch No. | Composition* | Manufacturer | P/L ratio | Mixing time (sec) |
|---|---|---|---|---|
| Ketac Molar EasyMix/56908 | Powder: glass powder, polycarboxylic acid, pigments | 3M ESPE, St. Paul, MN, USA | 1/1 | 60 |
| Liquid: water, tartaric acid, conservation agents | ||||
| Ketac Cem Easy Mix/56908 | Powder: glass powder, polycarboxylic acid, pigments | 3M ESPE, St. Paul, MN, USA | 1/2 | 60 |
| Liquid: water, tartaric acid, conservation agents | ||||
| Vitremer/544223 | Powder: radiopaque fluoroaluminosilicate glass, microencapsulated potassium persulfate, ascorbic acid | 3M ESPE, St. Paul, MN, USA | 1/1 | 45 |
| Liquid: aqueous solution of a polycarboxylic acid modified with pendant methacrylate groups, water, hydroxyethylmethacrylate, photoinitiators | ||||
| Vitro Fil/14111774 | Powder: strontium aluminum silicate, dehydrated polyacrylic acid, iron oxide | NOVA DFL, Rio de Janeiro, RJ, Brazil | 1/1 | 60 |
| Liquid: polyacrylic acid, tartaric acid, distilled water | ||||
| Vitro Molar/15030424 | Powder: barium aluminum silicate, dehydrated polyacrylic acid, iron oxide | NOVA DFL, Rio de Janeiro, RJ, Brazil | 1/1 | 20 |
| Liquid: polyacrylic acid, tartaric acid, distilled water | ||||
| Vidrion R/0321114 | Powder: sodium-calcium-fluoroaluminosilicate glass, polyacrylic acid and pigments | SS White, Rio de Janeiro, RJ, Brazil | 1/1 | 60 |
| Liquid: tartaric acid, distilled water | ||||
| Maxxion R/031214 | Powder: fluoroaluminosilicate glass, polycarboxylic acid, calcium fluoride, radiopacifiers | FGM, Joinville, SC, Brazil | 1/1 | 60 |
| Liquid: polyacrylic acid, tartaric acid, distilled water |
*This information was provided by the manufacturers in the Material Safety Data Sheet (MSDS) and instruction sheets.
Median, interquartile range, and percentages of water uptake (WTK) and weight loss (WL)
| Group | WTK (µg) | WL (µg) | ||||||
|---|---|---|---|---|---|---|---|---|
| Not treated | % | Treated | % | Not treated | % | Treated | % | |
| Ketac Molar | 1.3 (1.2–1.65)Aab | 4.8 | 1.0 (1.0–1.1)Aa | 4.3 | 0.9 (0.38–1.15)Aab | 2.3 | 0.7 (0.45–0.83)Aab | 2.6 |
| Ketac Cem | 0.5 (0.38–1.0)Aab | 1.7 | 1.2 (1.15–1.55)Aa | 5.2 | 0.8 (0.53–1.33)Aab | 2.4 | 0.9 (0.18–2.85)Aab | 5.5 |
| Vitremer | 3.6 (3.53–4.38)Aa | 11.7 | 2.5 (2.38–2.6)Aa | 11.1 | 0.1 (0.08–0.58)Ab | 1.4 | 0.1 (0.0–0.23)Ab | 0.5 |
| Maxxion R | 0.1 (0.1–0.85)Bb | 2.2 | 4.7 (4.48–4.73)Aa | 23.4 | 5.0 (2.0–5.4)Aa | 19.3 | 3.0 (2.98–3.33)Aa | 15.8 |
| Vidrion R | 0.4 (0.1–0.85)Ab | 1.9 | 1.0 (1.0–1.1)Aa | 5.2 | 0.8 (0.58–3.45)Aab | 8.1 | 0.7 (0.45–0.83)Aab | 3.3 |
WTK (M2–M3) and water solubility (M1–M3) of the specimens were calculated in micrograms (µg) from the differences in weight gain or loss during the immersion in water and drying cycles. Different uppercase superscript letters indicate a statistically significant difference within the row (p < 0.05). Different lowercase superscript letters indicate a statistically significant difference within the column (p < 0.05).
