I. Introduction
The oral asaccharolytic
Eubacterium spp. are a diverse group of Gram-positive rods that are frequently isolated from oral infections such as periodontitis and dento-alveolar infections.
1,
2) In a study of sixty-five patients with necrotic pulp and periapical lesions,
Eubacterium-specifically
Eubacterium alactolyticum(currently
Pseudoramibacter alactolyticus) and
Eubacterium lentum-were identified in a third of the samples.
3) Moreover, the prevalence of
Eubacterium spp., mostly
Eubacterium lentum, in cases of symptomatic or painfully infected root canals has been reported to range from 17-81% of teeth.
4-
6) Five years after treatment, it was noted that
Eubacterium spp. were identified at the time of root canal obturation in a higher proportion in the cases that eventually failed compared to cases that were successful.
7) Recently, on the basis of 16S rRNA sequence data and the phenotypic characters,
Eubacterium lentum, an organism of high incidence of symptomatic root canal infections, as noted before, was reclassified as
Eggerthella lenta.
8) In a previous study we used PCR-based identification to determine the presence of the following ten putative bacterial species from root canal of pulp necrosis and apical periodontitis:
Bacteroides forsythus, Fusobacterium nucleatum, Peptostreptococcus micros, Porphyromonas endodontalis, Porphyromonas gingivalis, Prevotella nigrescens, Prevotella intermedia, Treponema denticola and members of the
Enterococcus and
Streptococcus genera.
9) Recently techniques utilizing the 16S rRNA gene PCR assay have been provided a new tool for evaluating the members of diverse microbial communities including uncultivable microorganisms in the field of microbial ecology.
10) Therefore, the purposes of this study were to use the more sensitive technique of PCR to detect the presence of
Eubacterium spp. and
Eggerthella lenta in human samples from root canals with necrotic pulp and to study the association of these microorganisms with pre-operative clinical symptoms and the history of diabetes mellitus, and finally to study the association of
Eubacterium spp. with a selected group of other putative microorganisms previously identified in the same patient specimens.
II. Materials and Methods
Patient Selection and Sample Collection
Patient samples were obtained from consenting endodontic patients in accordance with protocols approved by the Institutional Review Board of the University of Connecticut Health Center as was previously described.
9) The teeth involved had pulp necrosis and apical periodontitis, did not have any previous endodontic procedures and had not have any been on antibiotics for at least three months. The original sample size of 24 was reduced to 22 after a PCR test utilizing 16s rDNA universal eubacterial primers showed that two of the samples was negative for any bacteria. These two specimens were excluded from any further analysis. Eight patients reported pre-operative pain and/or swelling and five patients were diabetic (two type 1 and three type 2). The technique for sample collection was as follows: Following rubber dam isolation of the tooth involved, the field was disinfected with 30% H
2O
2 then 5% tincture of iodine. Caries and/or existing restorations, if present, were removed, then the cavity was wiped with a sterile cotton pellet slightly wet with 1% buffered NaOCl, with care that it did not seep into the canal. The halogen disinfectants were then inactivated using 5% sodium thiosulphate. The pulp chamber was then accessed with a new sterile bur. If purulence or serous fluid was present in the canal, this was directly sampled using three size Fine paper points. Otherwise, sterile saline was deposited in the canal making sure it did not overflow. A size 15 - 30 K-type file(Maillefer, Ballaigus, Switzerland) was used to negotiate the canal to the estimated length. Three Fine paper points were then used to obtain the sample. The last paper point was left for 30 seconds in the canal. In multicanaled teeth, one paper point sample was obtained from each canal unless the canals were very calcified, in which case sampling of the canal in the root with the largest PA lesion and the largest canal was done. The paper points were placed in sterile, DNA/RNA-free vials containing 1mL filter-sterilized 10mM Tris-HCl; 1mM EDTA (pH =8) and 0.5g (0.71-1.18mm) sterile glass beads. The vials were frozen at -70℃ until used.
