Articles
- Page Path
- HOME > Restor Dent Endod > Volume 43(3); 2018 > Article
-
Research Article
Retention of BioAggregate and MTA as coronal plugs after intracanal medication for regenerative endodontic procedures: an
ex vivo study -
Suzan Abdul Wanees Amin
 , Shaimaa Ismail Gawdat
, Shaimaa Ismail Gawdat
-
Restor Dent Endod 2018;43(3):e18.
DOI: https://doi.org/10.5395/rde.2018.43.e18
Published online: April 26, 2018
Department of Endodontics, Faculty of Dentistry, Cairo University, Cairo, Egypt.
- Correspondence to Suzan Abdul Wanees Amin, BDS, MSc, PhD. Associate Professor, Department of Endodontics, Faculty of Dentistry, Cairo University, 12 El Saraya Street, Manial ElRoda, 11451 Cairo, Egypt. swanees@rocketmail.com
Copyright © 2018. The Korean Academy of Conservative Dentistry
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,675 Views
- 9 Download
- 8 Crossref
Abstract
-
Objectives This study compared the retention of BioAggregate (BA; Innovative BioCeramix) and mineral trioxide aggregate (MTA; Angelus) as coronal plugs after applying different intracanal medications (ICMs) used in regenerative endodontics.
-
Materials and Methods One-hundred human maxillary central incisors were used. The canals were enlarged to a diameter of 1.7 mm. Specimens were divided into 5 groups (n = 20) according to the ICM used: calcium hydroxide (CH), 2% chlorhexidine (CHX), triple-antibiotic paste (TAP), double-antibiotic paste (DAP), and no ICM (control; CON). After 3 weeks of application, ICMs were removed and BA or MTA were placed as the plug material (n = 10). The push-out bond strength and the mode of failure were assessed. The data were analyzed using 2-way analysis of variance, the Tukey's test, and the χ2 test; p values < 0.05 indicated statistical significance.
-
Results The type of ICM and the type of plug material significantly affected bond strength (p < 0.01). Regardless of the type of ICM, BA showed a lower bond strength than MTA (p < 0.05). For MTA, CH showed a higher bond strength than CON, TAP and DAP; CHX showed a higher bond strength than DAP (p < 0.01). For BA, CH showed a higher bond strength than DAP (p < 0.05). The mode of failure was predominantly cohesive for BA (p < 0.05).
-
Conclusions MTA may show better retention than BA. The mode of bond failure with BA can be predominantly cohesive. BA retention may be less affected by ICM type than MTA retention.
INTRODUCTION
MATERIALS AND METHODS
RESULTS
The push-out bond strength (MPa) of mineral trioxide aggregate (MTA) and BioAggregate (BA) after the prior use of different intracanal medications (ICMs)
Mode of failure distribution of mineral trioxide aggregate (MTA) and BioAggregate (BA) after intracanal medication (ICM)
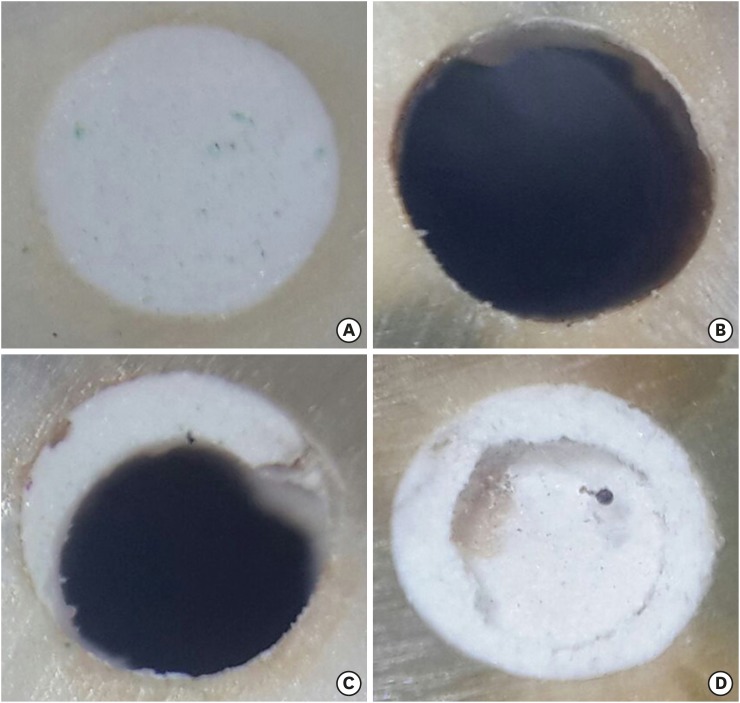
Representative specimens of the different modes of bond failure. (A) A specimen with the material in the root canal before displacement in the push-out test; (B) A specimen showing an adhesive mode of failure; (C) A specimen showing a mixed mode of failure; (D) A specimen showing a cohesive mode of failure.
