Articles
- Page Path
- HOME > Restor Dent Endod > Volume 41(3); 2016 > Article
- Research Article Cytotoxicity and biocompatibility of Zirconia (Y-TZP) posts with various dental cements
- Hyeongsoon Shin, Hyunjung Ko, Miri Kim
-
2016;41(3):-175.
DOI: https://doi.org/10.5395/rde.2016.41.3.167
Published online: May 30, 2016
Department of Conservative Dentistry, Asan Medical Center, Ulsan University, Seoul, Korea.
- Correspondence to Miri Kim, DDS, PhD. Associate professor, Department of Conservative Dentistry, Asan Medical Center, University of Ulsan, 43 gil 88, Olympic-ro, Songpa-gu, Seoul, Korea 05505. TEL, +82-2-3010-3850; FAX, +82-2-3010-6967; kmrne@hanmail.net
• Received: January 13, 2016 • Accepted: April 22, 2016
©Copyrights 2016. The Korean Academy of Conservative Dentistry.
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 2,303 Views
- 17 Download
- 30 Crossref
Abstract
-
Objectives Endodontically treated teeth with insufficient tooth structure are often restored with esthetic restorations. This study evaluated the cytotoxicity and biological effects of yttria partially stabilized zirconia (Y-TZP) blocks in combination with several dental cements.
-
Materials and Methods Pairs of zirconia cylinders with medium alone or cemented with three types of dental cement including RelyX U200 (3M ESPE), FujiCEM 2 (GC), and Panavia F 2.0 (Kuraray) were incubated in medium for 14 days. The cytotoxicity of each supernatant was determined using 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays on L929 fibroblasts and MC3T3-E1 osteoblasts. The levels of interleukin-6 (IL-6) mRNA were evaluated by reverse transcription polymerase chain reaction (RT-PCR), and IL-6 protein was evaluated by enzyme-linked immunosorbent assays (ELISA). The data were analyzed using one-way ANOVA and Tukey post-hoc tests. A p < 0.05 was considered statistically significant.
-
Results The MTT assays showed that MC3T3-E1 osteoblasts were more susceptible to dental cements than L929 fibroblasts. The resin based dental cements increased IL-6 expression in L929 cells, but reduced IL-6 expression in MC3T3-E1 cells.
-
Conclusions Zirconia alone or blocks cemented with dental cement showed acceptable biocompatibilities. The results showed resin-modified glass-ionomer based cement less produced inflammatory cytokines than other self-adhesive resin-based cements. Furthermore, osteoblasts were more susceptible than fibroblasts to the biological effects of dental cement.
Introduction
Many endodontically treated teeth show a significant loss of tooth structures. Thus, a full-coverage crown restoration is often needed to prevent undesirable tooth fractures. If there is extensive destruction of the tooth structure, then a post and core system is required to provide appropriate retention and support of the crown.1 Over the past several decades the patient's need for esthetic dental restorations have increased, which resulted in the development of several tooth colored restorative materials.2 More recently, a wide range of esthetic posts have become commercially available including fiber reinforced composite resin posts (FRC) and yttrium stabilized zirconia (Y-TZP) based ceramic posts.3,4 Zirconia posts are composed primarily of zirconium dioxide and exhibit high flexural strength and chemical durability.5 Although these posts are used frequently, there are questions regarding their adhesion to the tooth substance. The ability of dental cements to bond to zirconia is uncertain.6,7 The most common cause of failures in endodontic dowels was dowel de-bonding (37%).8 Therefore, it is important to optimize bonding techniques to provide sufficient bond strength for the retention of restorations, prevent microleakage, and increase resistance to fracture/fatigue.9 A failure of the zirconia restorations to bond with dental cements can result in the release of excess or residue cement into adjacent tissues. Furthermore, bonding failure of zirconia restoration with cement may result in tooth discoloration, periodontal problems, or secondary dental caries.10,11
The in vitro cytotoxicity evaluation for dental cements and other dental materials requires selecting the most appropriate cells and examining the secretion of inflammatory cytokines.12 Inflammation causes the secretion of bone resorbing and inflammatory cytokines such as interleukin-6 (IL-6).13 IL-6 was shown to be present in cells of patients with apical periodontitis and the levels were proportional to the size of the periapical lesions.14
With such a broad range of dental materials, there are a number of factors to consider in making an appropriate choice. Although many studies have shown the biocompatibility of zirconia,15,16 the biological effects of zirconia restorations bonded with dental cements in clinical situations have not been reported. In this respect, this study evaluated the cytotoxicity and biological effects of zirconia restorations in combination with various dental cements.
Materials and Methods
We prepared 96 zirconia cylinders (Lava, 3M ESPE, Seefeld, Germany) that measured 5 mm in diameter and 12 mm in height. The cylinders were densely sintered and washed with acetone in an ultrasonic bath. The cylinders were then rinsed with distilled water, and sterilized by autoclaving at 130℃ for 15 minutes. Then, the cylinders were randomly divided into five groups of 12 cylinders. Group 1 was the negative control group consisting medium alone. Group 2 was the positive control group consisting pairs of cylinders without cement. Groups 3, 4, and 5 consisted pairs of cylinders cemented with RelyX U200 (3M ESPE, St. Paul, MN, USA), FujiCEM 2 (GC, Tokyo, Japan), and Panavia F 2.0 (Kuraray, Okayama, Japan), respectively (Table 1). Two cylinders were cemented under pressure and each cement gap was adjusted to 100 µm film thickness.