The vials with paper point specimens were vortexed for 2 minutes to disperse microbial cellular material into suspension, then centrifuged for 10 minutes at 7,500 rpm, and the supernatant was again removed. From the cellular pellet, DNAs were extracted using the Chelex extraction and boiling technique
11), or the enzymatic extraction method, according to the Quagen-QIAamp DNA mini kit protocol(Qiagen, Valencia, CA). Extraction yielded 400µL that were aliquoted in sterile, DNA-, RNA-free conical tubes, and frozen at -20℃ until use. The yield of extracted DNA ranged from 2-33 ng/µL for the stock bacterial strains and 1-19.5 ng/µL for the clinical specimens.
Initially the presence of bacteria was then verified in the sample using a 16S rDNA universial eubacterial primer pair. Subsequently previously published genus-level oligonucleotide primers for
Eubacterium spp. and species-level oligonucleotide primers for
Eggerthella lenta were selected for specific PCR amplification of 16S rRNA genes(
Table 1).
In pilot experiments, the conditions for the primers were optimized using DNA extracted from corresponding control bacterial stains obtained from the American Type Culture Collection(ATCC). For Eubacterium primer pair several controls were employed. The positive control for the Eubacterium primers was DNA extracted from Eubacterium nodatum stock strain(ATCC 33099) and Eggerthella lenta (ATCC 43055). The latter organism also served as positive control for the specific primers for Eggerthella lenta.
The negative controls included ATCC strains of the following bacterial species: Actinomyces israelii, B. forsythus, Enterococcus faecalis, F. nucleatum, P. endodontalis, P. gingivalis, P. intermedia, P. nigrescens, P. micros, and T. denticola, as well as ultra pure water(no DNA). All negative controls were negative with the specific primers. Except for water, all negative controls were positive with the universal 16S primers.
PCR amplification was performed in a thermal cycler(PE9700 or PE2400, Perkin Elmer-Biosystems, Foster City, CA) using final reaction volume 50µL containing 10µL extracted specimen(target) DNA(or 5µL extracted control stock bacterial DNA), 5µL 10x PCR buffer, 0.5µL (5U/µL) HotStar Taq (Qiagen, Valencia, CA), 1.5mM MgCl
2, 0.2mM each of the 4 deoxynucleotide triphosphates(dNTPs, Takara, Otsu, Shiga, Japan), 0.5µM of each primer and sterile ultra pure water. The PCR conditions used in this study were as follows: the initial denaturation was at 95℃ for 15 min with HotStar Taq. Thirty amplification cycles were then performed: denaturation for 30 sec (at 94℃), annealing for 30sec(temperature depended on the primer set-see
Table 1), and extension for 1 min(at 72℃). The final extension was at 72℃ for 5 min, and the products were cooled to 4℃. Successful amplification was verified by 2% agarose gel electrophoresis in TAE buffer(40mM Tris-acetate, 2mM EDTA; pH 8.3). The Power Pac 200 apparatus (Bio-Rad, Hercules, Calif.) was set at 110mA for 2 h or 95 V for 1 h. The gels were stained with 0.5µg of ethidium bromide per ml for 30 min and destained with water for 20 min. The PCR products were visualized under UV light using an Alpha Imager(Alpha Innotech Corp., San Leandro, CA).
For Eubacterium direct sequencing of PCR products reaction mixtures were prepared with the following protocol: Eubacterium genus level primer forward only(Universal 27F: 5'-AGA GTT TGA TCC TGG CTC AG-3', 0.7µl of working 5 mM primer) which is same sequence as the universal forward primer, DNA from reamplified PCR products to yield 80 ng/reaction mix, ultrapure water used to reach final volume of 12µl. The directly sequenced PCR products were purified using the Concert Rapid PCR purification system(Life Technologies, GibcoBRL, Rockville MD). The purified DNA was sequenced in the University of Connecticut Health Center Molecular Core Facility using the ABI Prism 3100 Genetic Analyzer(PE Applied Biosystems, Foster City, CA), with primers 27F and 557R. The resulting sequences were used to search databases available through NCBI.