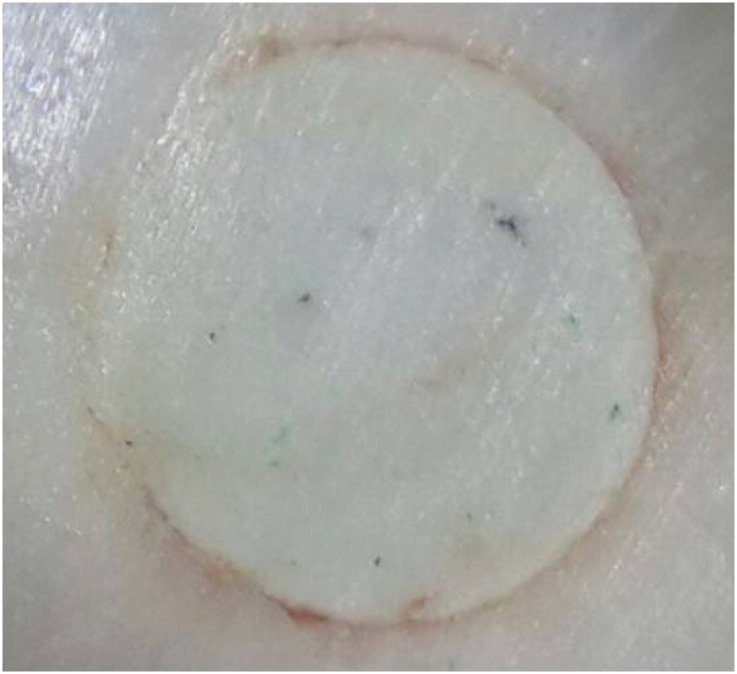
A specimen showing discoloration at the material-dentin interface with triple-antibiotic paste (TAP) as the intracanal medication (ICM).
DISCUSSION
CONCLUSIONS
-
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
-
Author Contributions:
Conceptualization: Amin SAW, Gawdat SI.
Data curation: Amin SAW, Gawdat SI.
Formal analysis: Amin SAW, Gawdat SI.
Funding acquisition: Amin SAW, Gawdat SI.
Investigation: Amin SAW, Gawdat SI.
Methodology: Amin SAW, Gawdat SI.
Project administration: Gawdat SI.
Resources: Amin SAW, Gawdat SI.
Software: Amin SAW, Gawdat SI.
Supervision: Amin SAW.
Validation: Amin SAW, Gawdat SI.
Visualization: Amin SAW, Gawdat SI.
Writing - original draft: Amin SAW, Gawdat SI.
Writing - review & editing: Amin SAW, Gawdat SI.