Each pair of cylinders was immersed in serum-free medium at a volume/surface area ratio of 1 cm2/mL for 14 days at 37℃ in a sealed container. The medium without zirconia was maintained under the same conditions and used as the negative control.
L929 cells (American Type Culture Collection, Manassas, VA, USA) were thawed and then plated in 100 mm culture dishes containing RPMI 1640 medium (HyClone, Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS, Gibco, Grand Island, NY, USA) and 1% penicillin-streptomycin. The cells were cultured in a humidified atmosphere containing 5% CO2 at 37℃. MC3T3-E1 cells (American Type Culture Collection) were cultured in α-MEM medium (Gibco) under the same conditions. The media for both cells were changed every other day.
Cultured L929 and MC3T3-E1 cells were seeded at an initial density of 1 × 105 cells/mL in their respective media containing 10% FBS in three 96-well culture plates. After 24 hours for attachment period, a 100 µL aliquot of test specimen was added to each well. The cell viability was determined using 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay kits (Trevigen Inc., Gaithersburg, MD, USA) according to the manufacturer's instructions following incubation for one and three days. The colorimetric changes were quantified at 540 nm using a microplate reader (Molecular Devices, Sunnyvale, CA, USA). Each sample was tested in triplicate, and the entire assay was repeated twice. The data were analyzed using one-way analysis of variance (ANOVA) and Tukey post-hoc tests (p = 0.05).
Cultured L929 and MC3T3-E1 cells were seeded at an initial density of 2 × 105 cells/mL in their respective media containing 10% FBS. After 24 hours, the medium was collected, and supernatants of test or control specimens were added. The supernatants were removed after three days and then total RNA was extracted using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA). The RNA samples were reverse-transcribed to cDNA (MP Biomedicals, Santa Ana, CA, USA). The cDNA was examined with gene-specific PCR for IL-6 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression (TaKaRa BIO Inc., Shiga, Japan) using the primers and reaction with the Gene AMP PCR system 9700 (Perkin-Elmer, Norwalk, CT, USA). The gels were photographed under ultraviolet illumination, and the bands were quantified. The IL-6 mRNA expression was normalized relative to GAPDH mRNA expression in the same samples. The data were analyzed using one-way ANOVA and Tukey tests (p = 0.05, SPSS 12.0, SPSS GmbH, Munich, Germany).
The total protein content in culture supernatants was measured using BCA assay kits (Pierce Biotechnology, Rockford, IL, USA). The concentrations of IL-6 in cell culture supernatants were determined using commercially available Quantikine Enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems Inc., Minneapolis, MV, USA) according to the manufacturer's instructions. The absorbance was measured at 450 nm using a microplate reader. The data were analyzed using one-way ANOVA and Tukey tests (p = 0.05).
L929 and MC3T3-E1 cells were seeded in 35 mm dishes (Ibidi, Martinsried, Germany) at densities of 1 × 105 cells/well and 5 × 104 cells/well, respectively. After 24 hours, the medium was removed and the supernatants of the test or control specimens were added. The cells were then cultured for additional 24 hours. The supernatants were removed and the cells were fixed with 3.7% formaldehyde for 10 minutes. The cells were permeabilized with 0.1% Triton X-100 (Sigma-Aldrich, St Louis, MO, USA) for 5 minutes. The cells were then incubated with 1% bovine serum albumin for 30 minutes to block non-specific binding. The cells were stained for 20 minutes with rhodamine phalloidin (1:200, Molecular Probes, Eugene, OR, USA) to stain F-actin filaments and 4',6-diamidino-2-phenylindole (DAPI, Molecular Probes) to stain nuclei. The cells were washed with phosphate buffered saline (Gibco) and observed by fluorescence microscopy (Olympus, Tokyo, Japan) at x250 magnification. Total cell counts of DAPI staining were estimated using the fluorescence microscope. The proportion of damaged cells was measured using Image J (National Institutes of Health, Bethesda, MD, USA). Thirty specimens of each group were observed by the manual method of Image J program. Cell images were estimated by well-trained single observer, and the procedure was repeated three times.
Data were analyzed by one-way ANOVA and the Tukey HSD test at a 0.05 significance level. Statistical analysis was performed with SPSS for Windows (SPSS 12.0, SPSS GmbH).
Results
In MTT assay, L929 cell viability was significantly lower in Group 5 than in Group 1 after one day (p < 0.001). The cell viability was significantly lower in Groups 3, 4, and 5 than in Group 1 after three days (p = 0.005, p = 0.029, and p = 0.008, respectivley, Figure 1a). The viability of MC3T3-E1 cells decreased gradually from one to three days in all groups except the negative control (Group 1). The differences were significant after three days (p < 0.001, Figure 1b). A comparison of the two cell lines showed that MC3T3-E1 osteoblasts were more vulnerable to dental cements than L929 fibroblasts.
The expression of IL-6 mRNA in L929 cells was higher in Groups 2 through 5 than in the negative control group after one day. After three days, IL-6 mRNA levels were higher in Groups 2 and 3 than in the negative control (p < 0.05). However, the IL-6 mRNA levels were lower in Groups 4 and 5 than in the negative control (p < 0.05, Figures 2a and 2c). In MC3T3-E1 cells, the expression of IL-6 mRNA was higher in Groups 2 and 5, but lower in Groups 3 and 4 than in the negative control after one day. After three days, the IL-6 mRNA expression was significantly higher in Groups 2 and 3 (p < 0.05) than the negative control. However, the mRNA levels were significantly lower in Groups 4 and 5 (p < 0.05) than in the negative control (Figures 2b and 2d).