Phylogenetic analyses
The resulting bi-directional consensus sequences, consisting of about 500-800 base pairs, were characterized using the BLAST algorithm. Although these sequences do not represent the complete 16S gene (about 1500 bp), partial sequences can suffice for determining the identity of microorganisms
12,
13), particularly, as in this case, when targeting a limited range of microorganisms with genus-specific primers. Each sequence was aligned to the 15-20 closest sequences with ClustalW in MacVector(GCG, Oxford Molecular Co.). A Neighbor-Joining (NJ) phylogenetic tree was constructed from the alignment using MacVector (GCG, Oxford Molecular Co.). A distance matrix was constructed using a Tamura-Nei model without Gamma correction and with gaps distributed proportionately. NJ Bootstrap values were derived from 1000 replications and were added to the tree.
The associations between the positive identification of a bacterial species or genus and symptoms, and the history of diabetes were analyzed using odds ratio(OR) analysis. OR associations of 2 or more were considered positive associations.
3,
14) These positive associations were further analyzed using a Chi-square analysis to determine their statistical significance. Furthermore, similar analysis was performed of the association of organisms with each other.
III. Results
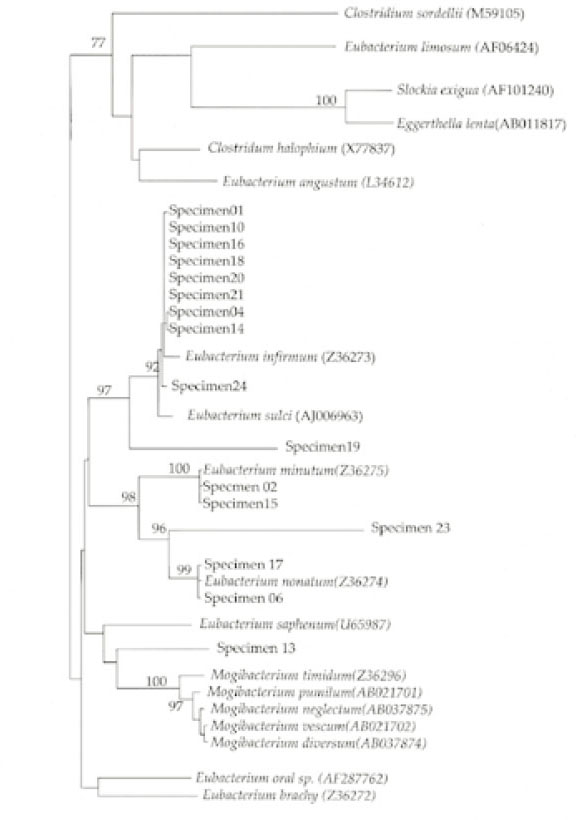
Sixteen of 22 or (73%) of the patient specimens were positive for
Eubacterium spp. None of the specimens contained
Eggerthella lenta. The phylogenetic analysis showed that the most frequently amplified
Eubacterium sequence was virtually identical with that of
Eubacterium infirmum(9 of 16 or 56%) (
Fig. 1). Of the sixteen specimens that were positive for the presence of
Eubacterium spp., five samples were from patients who experienced pre-operative pain and/or swelling and four from patients who had diabetes mellitus(
Table 2). Analysis using OR and Chi-square showed no significant associations between the combined presence or absence of all
Eubacterium spp. and the history of symptoms or diabetes. However, a history of diabetes was significantly associated with the presence of
Eubacterium infirmum (OR = 9.6; Chi-square, p = 0.04). Four of the nine specimens positive for
E. infirmum were from patients with preoperative symptoms(OR = 1.8; Chi-square, p > 0.05). Three specimens had
Eubacterium sequences that were related to certain organisms with relatively low homology: specimen 13 was closest to
Mogibacterium timidum(86% homology); specimen 19 was closest to
Eubacterium sulci (83% homology); and specimen 23 was closest to
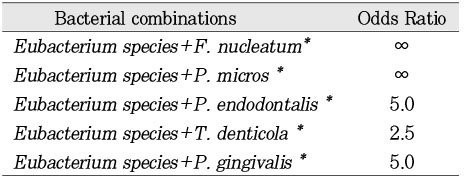
Eubacterium nodatum(80% homology). Odds ratio (OR) analysis also showed a positive association between the presence of
Eubacterium spp. with the presence of
F. nucleatum, P. micro, P. nigrescens, P. endodontalis, B. forsythus, T. denticola, P. gingivalis(OR>2). Furthermore, a positive association was observed between the presence of diabetes mellitus and the combination of
Eubacterium spp. with
F. nucleatum, P. micro, P. endodontalis, T. denticolar, or P. gingivalis (
Table 3).