- 1. Galler KM. Clinical procedures for revitalization: current knowledge and considerations. Int Endod J 2016;49:926-936.ArticlePubMed
- 2. Kontakiotis EG, Filippatos CG, Tzanetakis GN, Agrafioti A. Regenerative endodontic therapy: a data analysis of clinical protocols. J Endod 2015;41:146-154.ArticlePubMed
- 3. Kahler B, Rossi-Fedele G. A review of tooth discoloration after regenerative endodontic therapy. J Endod 2016;42:563-569.ArticlePubMed
- 4. Tagelsir A, Yassen GH, Gomez GF, Gregory RL. Effect of antimicrobials used in regenerative endodontic procedures on 3-week-old Enterococcus faecalis biofilm. J Endod 2016;42:258-262.ArticlePubMed
- 5. Prabhakar A, Taur S, Hadakar S, Sugandhan S. Comparison of antibacterial efficacy of calcium hydroxide paste, 2% chlorhexidine gel and turmeric extract as an intracanal medicament and their effect on microhardness of root dentin: an in vitro study. Int J Clin Pediatr Dent 2013;6:171-177.ArticlePubMedPMC
- 6. Abu Zeid ST, Khafagi MG, Abou Neel EA. Effect of root canal medications on maturation and calcification of root canal dentin’ hydroxyapatite. Spectrosc Lett 2016;49:135-139.Article
- 7. Camilleri J, Grech L, Galea K, Keir D, Fenech M, Formosa L, Damidot D, Mallia B. Porosity and root dentine to material interface assessment of calcium silicate-based root-end filling materials. Clin Oral Investig 2014;18:1437-1446.ArticlePubMedPDF
- 8. Camilleri J, Sorrentino F, Damidot D. Characterization of un-hydrated and hydrated BioAggregate and MTA Angelus. Clin Oral Investig 2015;19:689-698.ArticlePubMedPDF
- 9. Zhang H, Pappen FG, Haapasalo M. Dentin enhances the antibacterial effect of mineral trioxide aggregate and bioaggregate. J Endod 2009;35:221-224.ArticlePubMed
- 10. Hashem AA, Wanees Amin SA. The effect of acidity on dislodgment resistance of mineral trioxide aggregate and bioaggregate in furcation perforations: an in vitro comparative study. J Endod 2012;38:245-249.ArticlePubMed
- 11. Huan Z, Chang J. Novel tricalcium silicate/monocalcium phosphate monohydrate composite bone cement. J Biomed Mater Res B Appl Biomater 2007;82:352-359.ArticlePubMed
- 12. Schembri-Wismayer P, Camilleri J. Why biphasic? Assessment of the effect on cell proliferation and expression. J Endod 2017;43:751-759.ArticlePubMed
- 13. Tian J, Zhang Y, Lai Z, Li M, Huang Y, Jiang H, Wei X. Ion release, microstructural, and biological properties of iRoot BP Plus and ProRoot MTA exposed to an acidic environment. J Endod 2017;43:163-168.ArticlePubMed
- 14. Guven Y, Tuna EB, Dincol ME, Ozel E, Yilmaz B, Aktoren O. Long-term fracture resistance of simulated immature teeth filled with various calcium silicate-based materials. Biomed Res Int 2016;2016:2863817.ArticlePubMedPMCPDF
- 15. Keskin C, Demiryurek EO, Ozyurek T. Color stabilities of calcium silicate-based materials in contact with different irrigation solutions. J Endod 2015;41:409-411.ArticlePubMed
- 16. Tuloglu N, Bayrak S. Comparative evaluation of mineral trioxide aggregate and bioaggregate as apical barrier material in traumatized nonvital, immature teeth: a clinical pilot study. Niger J Clin Pract 2016;19:52-57.ArticlePubMed
- 17. Topçuoğlu HS, Arslan H, Akçay M, Saygili G, Çakici F, Topçuoğlu G. The effect of medicaments used in endodontic regeneration technique on the dislocation resistance of mineral trioxide aggregate to root canal dentin. J Endod 2014;40:2041-2044.ArticlePubMed
- 18. Turk T, Fidler A. Effect of medicaments used in endodontic regeneration technique on push-out bond strength of MTA and Biodentine. Biotechnol Biotechnol Equip 2016;30:140-144.Article
- 19. Turk T, Ozisik B, Aydin B. Time-dependent effectiveness of the intracanal medicaments used for pulp revascularization on the dislocation resistance of MTA. BMC Oral Health 2015;15:130-135.ArticlePubMedPMCPDF
- 20. Nagas E, Cehreli ZC, Uyanik MO, Vallittu PK, Lassila LV. Effect of several intracanal medicaments on the push-out bond strength of ProRoot MTA and Biodentine. Int Endod J 2016;49:184-188.PubMed