IL-6 secretion by L929 cells was significantly higher after one and three days in all four experimental groups compared with the negative control group (p < 0.001, Figure 2e). Conversely, IL-6 expression by MC3T3-E1 cells was significantly lower in Groups 3, 4, and 5 than in the negative control group (p < 0.001, Figure 2f).
Total undamaged cell numbers of L929 (Figure 3a) and MC3T3-E1 (Figure 3b) were lower in Groups 3 and 5 than in the negative control group (p < 0.05). These reductions were more pronounced for MC3T3-E1 cells (p < 0.05). Although the cells maintained the same density in the presence of resin-modified glass-ionomer cements (RMGI), the cells treated with resin cement extract did not display same densities. A few cells with treated resin cements (RelyX U200 and Panavia F 2.0) condensed nuclear morphology partially, and cells had strikingly decreased numbers.
Discussion
Extensive loss of tooth structure is often encountered when restoring endodontically involved anterior teeth. This loss has a deleterious effect on esthetic results.17 Zirconia restorations are esthetic,18 partially adhesive, very rigid, and brittle. These zirconia post & core cannot be etched and do not bond effectively to resins, which makes them less predictable and requires suitable bonding methods.19 Dental materials such as cements and restorative materials should be evaluated in cytotoxicity tests for its biocompatibility.20 The health risk of dental materials is very important and a continuously emerging issue. Historically, safety issues of dental materials like amalgam, heavy metals in dental cement, and bisphenol in dental monomers have been raised. For example, it was reported that the cellular response to a biomaterial can be affected by cross-linked material and soluble monomers that may leach from the material.21 In this experiment, different responses of two cell types were investigated.
The MTT results in our study indicated that the cytotoxicity was different for L929 and MC3T3-E1 cells, which is consistent with previous findings. Previous studies have demonstrated that five different endodontic sealers had different cytotoxicity to L929 mouse fibroblasts, ROS 17/2.8 rat osteoblasts, and MC3T3-E1 mouse osteoblasts.22 Although the cellular responses to these sealers differed, mouse osteoblasts were more vulnerable to cytotoxic agents than fibroblasts.22 These findings suggest more than one cell line should be involved in assessing the cytotoxicity of dental materials. We also found that MC3T3-E1 cells were susceptible to supernatants from zirconia blocks alone in the absence of dental cement. However, several previous studies confirmed that zirconia is not cytotoxic.7,23,24,25 Although pure components of zirconia are not cytotoxic, responses to zirconia blocks may differ among cell lines. In previous report, CAD-CAM all-ceramic materials had presented different cell viabilities between human gingival fibroblasts and oral keratinocytes.26 The study demonstrated that the cell viability and migration ability of keratinocyte were negatively influenced by the tested CAD/CAM zirconia ceramics, whereas gingival fibroblast cell functionality was overall not negatively influenced. Biocompatibility of zirconia was well reported that it seemed to be suitable for making dental implants. However, some studies also point out its drawbacks. It was also found that most of the studies on zirconia dental implants are short-term studies and there is a need for more long-term clinical trials to prove that zirconia is worth enough to replace titanium as a biomaterial in dental implantology.
Resin-based composite cements have become standard materials for attaching ceramic prosthetics to tooth structures. Panavia F 2.0 contains 10-methacryl-oyloxydecyl-dihydrogen-phosphate (MDP), and RelyX U200 is another phosphate monomer-containing resin cement.8 Resin cements have shown favorable mechanical retention. MDP-containing resin cements are popular for ZrO2 prosthetics in clinical applications due to their low rates of failure and minimum loss of retention.27 Panavia F 2.0 was reported to provide better shear bond strengths than other glass-ionomer or RMGI-based cements.28 However, we showed that Panavia F 2.0 and RelyX U200 were more cytotoxic and induced higher levels of IL-6 than the RMGI FujiCEM 2. This result differs from that of a previous study,29 which found that Panavia F 2.0 was the least cytotoxic cement. The study did not assess RMGI cements.
The release of IL-6 was reported to play an important role in the pathogenesis of inflammation.30 IL-6 may be useful in making a differential diagnosis or may function as a biomarker that can predict the progression of bone resorption.14 IL-6 is considered as both pro- and anti-inflammatory cytokine because it is produced during inflammation and after secretion of tumor necrosis factor (TNF)-α and IL-1. IL-6 subsequently inhibits the secretion of TNF-α and IL-1.31 IL-6 was shown to interfere with programmed cell death in circulating mature neutrophils32 and was implicated in the regulation of enhanced neutrophil-mediated cytotoxicity. Besides proinflammatory mediators such as nitric oxide (NO), prostaglandin E2 (PGE2), and IL-8 were suggested indicators of inflammatory reactions.33 Further studies are needed to elucidate the exact mechanisms of another cytokines. Our results suggest that dental resin cements, but not glass-ionomer based cement, may affect cell survival by inducing the secretion of IL-6.
The results showed that L929 cells were more viable than MC3T3-E1 cells in the presence of dental cements. Resin cements were more cytotoxic to MC3T3-E1 cells and increased the expression of IL-6 mRNA than the glass-ionomer based cement. The expression of IL-6 mRNA increased in Panavia F 2.0 group on 1 day, and RelyX U200 group on 3 days. Some of these results of RT-PCR of IL-6 were not exactly in accordance with the results of ELISA. It is thought that the results came from sensitivity and specificity of the tests.