IV. Discussion
The comparison of rRNA sequence is a powerful tool for deducing phylogenetic and evolutionary relationship among bacteria in oral infection.
9) In this study both universal and specific PCR primers targeting bacterial 16S rRNA genes were used to investigate the prevalence of
Eubacterium species and
Eggerthella lenta in infected root canals. In addition, the potential association of these organisms with clinical symptoms or the presence of diabetes was also evaluated.
The
Eubacterium primers that we used have been validated for identification of several species including:
E. brachy, E. infirmum, E. nodatum, E. saphenum, and
E. tardum.
15) E. timidum was not identified in that study. The authors attributed this to the presence of four mismatches between the reverse primer and the rRNA sequence of this organism. However, specimen 13 in this study was identified as being closest to
Mogibacterium timidum (
Fig. 1), which is the recent reclassification of
E. timidum to the new genus
Mogibacterium.
16) In present study the percentage of specimens with positive Eubacterium DNA identification was 73%. This is comparable to the upper limits of other published material when analyzing the prevalence of
Eubacterium species in total samples and not just cases with pre-operative symptoms.
17) The genus
Eubacterium currently includes a heterogenous group of Gram-positive, obligately anaerobic, non-spore-forming bacilli, many of which are slow growing, fastidious and generally unreactive in biochemical tests.
18) As consequence, cultivation and identification of isolates are difficult and the taxonomy of the group remains indifferent.
An intriguing feature of the present study is that most of the
Eubacterium species in the infected root canals were
Eubacterium infirmum(9/16 or 56%), which was recently isolated from human periodontal pockets with periodontitis by Cheeseman et al.
19) Two other recent studies, that closely examined
Eubacterium spp. in endodontic infections using molecular methods, did not identify this organism. In one study, molecular sequencing was performed on cultured isolates, where culturing conditions may not have allowed the cultivation of
E. infirmum18), and in the other study, only primers specific to
Slackia exigua,
M. timidum and
E. saphenum were used.
20) Therefore, this is the first reporting of the species
E. infirmum in endodontic infections, as far as we could determine. This organism was also significantly associated with the presence of a history of diabetes mellitus.
Eubacterium infirmum strains are obligate, anaerobic, Gram-positive short rod and are rarely detected at healthy oral sites
19), but little is known about any possible virulence factors produced by these organisms in endodontic infections. A previous study which studied about serum antibody response against oral
Eubacterium species in periodontal disease serum antibody levels against four
Eubacterium species were significantly elevated in periodontal disease.
21) The presence of an elevated systemic antibody suggests that
Eubacterium species have breached the host's defenses insufficient quantities to stimulate an immune response. Further work is necessary to elucidate the potential pathogenic mechanisms of
E. infirmum and other
Eubacterium species in endodontic infections, and whether specific microorganisms preferentially favor the diabetic host environment. We have recently reported that in patients with periradicular endodontic infections, a history of diabetes is associated with a significantly lower prognosis of endodontic treatment.