- 21. Saghiri MA, Garcia-Godoy F, Gutmann JL, Lotfi M, Asatourian A, Ahmadi H. Push-out bond strength of a nano-modified mineral trioxide aggregate. Dent Traumatol 2013;29:323-327.ArticlePubMed
- 22. Saghiri MA, Asatourian A, Garcia-Godoy F, Gutmann JL, Sheibani N. The impact of thermocycling process on the dislodgement force of different endodontic cements. Biomed Res Int 2013;2013:317185.ArticlePubMedPMCPDF
- 23. Majeed A, AlShwaimi E. Push-out bond strength and surface microhardness of calcium silicate-based biomaterials: an in vitro study. Med Princ Pract 2017;26:139-145.ArticlePubMedPDF
- 24. Shokouhinejad N, Razmi H, Nekoofar MH, Sajadi S, Dummer PM, Khoshkhounejad M. Push-out bond strength of bioceramic materials in a synthetic tissue fluid. J Dent (Tehran) 2013;10:540-547.PubMedPMC
- 25. Chedella SC, Berzins DW. A differential scanning calorimetry study of the setting reaction of MTA. Int Endod J 2010;43:509-518.ArticlePubMed
- 26. Amin SA, Seyam RS, El-Samman MA. The effect of prior calcium hydroxide intracanal placement on the bond strength of two calcium silicate-based and an epoxy resin-based endodontic sealer. J Endod 2012;38:696-699.ArticlePubMed
- 27. Grattan-Bellew PE. Microstructural investigation of deteriorated Portland cement concretes. Construct Build Mater 1996;10:3-16.Article
- 28. Shokouhinejad N, Nekoofar MH, Iravani A, Kharrazifard MJ, Dummer PM. Effect of acidic environment on the push-out bond strength of mineral trioxide aggregate. J Endod 2010;36:871-874.ArticlePubMed
- 29. Shokouhinejad N, Yazdi KA, Nekoofar MH, Matmir S, Khoshkhounejad M. Effect of acidic environment on dislocation resistance of Endosequence root repair material and mineral trioxide aggregate. J Dent (Tehran) 2014;11:161-166.PubMedPMC
- 30. Shokouhinejad N, Nekoofar MH, Razmi H, Sajadi S, Davies TE, Saghiri MA, Gorjestani H, Dummer PM. Bioactivity of EndoSequence root repair material and bioaggregate. Int Endod J 2012;45:1127-1134.ArticlePubMed
- 31. Sideris K, Manita P. Estimation of ultimate modulus of elasticity and Poisson ratio of normal concrete. Cement Concr Compos 2004;26:623-631.Article
- 32. Twati WA, Wood DJ, Liskiewicz TW, Willmott NS, Duggal MS. An evaluation of the effect of non-setting calcium hydroxide on human dentine: a pilot study. Eur Arch Paediatr Dent 2009;10:104-109.ArticlePubMedPDF
- 33. Chen WP, Chen YY, Huang SH, Lin CP. Limitations of push-out test in bond strength measurement. J Endod 2013;39:283-287.ArticlePubMed
- 34. Pane ES, Palamara JE, Messer HH. Critical evaluation of the push-out test for root canal filling materials. J Endod 2013;39:669-673.ArticlePubMed
- 35. Saghiri MA, Garcia-Godoy F, Asatourian A, Lotfi M, Banava S, Khezri-Boukani K. Effect of pH on compressive strength of some modification of mineral trioxide aggregate. Med Oral Patol Oral Cir Bucal 2013;18:e714-e720.ArticlePubMedPMC
- 36. Bolhari B, Nekoofar MH, Sharifian M, Ghabrai S, Meraji N, Dummer PM. Acid and microhardness of mineral trioxide aggregate and mineral trioxide aggregate-like materials. J Endod 2014;40:432-435.ArticlePubMed
- 37. Jang YE, Lee BN, Koh JT, Park YJ, Joo NE, Chang HS, Hwang IN, Oh WM, Hwang YC. Cytotoxicity and physical properties of tricalcium silicate-based endodontic materials. Restor Dent Endod 2014;39:89-94.ArticlePubMedPMC
- 38. Shie MY, Huang TH, Kao CT, Huang CH, Ding SJ. The effect of a physiologic solution pH on properties of white mineral trioxide aggregate. J Endod 2009;35:98-101.ArticlePubMed
- 39. Yassen GH, Vail MM, Chu TG, Platt JA. The effect of medicaments used in endodontic regeneration on root fracture and microhardness of radicular dentine. Int Endod J 2013;46:688-695.ArticlePubMed
- 40. Berkhoff JA, Chen PB, Teixeira FB, Diogenes A. Evaluation of triple antibiotic paste removal by different irrigation procedures. J Endod 2014;40:1172-1177.ArticlePubMed