The biocompatibility was also confirmed by fluorescent staining. Generally, the DAPI staining of nucleic acids shows evidence of cell morphologic destruction.34 Cellular morphologic destructions can be detected by fluorescence microscopy using stains, such as DAPI, that bind to nucleic acids. In the present study, cells exposed to FujiCEM 2 showed very similar cell densities with control groups in DAPI staining, and the cells were as undamaged as in the control group. In contrast, a few of the nuclei of the RelyX U200 and Panavia F 2.0 treated cells did not display the typical morphology, but rather a strikingly decrease in cell numbers. Also we used rhodamine-conjugated phalloidin to visualize F-actin filaments. Changes in actin fibers are readily observed by fluorescence staining, indicating that intracellular changes in F-actin fibers occur before any gross morphological changes become evident.
Most of the cells exhibited original straight actin cables extending from the perinuclear region to the cell periphery, similar to those in control groups. These results indicate intracellular changes in F-actin fibers occurs prior to gross morphological changes.35 The influence of exudates on cells and spreading was confirmed by visualizing the actin cytoskeleton morphology and organization. Actin microfilaments are essential in maintaining cell shape, and the permeability of tight junctions.36
Conclusions
This study showed that zirconia posts alone, and posts with various dental cements are biocompatible with limited cytotoxicity. RMGI cements less produced inflammatory cytokines than resin based cements. Further, our data showed that the mouse osteoblasts were more susceptible than the mouse fibroblasts to potential cytotoxic dental cement materials.
Acknowledgement
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP, NRF-2015R1C1A2A01054748).
- 1. Hargreaves KM, Cohen S, Berman LH. Cohen's pathways of the pulp. 10th ed. St. Louis, MO: Mosby Elsevier; 2011. p. 781-787.
- 2. Tholey MJ, Swain MV, Thiel N. SEM observations of porcelain Y-TZP interface. Dent Mater 2009;25:857-862.ArticlePubMed
- 3. Bitter K, Kielbassa AM. Post-endodontic restorations with adhesively luted fiber-reinforced composite post systems: a review. Am J Dent 2007;20:353-360.PubMed
- 4. Oblak C, Jevnikar P, Kosmac T, Funduk N, Marion L. Fracture resistance and reliability of new zirconia posts. J Prosthet Dent 2004;91:342-348.ArticlePubMed
- 5. Ozkurt Z, Işeri U, Kazazoğlu E. Zirconia ceramic post systems: a literature review and a case report. Dent Mater J 2010;29:233-245.ArticlePubMed
- 6. McLaren EA, White SN. Glass-infiltrated zirconia/alumina-based ceramic for crowns and fixed partial dentures. Pract Periodontics Aesthet Dent 1999;11:985-994.PubMed
- 7. Manicone PF, Rossi Iommetti P, Raffaelli L. An overview of zirconia ceramics: basic properties and clinical applications. J Dent 2007;35:819-826.ArticlePubMed
- 8. Rasimick BJ, Wan J, Musikant BL, Deutsch AS. A review of failure modes in teeth restored with adhesively luted endodontic dowels. J Prosthodont 2010;19:639-646.ArticlePubMed
- 9. Thompson JY, Stoner BR, Piascik JR, Smith R. Adhesion/cementation to zirconia and other non-silicate ceramics: where are we now? Dent Mater 2011;27:71-82.ArticlePubMed
- 10. Ali Z, Eliyas S, Vere JW. Choosing the right dental material and making sense of the options: evidence and clinical recommendations. Eur J Prosthodont Restor Dent 2015;23:P150-P162.PubMed
- 11. Shiozawa M, Takahashi H, Asakawa Y, Iwasaki N. Color stability of adhesive resin cements after immersion in coffee. Clin Oral Investig 2015;19:309-317.ArticlePubMedPDF
- 12. Yesilsoy C, Feigal RJ. Effects of endodontic materials on cell viability across standard pore size filters. J Endod 1985;11:401-407.ArticlePubMed
- 13. Uematsu S, Mogi M, Deguchi T. Interleukin (IL)-1 beta, IL-6, tumor necrosis factor-alpha, epidermal growth factor, and beta 2-microglobulin levels are elevated in gingival crevicular fluid during human orthodontic tooth movement. J Dent Res 1996;75:562-567.ArticlePubMedPDF
- 14. Azuma MM, Samuel RO, Gomes-Filho JE, Dezan-Junior E, Cintra LT. The role of IL-6 on apical periodontitis: a systematic review. Int Endod J 2014;47:615-621.PubMed
- 15. Uo M, Sjögren G, Sundh A, Watari F, Bergman M, Lerner U. Cytotoxicity and bonding property of dental ceramics. Dent Mater 2003;19:487-492.ArticlePubMed
- 16. Gong SH, Lee H, Pae A, Noh K, Shin YM, Lee JH, Woo YH. Gene expression of MC3T3-E1 osteoblastic cells on titanium and zirconia surface. J Adv Prosthodont 2013;5:416-422.ArticlePubMedPMC