22) It is not known whether this may be, at least in part, due to the preferential presence of specific bacteria, such as
E. infirmum, in the necrotic pulp of diabetic patients.
We also evaluated the associations of these microorganisms with pre-operative clinical symptoms. In previous studies, which evaluated the association between the presence of
Eubacterium species and reported pain and swelling in symptomatic or painfully infected root canals, the presence of
Eubacterium spp. was ranged from 17 to 81 percent of teeth studied.
4-
6) In the sixteen samples that were positive for the presence of
Eubacterium species, five samples were from patients who experienced preoperative pain and/or swelling. Statistical analysis did not reveal any significant associations between the bacterial presence or absence of
Eubacterium spp. and pre-operative symptoms. Despite the fact that both sets of primers, specific for Eggerthella lenta and
Eubacterium spp., did amplify our positive control
E. lenta ATCC stock strain, we could not identify this organism in any specimen. The organism
Eubacterium lentum, which was recently reclassified as
E. lenta had been identified in a number of endodontic studies by culturing.
4-
6) This is a fecal organism that is uncommon in the oral cavity, and is difficult to distinguish from another closely related organism
Eubacterium exiguum, recently reclassified as
Slackia exigua, in that both organisms are generally not reactive to biochemical tests.
23,
24) This latter organism has been identified in periodontal and endodontic infections.
25) It is possible that the organism identified in previous studies as
E. lenta is the same as
S. exigua, or that these specimens did not contain
E. lenta. Further studies need to elucidate if
E. lenta is an endodontic pathogen.
A number of sequences were marked with a question mark in
table 2. These sequences either could not be matched to known sequences or had a number of ambiguous characters(designated as Ns) in hypervariable regions of the sequences. These Ns were not used by the software to perform phylogenetic analyses, but do contribute to the uncertainty in identifying some of the sequences. In future, cloning will be performed to singularize the sequences for optimal results on all sequences.
In previous study we analyzed for the presence of the following ten putative bacterial species from root canal of pulp necrosis and apical periodontitis in same patients:
F. nucleatum, P. micros, S. intermedia, P. nigrescens, P. endodontalis, B. forsythus, T. denticola, Enterococcus species, P. gingivalis and
P intermedia.
9) To investigate the association of
Eubacterium species with a selected group of above ten putative microorganisms, we used odds ratio analysis and the results showed a positive association between the presence of
Eubacterium species with the presence of
F. nucleatum, P. micro, P. nigrescens, P. endodontalis, B. forsythus, T. denticola, P. gingivalis. Furthermore, a positive association was observed between the presence of diabetes mellitus and the combination of
Eubacterium species with
F. nucleatum, P. micro, P. endodontalis, T. denticolar, or P. gingivalis.
Table 3 shows the odds ratio extremes of ∞ in a number of cases due to the high prevalence of some organisms (e.g.
F. nucleatum or P. micros). Because of the small number of positive identification in a number of cases and the relative small overall sample, this data is presented as revealing possible trends and should be further investigated.
In conclusion, Eubacterium spp., particularly E. infirmum, were highly prevalent organisms in endodontic infection, and the latter species was significantly associated with a history of diabetes. Further work is however, necessary to elucidate potential pathogenic mechanisms of Eubacterium species in endodontic infections.
-
This study was partly supported by a NIDCR grant #DE 14476-02.