- 41. Yassen GH, Chu TM, Eckert G, Platt JA. Effect of medicaments used in endodontic regeneration technique on the chemical structure of human immature radicular dentin: an in vitro study. J Endod 2013;39:269-273.ArticlePubMed
- 42. Yassen GH, Chu TM, Gallant MA, Allen MR, Vail MM, Murray PE, Platt JA. A novel approach to evaluate the effect of medicaments used in endodontic regeneration on root canal surface indentation. Clin Oral Investig 2014;18:1569-1575.ArticlePubMedPDF
- 43. Prather BT, Ehrlich Y, Spolnik K, Platt JA, Yassen GH. Effects of two combinations of triple antibiotic paste used in endodontic regeneration on root microhardness and chemical structure of radicular dentine. J Oral Sci 2014;56:245-251.ArticlePubMed
- 44. Yassen GH, Eckert GJ, Platt JA. Effect of intracanal medicaments used in endodontic regeneration procedures on microhardness and chemical structure of dentin. Restor Dent Endod 2015;40:104-112.ArticlePubMed
- 45. Yilmaz S, Dumani A, Yoldas O. The effect of antibiotic pastes on microhardness of dentin. Dent Traumatol 2016;32:27-31.ArticlePubMed
- 46. Yassen GH, Platt JA. The effect of nonsetting calcium hydroxide on root fracture and mechanical properties of radicular dentine: a systematic review. Int Endod J 2013;46:112-118.ArticlePubMed
- 47. Shetty S, Manjunath MK, Tejaswi S. An in-vitro evaluation of the pH change through root dentin using different calcium hydroxide preparations as an intracanal medicament. J Clin Diagn Res 2014;8:ZC13-ZC16.Article
- 48. Saghiri MA, Shokouhinejad N, Lotfi M, Aminsobhani M, Saghiri AM. Push-out bond strength of mineral trioxide aggregate in the presence of alkaline pH. J Endod 2010;36:1856-1859.ArticlePubMed
- 49. Arslan H, Akcay M, Capar ID, Ertas H, Ok E, Uysal B. Efficacy of needle irrigation, EndoActivator, and photon-initiated photoacoustic streaming technique on removal of double and triple antibiotic pastes. J Endod 2014;40:1439-1442.ArticlePubMed
- 50. Mohammadi Z, Abbott PV. On the local applications of antibiotics and antibiotic-based agents in endodontics and dental traumatology. Int Endod J 2009;42:555-567.ArticlePubMed
- 51. Tjäderhane L, Nascimento FD, Breschi L, Mazzoni A, Tersariol IL, Geraldeli S, Tezvergil-Mutluay A, Carrilho M, Carvalho RM, Tay FR, Pashley DH. Strategies to prevent hydrolytic degradation of the hybrid layer: a review. Dent Mater 2013;29:999-1011.ArticlePubMedPMC
- 52. Sabatini C, Pashley DH. Mechanisms regulating the degradation of dentin matrices by endogenous dentin proteases and their role in dental adhesion. A review. Am J Dent 2014;27:203-214.PubMedPMC
- 53. Ari H, Erdemir A, Belli S. Evaluation of the effect of endodontic irrigation solutions on the microhardness and the roughness of root canal dentin. J Endod 2004;30:792-795.ArticlePubMed
REFERENCES
Tables & Figures
REFERENCES
Citations

- An in vitro comparative evaluation of the effect of three intracanal medicaments – chlorhexidine gel, triple antibiotic paste, and calcium hydroxide paste on the push-out bond strength of MTA Plus, Biodentine, and calcium-enriched mixture
Gouthami Datta, Ramya Raghu, Ashish Shetty, Gautham P Manjunath, Dishant Patel, Subhashini Rajasekhara
Endodontology.2023; 35(1): 60. CrossRef - Effects of calcium hydroxide intracanal medicament on push‐out bond strength of endodontic sealers: A systematic review and meta‐analysis
Mohammed Nasser Alhajj, Fadhilah Daud, Sadeq Ali Al‐Maweri, Yanti Johari, Zuryati Ab‐Ghani, Mariatti Jaafar, Yoshihito Naito, Widyasri Prananingrum, Zaihan Ariffin
Journal of Esthetic and Restorative Dentistry.2022; 34(8): 1166. CrossRef - A Breakthrough in the Era of Calcium Silicate-Based Cements: A Critical Review
Payal S Chaudhari, Manoj G Chandak, Akshay A Jaiswal, Nikhil P Mankar, Priyanka Paul
Cureus.2022;[Epub] CrossRef - Modern Medicaments for Endodontic Treatment in Children
Н.В. Шаковец, О.С. Романова
Стоматология. Эстетика. Инновации.2021; (4): 408. CrossRef - Do intracanal medications used in regenerative endodontics affect the bond strength of powder-to-liquid and ready-to-use cervical sealing materials?