- 17. Heydecke G, Butz F, Strub JR. Fracture strength and survival rate of endodontically treated maxillary incisors with approximal cavities after restoration with different post and core systems: an in-vitro study. J Dent 2001;29:427-433.ArticlePubMed
- 18. Meyenberg KH, Lüthy H, Schärer P. Zirconia posts: a new all-ceramic concept for nonvital abutment teeth. J Esthetic Dent 1995;7:73-80.Article
- 19. Blatz MB, Sadan A, Kern M. Resin-ceramic bonding: a review of the literature. J Prosthet Dent 2003;89:268-274.ArticlePubMed
- 20. Houck KA, Kavlock RJ. Understanding mechanisms of toxicity: insights from drug discovery research. Toxicol Appl Pharmacol 2008;227:163-178.ArticlePubMed
- 21. Wang MO, Etheridge JM, Thompson JA, Vorwald CE, Dean D, Fisher JP. Evaluation of the in vitro cytotoxicity of cross-linked biomaterials. Biomacromolecules 2013;14:1321-1329.ArticlePubMedPMC
- 22. Brackett MG, Messer RL, Lockwood PE, Bryan TE, Lewis JB, Bouillaguet S, Wataha JC. Cytotoxic response of three cell lines exposed in vitro to dental endodontic sealers. J Biomed Mater Res B Appl Biomater 2010;95:380-386.ArticlePubMed
- 23. Dion I, Rouais F, Baquey C, Lahaye M, Salmon R, Trut L, Cazorla JP, Huong PV, Monties JR, Havlik P. Physico-chemistry and cytotoxicity of ceramics: part I: characterization of ceramic powders. J Mater Sci Mater Med 1997;8:325-332.ArticlePubMedPDF
- 24. Torricelli P, Verne E, Brovarone CV, Appendino P, Rustichelli F, Krajewski A, Ravaglioli A, Pierini G, Fini M, Giavaresi G, Giardino R. Biological glass coating on ceramic materials: in vitro evaluation using primary osteoblast cultures from healthy and osteopenic rat bone. Biomaterials 2001;22:2535-2543.ArticlePubMed
- 25. Lohmann CH, Dean DD, Köster G, Casasola D, Buchhorn GH, Fink U, Schwartz Z, Boyan BD. Ceramic and PMMA particles differentially affect osteoblast phenotype. Biomaterials 2002;23:1855-1863.ArticlePubMed
- 26. Pabst AM, Walter C, Grassmann L, Weyhrauch M, Brüllmann DD, Ziebart T, Scheller H, Lehmann KM. Influence of CAD/CAM all-ceramic materials on cell viability, migration ability and adenylate kinase release of human gingival fibroblasts and oral keratinocytes. Clin Oral Investig 2014;18:1111-1118.ArticlePubMedPDF
- 27. Özcan M, Bernasconi M. Adhesion to zirconia used for dental restorations: a systematic review and meta-analysis. J Adhes Dent 2015;17:7-26.PubMed
- 28. Kim MJ, Kim YK, Kim KH, Kwon TY. Shear bond strengths of various luting cements to zirconia ceramic: surface chemical aspects. J Dent 2011;39:795-803.ArticlePubMed
- 29. Mahasti S, Sattari M, Romoozi E, Akbar-Zadeh Baghban A. Cytotoxicity comparison of Harvard zinc phosphate cement versus Panavia F2 and Rely X Plus resin cements on rat L929-fibroblasts. Cell J 2011;13:163-168.PubMedPMC
- 30. Huang FM, Tsai CH, Yang SF, Chang YC. Induction of interleukin-6 and interleukin-8 gene expression by root canal sealers in human osteoblastic cells. J Endod 2005;31:679-683.ArticlePubMed
- 31. Schindler R, Mancilla J, Endres S, Ghorbani R, Clark SC, Dinarello CA. Correlations and interactions in the production of interleukin-6 (IL-6), IL-1, and tumor necrosis factor (TNF) in human blood mononuclear cells: IL-6 suppresses IL-1 and TNF. Blood 1990;75:40-47.ArticlePubMedPDF
- 32. Colotta F, Re F, Polentarutti N, Sozzani S, Mantovani A. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood 1992;80:2012-2020.ArticlePubMedPDF
- 33. Chang SW, Bae WJ, Yi JK, Lee S, Lee DW, Kum KY, Kim EC. Odontoblastic differentiation, inflammatory response, and angiogenic potential of 4 calcium silicate-based cements: Micromega MTA, ProRoot MTA, RetroMTA, and Experimental Calcium Silicate Cement. J Endod 2015;41:1524-1529.ArticlePubMed
- 34. Lee SJ, Chung J, Na HS, Park EJ, Jeon HJ, Kim HC. Characteristics of novel root-end filling material using epoxy resin and Portland cement. Clin Oral Investig 2013;17:1009-1015.ArticlePubMedPDF
- 35. Van Cruchten S, Van Den Broeck W. Morphological and biochemical aspects of apoptosis, oncosis and necrosis. Anat Histol Embryol 2002;31:214-223.ArticlePubMed
- 36. Stan MS, Memet I, Fratila C, Krasicka-Cydzik E, Roman I, Dinischiotu A. Effects of titanium-based nanotube films on osteoblast behavior in vitro. J Biomed Mater Res A 2015;103:48-56.ArticlePubMed
REFERENCES
Figure 1
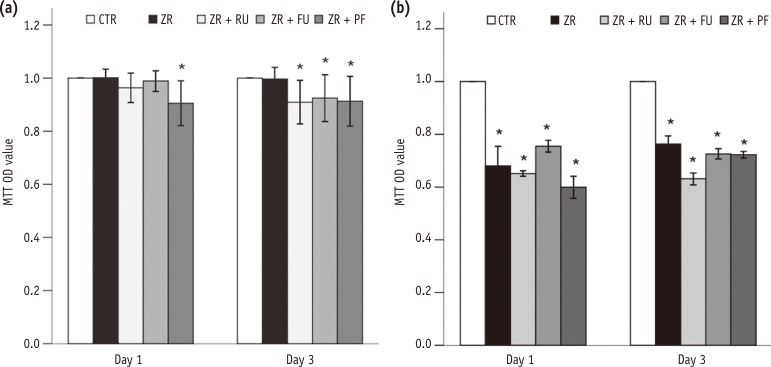
Effects of zirconia with or without an intermediate cement layer on the viability of (a) L929 and (b) MC3T3-E1 cells measured by MTT assays. Groups with the same lower case letters did not differ significantly on those days.