REFERENCES
- 1. Wade WG, Lewis MA, Cheeseman SL, Absi EG, Bishop PA. An unclassified Eubacterium taxon in acute dento-alveolar abscess. J Med Microbiol. 1994;40: 115-117.ArticlePubMed
- 2. Wade WG. The role of Eubacterium species in periodontal disease and other oral infections. Microb Ecol Health Dis. 1996;9: 367-370.Article
- 3. Sundqvist G. Associations between microbial species in dental root canal infections. Oral Microbiol Immunol. 1992;7: 257-262.ArticlePubMed
- 4. Wasfy MO, McMahon KT, Minah GE, Falkler WA Jr. Microbiological evaluation of periapical infections in Egypt. Oral Microbiol Immunol. 1992;7: 100-105.ArticlePubMed
- 5. Yoshida M, Fukushima H, Yamamoto K, Ogawa K, Toda T, Sagawa H. Correlation between clinical symptoms and microorganisms isolated from root canals of teeth with periapical pathosis. J Endod. 1987;13: 24-28.ArticlePubMed
- 6. Sundqvist G, Johansson E, Sjogren U. Prevalence of black-pigmented bacteroides species in root canal infections. J Endod. 1989;15: 13-19.ArticlePubMed
- 7. Sjögren U, Figdor D, Persson S, Sundqvist G. Influence of infection at the time of root filling on the outcome of endodontic treatment of teeth with apical periodontitis. Int Endod J. 1997;30: 297-306.ArticlePubMed
- 8. Kageyama A, Benno Y, Nakase T. Phylogenetic evidence for the transfer of Eubacterium lentum to the genus Eggerthella as Eggerthella lenta gen. nov., comb. nov. Int J Syst Bacteriol. 1999;49: 1725-1732.ArticlePubMed
- 9. Fouad AF, Barry J, Caimano M, Clawson C, Zhu Q, Carver R, Hazlett K, Radolf JD. PCR-based identification of bacteria in endodontic infections. J Clin Microbiol. 2002;40: 3223-3231.ArticlePubMedPMCPDF
- 10. Rolph HJ, Lennon A, Riggio MP, Saunders WP, MacKenzie D, Coldero L, Bagg J. Molecular identification of microorganisms from endodontic infections. J Clin Microbiol. 2001;39: 3282-3289.ArticlePubMedPMCPDF
- 11. Conrads G, Soffner J, Pelz K, Mutters R. Taxonomic update and clinical significance of species within the genus Peptostreptococcus. Clin Infect Dis. 1997;25: S94-S97.ArticlePubMed
- 12. Felsenstein J. PHYLIP (Phylogeny Inference Package) version 3.5c. 1993;Seattle: Department of Genetics, University of Washington; Distributed by the author.
- 13. Page RD. TreeView: an application to display phylogenetic trees on personal computers. Comput Appl Biosci. 1996;12: 357-358.ArticlePubMed
- 14. Socransky SS, Haffajee AD, Dzink JL, Hillman JD. Associations. between microbial species in subgingival plaque samples. Oral Microbiol Immunol. 1988;3: 1-7.ArticlePubMed
- 15. Spratt DA, Weightman AJ, Wade WG. Diversity of oral asaccharolytic Eubacterium species in periodontitis--identification of novel phylotypes representing uncultivated taxa. Oral Microbiol Immunol. 1999;14: 56-59.ArticlePubMedPDF