MarinaCarvalho Prado, Kevillin Martiniano, AndreaCardoso Pereira, KarineL Cortellazzi, MarinaA Marciano, Gabriel Abuna, Adriana de-Jesus-Soares
Journal of Conservative Dentistry.2021; 24(5): 464. CrossRef - In vivo Biocompatibility and Bioactivity of Calcium Silicate-Based Bioceramics in Endodontics
Wencheng Song, Wei Sun, Lili Chen, Zhenglin Yuan
Frontiers in Bioengineering and Biotechnology.2020;[Epub] CrossRef - Effect of Hydrogel-Based Antibiotic Intracanal Medicaments on Push-Out Bond Strength
Rayan B. Yaghmoor, Jeffrey A. Platt, Kenneth J. Spolnik, Tien Min Gabriel Chu, Ghaeth H. Yassen
European Journal of Dentistry.2020; 14(04): 575. CrossRef - Regenerative Endodontic Procedures, Disinfectants and Outcomes: A Systematic Review
Adam S. Kharchi, Nara Tagiyeva-Milne, Shalini Kanagasingam
Primary Dental Journal.2020; 9(4): 65. CrossRef

Figure 1
Figure 2
The push-out bond strength (MPa) of mineral trioxide aggregate (MTA) and BioAggregate (BA) after the prior use of different intracanal medications (ICMs)
| ICM | Material | ||
|---|---|---|---|
| MTA | BA | p value | |
| CON | 1.300 ± 0.529b,c | 0.698 ± 0.184a,b | 0.003* |
| CH | 3.592 ± 1.387a | 1.109 ± 0.810a | < 0.001* |
| CHX | 2.406 ± 0.600a,b | 0.953 ± 0.696a,b | < 0.001* |
| TAP | 1.641 ± 1.044b,c | 0.599 ± 0.282a,b | 0.007* |
| DAP | 0.533 ± 0.196c | 0.357 ± 0.191b | 0.060 |
| p value | < 0.001† | 0.043† | |
The values are presented as means ± standard deviations.
Different lowercase letters indicate that there are statistically significant differences between the groups within the column.
CON, control; CH, calcium hydroxide; CHX, chlorhexidine; TAP, triple-antibiotic paste; DAP, double-antibiotic paste; ANOVA, analysis of variance.
*Indicates statistical significance at p < 0.05 according to the Student's t-test; †Indicates statistical significance at p < 0.05 according to 1-way ANOVA.
Mode of failure distribution of mineral trioxide aggregate (MTA) and BioAggregate (BA) after intracanal medication (ICM)
| ICM | Material | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| MTA | BA | Total/ICM (n = 20) | |||||||
| A | M | C | A | M | C | A | M | C | |
| CON | 3 | 6 | 1 | 2 | 5 | 3 | 5/20 | 11/20 | 4/20 |
| CH | 3 | 4 | 4 | 0 | 3 | 6 | 3/20 | 7/20 | 10/20 |
| CHX | 5 | 4 | 2 | 2 | 3 | 4 | 7/20 | 7/20 | 6/20 |
| TAP | 3 | 4 | 2 | 1 | 4 | 6 | 4/20 | 8/20 | 8/20 |
| DAP | 4 | 3 | 2 | 2 | 4 | 5 | 6/20 | 7/20 | 7/20 |
| Total/material (n = 50) | 18/50 | 21/50 | 11/50 | 7/50 | 19/50 | 24/50 | |||
The values represent the counts of specimens with each mode of failure.
A, adhesive; M, mixed; C, cohesive; CON, control; CH, calcium hydroxide; CHX, chlorhexidine; TAP, triple-antibiotic paste; DAP, double-antibiotic paste.
The values are presented as means ± standard deviations.
Different lowercase letters indicate that there are statistically significant differences between the groups within the column.
CON, control; CH, calcium hydroxide; CHX, chlorhexidine; TAP, triple-antibiotic paste; DAP, double-antibiotic paste; ANOVA, analysis of variance.
*Indicates statistical significance at
The values represent the counts of specimens with each mode of failure.
A, adhesive; M, mixed; C, cohesive; CON, control; CH, calcium hydroxide; CHX, chlorhexidine; TAP, triple-antibiotic paste; DAP, double-antibiotic paste.

 KACD
KACD
 ePub Link
ePub Link Cite
Cite