*Significant difference from the negative control group at each time period, according to Tukey tests. Error bars mean ± 1.0 standard deviations.
MTT, 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide; OD, optical density; CTR, Group 1, negative control; ZR, Group 2, positive control; ZR + RU, Group 3, zirconia with RelyX U200; ZR + FU, Group 4, zirconia with FujiCEM 2; ZR + PF, Group 5, zirconia with Panavia F 2.

Figure 2
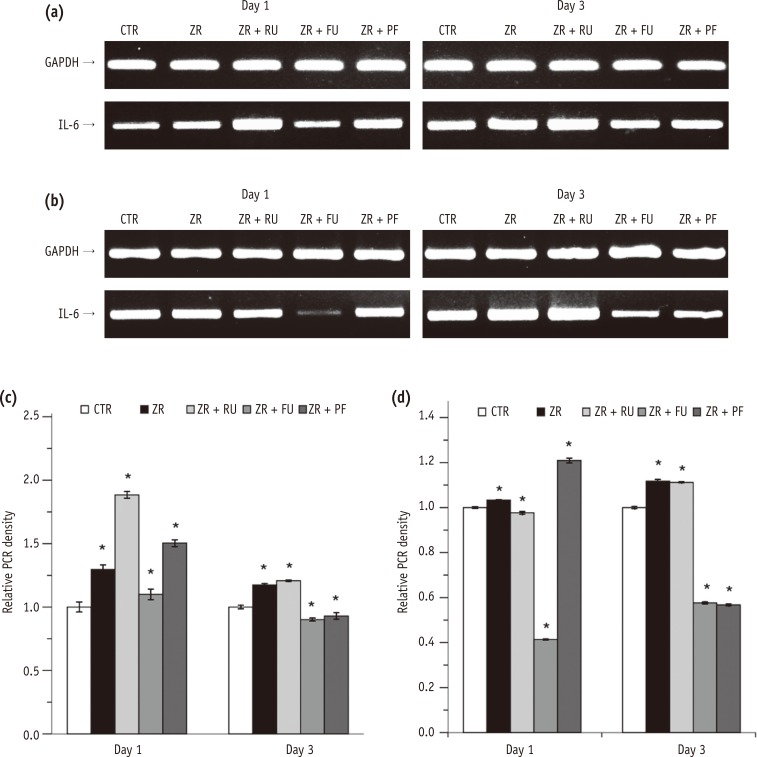
Effects of zirconia with or without an intermediate cement layer on IL-6 expression by (a) L929 and (b) MC3T3-E1 cells. The graphs in (c) and (d) show the densitometric quantification of protein expression of the bands shown in (a) and (b). The results are presented as fold increases relative to control. The graphs in e and f present ELISA results.
*Significant difference from the negative control group at each time period, according to Tukey tests. Different lower case letters indicate significant between group differences.
GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IL, interleukin-6; PCR, polymerase chain reaction; CTR, Group 1, negative control; ZR, Group 2, positive control; ZR + RU, Group 3, zirconia with RelyX U200; ZR + FU, Group 4, zirconia with FujiCEM 2; ZR + PF, Group 5, zirconia with Panavia F 2.0.

Figure 3
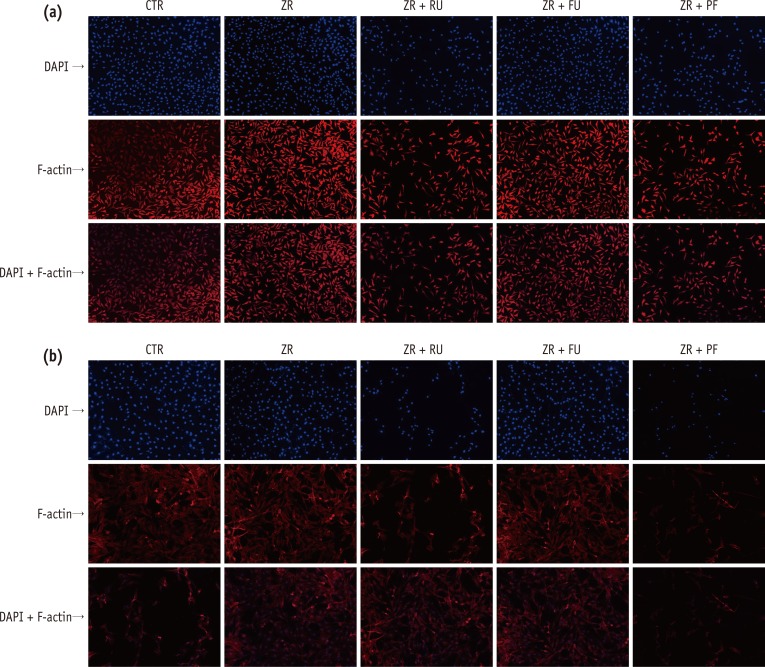
Fluorescent staining of (a) L929 and (b) MC3T3-E1 cells treated with medium only (CTR) or the supernatant of each test group (ZR, ZR + RU, ZR + FU, and ZR + PF). Rows represent cells stained with DAPI, rhodamine-conjugated phalloidin, and both after three days in culture. Cells exposed to FujiCEM 2 (ZR + FU) showed very similar cell densities with control groups in DAPI staining, and the cells were as undamaged as in the control group. In contrast, a few of the nuclei of the RelyX U200 (ZR + RU) and Panavia F 2.0 (ZR + PF) extract-treated cells remarkably decreased in cell numbers. Damaged cells showed flat and thin shapes.