- 16. Nakazawa F, Sato M, Poco SE, Hashimura T, Ikeda T, Kalfas S, Sundqvist G, Hoshino E. Description of Mogibacterium pumilum gen. nov., sp. nov. and Mogibacterium vescum gen. nov., sp. nov., and reclassification of Eubacterium timidum (Holdeman et al. 1980) as Mogibacterium timidum gen. nov., comb. nov. Int J Syst Evol Microbiol. 2000;50(Pt 2):679-688.ArticlePubMed
- 17. Haapasalo M, Kerosuo E, Lounatmaa K. Hydrophobicities of human PMN leukocytes and oral Bacteroides and Porphyromonas spp., Wolinella recta, and E. yurii with special reference to bacterial surface structures. Scand J Dent Res. 1990;98: 472-481.PubMed
- 18. Downes J, Munson MA, Spratt DA, Kononen E, Tarkka E, Jousimies-Somer H, Wade WG. Characterization of Eubacterium-like strains isolated from oral infections. J Med Microbiol. 2001;50: 947-951.PubMed
- 19. Cheeseman SL, Hiom SJ, Weightman AJ, Wade WG. Phylogeny of oral asaccarolytic Eubacterium species determined by 16S rRNA sequence comparision and proposal of Eubacterium infirmum sp.nov. and Eubacterium tardum sp.nov. Int J Syst Bacteriol. 1996;46(4):957-959.PubMed
- 20. Hashimura T, Sato M, Hoshino E. Detection of Slackia exigua, Mogibacterium timidum and Eubacterium saphenum from pulpal and periradicular samples using the Polymerase Chain Reaction (PCR) method. Int Endod J. 2001;34: 463-470.ArticlePubMedPDF
- 21. Smith AJ, Wade WG. Serum antibody response against oral Eubacterium species. J Periodontal Res. 1999;34: 175-178.PubMed
- 22. Fouad AF, Burleson J. The effct of diabetes mellitus on endodontic treatment outcome. J Am Dent Assoc. 2003;134: 43-51.PubMed
- 23. Wade WG, Downes J, Dymock D, Hiom SJ, Weightman AJ, Dewhirst FE, Paster BJ, Tzellas N, Coleman B. The family Coriobacteriaceae: reclassification of Eubacterium exiguum (Poco et al. 1996) and Peptostreptococcus heliotrinreducens (Lanigan 1976) as Slackia exigua gen. nov., comb. nov. and Slackia heliotrinireducens gen. nov., comb. nov., and Eubacterium lentum (Prevot 1938) as Eggerthella lenta gen. nov., comb. nov. Int J Syst Bacteriol. 1999;49: 595-600.ArticlePubMed
- 24. Wade WG, Slayne MA, Aldred MJ. Comparison of identification methods for oral asaccharolytic Eubacterium species. J Med Microbiol. 1990;33: 239-242.ArticlePubMed
- 25. Wade WG. The role of Eubacterium species in periodontal disease and other oral infections. Microb Ecol Health Dis. 1997;9: 367-370.Article
- 26. Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 16S rDNA amplification for phylogenetic study. J Bacteriol. 1991;173: 697-703.ArticlePubMedPMCPDF
Fig. 1Phylogenetic analysis of 16S rRNA gene sequences with oral asaccharolytic Eubacterium-branch specific primer 557R and universal primer 27F, sequences of oral asaccharolytic Eubacterium species and most closely related species retrieved from sequence databases.
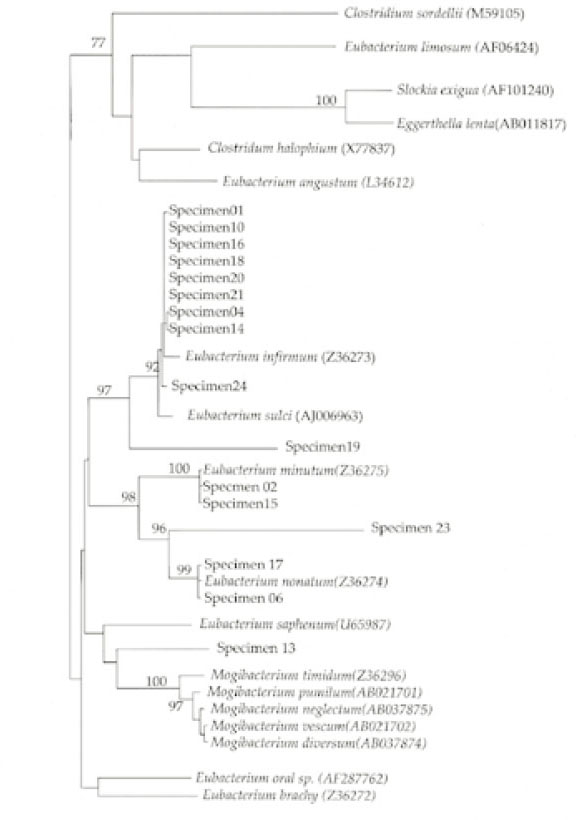
Table 1Oligonucleotide primers used. Top primer is sense, bottom is antisense.