Compressive strength (MPa) of glass-ionomer cement (GIC) restoratives used in this study
| GIC | Untreated | Treated |
|---|---|---|
| Vitremer | 113.8 ± 8.1Aa | 92.9 ± 15.9Ba |
| Ketac Molar | 112.6 ± 15.1Aa | 72.7 ± 16.7Bb |
| Ketac Cem | 112.4 ± 12.6Aa | 55.4 ± 15.0Bb |
| Vitro Molar | 75.0 ± 8.5Ab | 64.9 ± 14.3Ab |
| Vitro Fil | 66.5 ± 7.5bc | -* |
| Maxxion R | 50.9 ± 4.7Ac | 64.1 ± 17.5Ab |
| Vidrion R | 46.5 ± 14.9c | - |
Data are shown as means ± standard deviations. Different uppercase superscript letters indicate a statistically significant difference within the row (p < 0.05). Different lowercase superscript letters indicate a statistically significant difference within the column (p < 0.05).
*Vitro Fil and Vidrion R were not tested after the bleaching protocol because the specimens disintegrated.
Knoop hardness number of glass-ionomer cement (GIC) restoratives used in this study after different bleaching sessions
| GIC | Bleaching treatment | Time (session) | |||
|---|---|---|---|---|---|
| 24 hr (before the protocol) | 24 hr (first session) | 48 hr (second session) | 72 hr (third session) | ||
| Vidrion R | − | 49.8 ± 5.4Ba | 53.4 ± 5.2ABa | 57.8 ± 3.3Aa | 49.8 ± 5.2Ba |
| + | 53.1 ± 4.8ABa | 56.0 ± 5.2ABa | 58.1 ± 6.6Aa | 50.7 ± 6.1Ba | |
| Vitremer | − | 96.8 ± 11.0Aa | 97.6 ± 12.3Ab | 91.2 ± 10.0Aa | 106.2 ± 10.7Aa |
| + | 100.6 ± 9.8Ba | 119.5 ± 20.1Aa | 100.9 ± 19.8Ba | 113.1 ± 12.1ABa | |
| Vitro Molar | − | 51.1 ± 5.2Ca | 57.1 ± 7.0BCa | 60.2 ± 5.9Aa | 60.5 ± 9.4Aba |
| + | 51.0 ± 5.6Ba | 58.1 ± 5.9ABa | 63.7 ± 9.5Aa | 53.3 ± 8.6Ba | |
| Ketac Cem | − | 74.1 ± 8.8Ba | 83.2 ± 10.6ABa | 77.2 ± 8.8ABa | 86.7 ± 8.3Aa |
| + | 80.7 ± 11.1Aa | 87.3 ± 5.9Aa | 80.7 ± 7.6Aa | 82.9 ± 5.5Aa | |
| Ketac Molar | − | 143.5 ± 25.6Aa | 86.6 ± 17.2Bb | 80.8 ± 11.2Bb | 77.1 ± 11.1Bb |
| + | 148.9 ± 23.9Aa | 120.9 ± 16.6Ba | 119.9 ± 26.5Ba | 114.1 ± 10.9Ba | |
| Vitro Fil | − | 49.5 ± 5.8Ba | 60.0 ± 7.2Aa | 51.8 ± 6.3Ba | 42.0 ± 4.5Ca |
| + | 48.0 ± 2.7Ba | 60.0 ± 7.7Aa | 44.8 ± 6.1Ba | 37.2 ± 6.5Ca | |
| Maxxion R | − | 41.7 ± 7.5Aa | 40.2 ± 2.2Aa | 40.1 ± 4.5Aa | 40.5 ± 4.5Aa |
| + | 48.9 ± 6.2Aa | 41.1 ± 9.4ABa | 39.1 ± 4.8Ba | 38.2 ± 4.7Ba | |
Data are shown as means ± standard deviations. Different uppercase superscript letters indicate a statistically significant difference within each row, that is, within each cement separately (p < 0.05). Different lowercase superscript letters indicate a statistically significant difference between the presence and absence of the application of each bleaching agent (p < 0.05).
Distribution of the chemical elements of the composition of the glass-ionomer cements (GICs) in relative percentage by weight (wt%).