CTR, negative control; ZR, positive control; ZR + RU, zirconia with RelyX U200; ZR + FU, zirconia with FujiCEM 2; ZR + PF, zirconia with Panavia F 2.0, DAPI, 4',6-diamidino-2-phenylindole.

Table 1
Type, composition, and batch number of tested cements
Tables & Figures
REFERENCES
Citations
Citations to this article as recorded by 

- Digital light processing 3D printing of yttria stabilized zirconia ceramics: microstructures, characterizations, and cytocompatibility
Luke Wadle, Mena Asha Krishnan, Ryan Wall, Lanh Trinh, Bin Duan, Bai Cui
Emergent Materials.2025; 8(2): 1023. CrossRef - Doping of casted silk fibroin membranes with extracellular vesicles for regenerative therapy: a proof of concept
Sandra Fuest, Amanda Salviano-Silva, Cecile L. Maire, Yong Xu, Christian Apel, Audrey Laure Céline Grust, Arianna Delle Coste, Martin Gosau, Franz L. Ricklefs, Ralf Smeets
Scientific Reports.2024;[Epub] CrossRef - 3D printing of ceramics: Advantages, challenges, applications, and perspectives
Susmita Bose, Enver Koray Akdogan, Vamsi K. Balla, Sushant Ciliveri, Paolo Colombo, Giorgia Franchin, Nicholas Ku, Priya Kushram, Fangyong Niu, Joshua Pelz, Andrew Rosenberger, Ahmad Safari, Zachary Seeley, Rodney W. Trice, Lionel Vargas‐Gonzalez, Jeffrey
Journal of the American Ceramic Society.2024; 107(12): 7879. CrossRef - A Review on Biocompatibility of Dental Restorative and Reconstruction Materials
Pune Nina Paqué, Mutlu Özcan
Current Oral Health Reports.2024; 11(1): 68. CrossRef - Enhancement of Human Gingival Fibroblasts Bioactivity and Proliferation on Plasma Sprayed Yttria-Stabilised Zirconia/TiO2 Surface Coating of Titanium Alloys: An In-Vitro Study
Afida Jemat, Masfueh Razali, Yuichi Otsuka, Mariyam Jameelah Ghazali
Coatings.2023; 13(10): 1746. CrossRef - Material extrusion-based additive manufacturing of zirconia toughened alumina: Machinability, mechanical properties and biocompatibility
Tianyu Yu, Xiaolong Zhu, Hongwei Yu, Pan Wu, Chun Li, Xiaoxiao Han, Mingjun Chen
Journal of Manufacturing Processes.2023; 94: 120. CrossRef - Green synthesis and characterization of zirconium nanoparticlefor dental implant applications
Mohammad Asaduzzaman Chowdhury, Nayem Hossain, Md. Golam Mostofa, Md. Riyad Mia, Md. Tushar, Md. Masud Rana, Md. Helal Hossain
Heliyon.2023; 9(1): e12711. CrossRef - The role of Y2O3 in the bioactivity of YSZ/PLLA composites
Elia Marin, Giada Bassi, Orion Yoshikawa, Francesco Boschetto, Wenliang Zhu, Arianna Rossi, Alex Lanzutti, Huaizhong Xu, Monica Montesi, Silvia Panseri, Giuseppe Pezzotti
Journal of Materials Science.2023; 58(27): 11218. CrossRef - Nano-β-tricalcium phosphate incorporated root dentin adhesive in the bonding interface of yttria-stabilized tetragonal zirconia polycrystalline post
Amal S. Al-Qahtani, Huda I. Tulbah, Mashael Binhasan, Mai M. Alhamdan, Imran Farooq, Fahim Vohra, Tariq Abduljabbar
Applied Nanoscience.2022; 12(11): 3489. CrossRef - Outcome of teeth restored with CAD/CAM zirconium dioxide post-cores: a retrospective study with a follow-up period of 3–6 years
Shunv Ying, Song Chen, Siyuan Wang, Lingli Xu, Xiaofeng Wang, Fuming He, Wei Liu
BMC Oral Health.2022;[Epub] CrossRef - Review of Physical, Mechanical, and Biological Characteristics of 3D-Printed Bioceramic Scaffolds for Bone Tissue Engineering Applications
Mahendran Thangavel, Renold Elsen Selvam
ACS Biomaterials Science & Engineering.2022; 8(12): 5060. CrossRef - Comparison of Regular and Speed Sintering on Low-Temperature Degradation and Fatigue Resistance of Translucent Zirconia Crowns for Implants: An In Vitro Study
Suchada Kongkiatkamon, Chaimongkon Peampring
Journal of Functional Biomaterials.2022; 13(4): 281. CrossRef - Cytotoxicity and Bonding Property of Bioinspired Nacre-like Ceramic-Polymer Composites
Hui Sun, Kefeng Gao, Zhe Yi, Chengwei Han, Zengqian Liu, Qiang Wang, Qing Zhou, Zhefeng Zhang
Frontiers in Bioengineering and Biotechnology.2022;[Epub] CrossRef - Characterisation of Selected Materials in Medical Applications