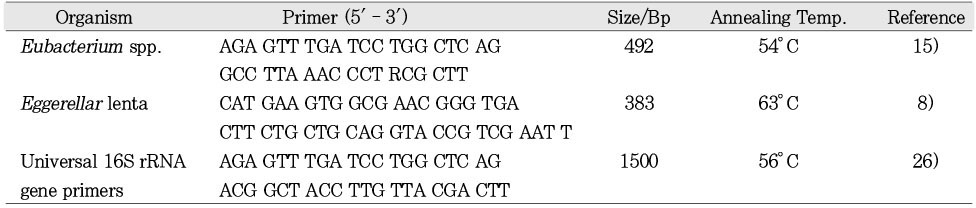
Table 2PCR results of Eubacterium spp at the species level from 16 Eubacterium positive specimens. Diabetes Mellitus (DM) type, (ND: non-diabetic); preoperative pain and/or swelling experience (symptomatic) for 22 patient specimens. §Specimens 5 and 8 had very little bacterial DNA. † Specimens #9 and 12 had no bacterial DNA (see text). The question marks indicate that identity could not be established on these organisms, or that there is a potential for mixed sequences.
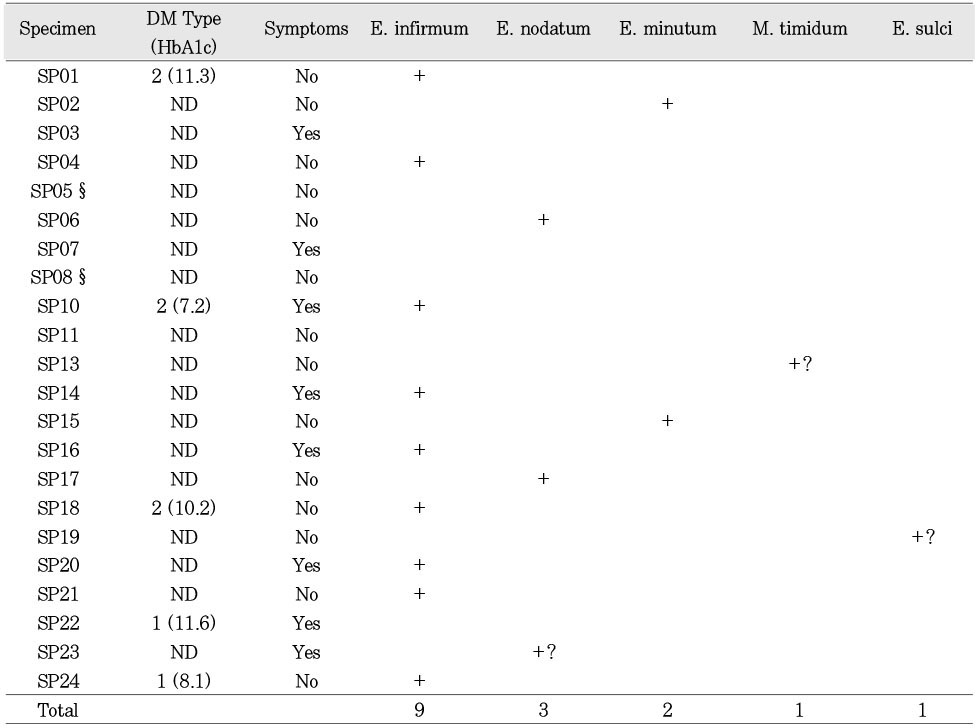
Table 3Odds Ratio analysis of the association between diabetes and bacterial combinations.




 KACD
KACD


 ePub Link
ePub Link Cite
Cite