| GIC | Time (sessions) | Chemical elements (wt%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C | O | F | Na | Al | Si | Ca | Nb | W | Ba-L | ||
| Vidrion R | Untreated | 30.7 | 31.1 | 7.4 | 2.4 | 9.9 | 5.5 | 8.2 | - | - | 4.9 |
| 24 hr | 35.6 | 30.4 | 7.7 | 2.5 | 9.7 | 6.4 | 7.7 | - | - | - | |
| 48 hr | 31.4 | 34.0 | 7.7 | 2.5 | 10.5 | 6.7 | 7.3 | - | - | - | |
| 72 hr | 29.9 | 28.8 | 5.6 | 1.5 | 6.5 | 3.1 | 5.7 | - | - | - | |
| Vitremer | Untreated | 46.5 | 25.2 | 8.9 | 1.4 | 6.6 | 11.5 | - | 26.7 | - | - |
| 24 hr | 46.7 | 16.1 | 3.3 | 0.9 | 2.6 | 3.7 | - | 26.7 | - | - | |
| 48 hr | 47.2 | 21.1 | 4.3 | 0.7 | 4.0 | 4.2 | - | 18.6 | - | - | |
| 72 hr | 35.0 | 23.7 | 4.7 | 0.8 | 5.3 | 9.0 | - | 21.5 | - | - | |
| Vitro Molar | Untreated | 35.1 | 32.5 | 6.1 | 2.3 | 8.9 | 7.1 | 8.3 | - | - | - |
| 24 hr | 33.6 | 34.2 | 6.9 | 2.2 | 8.0 | 6.4 | 8.6 | - | - | - | |
| 48 hr | 27.2 | 28.2 | 5.8 | 1.2 | 7.8 | 6.2 | 6.5 | 17.1 | - | - | |
| 72 hr | 34.4 | 29.1 | 6.3 | 2.2 | 9.8 | 8.3 | 9.9 | - | - | - | |
| Ketac Cem | Untreated | 27.6 | 32.4 | 10.3 | 2.7 | 7.9 | 9.1 | 9.9 | - | - | - |
| 24 hr | 26.3 | 34.3 | 8.9 | 2.3 | 9.2 | 9.7 | 9.2 | 22.3 | - | - | |
| 48 hr | 29.9 | 32.4 | 8.7 | 2.4 | 9.0 | 10.6 | 6.9 | 18.3 | - | - | |
| 72 hr | 25.3 | 30.1 | 6.0 | 1.4 | 5.4 | 7.7 | 2.4 | 19.1 | - | - | |
| Ketac Molar | Untreated | 29.1 | 37.3 | 6.5 | 2.0 | 9.4 | 7.5 | 8.1 | - | - | - |
| 24 hr | 22.1 | 29.5 | 5.4 | 2.1 | 6.3 | 4.5 | 7.3 | 22.4 | - | - | |
| 48 hr | 19.9 | 33.2 | 6.5 | 1.4 | 7.1 | 7.8 | 5.8 | 18.3 | - | - | |
| 72 hr | 21.1 | 33.5 | 8.7 | 1.6 | 6.2 | 8.2 | 6.5 | 14.2 | - | - | |
| Vitro Fil | Untreated | 28.8 | 25.7 | 4.3 | 1.6 | 5.4 | 7.2 | 1.9 | 12.1 | 11.9 | - |
| 24 hr | 20.2 | 22.4 | 5.4 | 1.5 | 6.0 | 5.4 | 1.8 | 17.6 | 14.0 | - | |
| 48 hr | 43.5 | 18.2 | 3.1 | 1.1 | 3.6 | 4.6 | - | 19.8 | - | - | |
| 72 hr | 22.5 | 23.8 | 6.5 | 1.2 | 8.4 | 6.3 | - | - | 33.3 | - | |
| Maxxion | Untreated | 26.2 | 25.9 | 6.8 | 3.4 | 7.6 | 3.0 | 3.7 | 23.2 | - | - |
| 24 hr | 33.5 | 30.9 | 10.3 | 4.7 | 11.1 | 5.1 | 4.3 | - | - | - | |
| 48 hr | 32.3 | 31.0 | 9.7 | 4.6 | 11.4 | 5.9 | 5.0 | - | - | - | |
| 72 hr | 27.1 | 29.1 | 6.1 | 3.2 | 7.2 | 6.7 | 2.7 | 17.8 | - | - | |
Although there are limitations of energy-dispersive X-ray spectroscopy in identifying and quantifying chemical elements with low atomic numbers, such as C, the relative quantities (wt%) of elements were obtained using the χ2 test.
*This information was provided by the manufacturers in the Material Safety Data Sheet (MSDS) and instruction sheets.
WTK (M2–M3) and water solubility (M1–M3) of the specimens were calculated in micrograms (µg) from the differences in weight gain or loss during the immersion in water and drying cycles. Different uppercase superscript letters indicate a statistically significant difference within the row (
Data are shown as means ± standard deviations. Different uppercase superscript letters indicate a statistically significant difference within the row (
*Vitro Fil and Vidrion R were not tested after the bleaching protocol because the specimens disintegrated.
Data are shown as means ± standard deviations. Different uppercase superscript letters indicate a statistically significant difference within each row, that is, within each cement separately (
Although there are limitations of energy-dispersive X-ray spectroscopy in identifying and quantifying chemical elements with low atomic numbers, such as C, the relative quantities (wt%) of elements were obtained using the χ2 test.

 KACD
KACD
 ePub Link
ePub Link Cite
Cite