Kacper Kroczek, Paweł Turek, Damian Mazur, Jacek Szczygielski, Damian Filip, Robert Brodowski, Krzysztof Balawender, Łukasz Przeszłowski, Bogumił Lewandowski, Stanisław Orkisz, Artur Mazur, Grzegorz Budzik, Józef Cebulski, Mariusz Oleksy
Polymers.2022; 14(8): 1526. CrossRef - Adhesive bond integrity of Y‐TZP post with calcium fluoride infiltrated resin dentin adhesive: An SEM, EDX, FTIR and micro‐Raman study
Eman M. AlHamdan, Samar Al‐Saleh, Mohammad H. AlRefeai, Imran Farooq, Eisha Abrar, Fahim Vohra, Tariq Abduljabbar
Surface and Interface Analysis.2021; 53(11): 956. CrossRef - Additive Manufacturing (3D PRINTING) Methods and Applications in Dentistry
Elif DEMİRALP, Gülsüm DOĞRU, Handan YILMAZ
Clinical and Experimental Health Sciences.2021; 11(1): 182. CrossRef - Interleukin-1β activity in gingival crevicular fluid of abutment teeth with temporary fixed restorations versus final fixed restorations: Prospective observational study
Amal Abdallah A. Abo-Elmagd, Dina Sabry, Ebtehal Mohammed
The Saudi Dental Journal.2021; 33(6): 322. CrossRef - Change in the Microhardness of Composite Ceramics at the CaO–ZrO2/CaO–ZrO2 + Al2O3 Interface
A. A. Dmitrievskiy, D. G. Zhigacheva, G. V. Grigoriev, P. N. Ovchinnikov
Journal of Surface Investigation: X-ray, Synchrotron and Neutron Techniques.2021; 15(S1): S137. CrossRef - Cytotoxicity of two self-adhesive flowable composites on bovine dental pulp-derived cells
Firdevs KAHVECİOĞLU, Türkay KÖLÜŞ, Fatma SAĞ GÜNGÖR, Hayriye Esra ÜLKER
Journal of Health Sciences and Medicine.2021; 4(2): 209. CrossRef - Cytotoxicity and biocompatibility of high mol% yttria containing zirconia
Gulsan Ara Sathi Kazi, Ryo Yamagiwa
Restorative Dentistry & Endodontics.2020;[Epub] CrossRef - Cytotoxic effects of different self-adhesive resin cements: Cell viability and induction of apoptosis
Soner Şişmanoğlu, Mustafa Demirci, Helmut Schweikl, Gunes Ozen-Eroglu, Esin Cetin-Aktas, Serap Kuruca, Safa Tuncer, Neslihan Tekce
The Journal of Advanced Prosthodontics.2020; 12(2): 89. CrossRef - Effects of nano-zirconia fillers conditioned with phosphate ester monomers on the conversion and mechanical properties of Bis-GMA- and UDMA-based resin composites
Jiaxue Yang, Jiadi Shen, Xinyi Wu, Feng He, Haifeng Xie, Chen Chen
Journal of Dentistry.2020; 94: 103306. CrossRef - Effect of APTES- or MPTS-Conditioned Nanozirconia Fillers on Mechanical Properties of Bis-GMA-Based Resin Composites
Jiaxue Yang, Mengyuan Liao, Gaoying Hong, Shiqi Dai, Jiadi Shen, Haifeng Xie, Chen Chen
ACS Omega.2020; 5(50): 32540. CrossRef - In Vitro Cytotoxicity of Self-Adhesive Dual-Cured Resin Cement Polymerized Beneath Three Different Cusp Inclinations of Zirconia
Chang-Yuan Zhang, Yi-Ling Cheng, Xin-Wen Tong, Hao Yu, Hui Cheng
BioMed Research International.2019; 2019: 1. CrossRef - Investigations on the corrosion behaviour and biocompatibility of magnesium alloy surface composites AZ91D-ZrO2 fabricated by friction stir processing
R. Vaira Vignesh, R. Padmanaban, M. Govindaraju, G. Suganya Priyadharshini
Transactions of the IMF.2019; 97(5): 261. CrossRef - Microwave assisted synthesis and antimicrobial activity of Fe3O4-doped ZrO2 nanoparticles
M. Imran, Saira Riaz, Ifra Sanaullah, Usman Khan, Anjum N. Sabri, Shahzad Naseem
Ceramics International.2019; 45(8): 10106. CrossRef - Additive manufacturing of ceramics for dental applications: A review
Raquel Galante, Celio G. Figueiredo-Pina, Ana Paula Serro
Dental Materials.2019; 35(6): 825. CrossRef - Reinforcement of dental resin composite via zirconium hydroxide coating and phosphate ester monomer conditioning of nano-zirconia fillers
Xinyi Wu, Shiqi Dai, Ying Chen, Feng He, Haifeng Xie, Chen Chen
Journal of the Mechanical Behavior of Biomedical Materials.2019; 94: 32. CrossRef - Effects of TiO2 on microstructural, mechanical properties and in-vitro bioactivity of plasma sprayed yttria stabilised zirconia coatings for dental application
A. Jemat, M.J. Ghazali, M. Razali, Y. Otsuka, A. Rajabi
Ceramics International.2018; 44(4): 4271. CrossRef - In vitro evaluation of cytotoxicity of different self-adhesive resin cements
Necla Demir, Firdevs Kahvecioğlu, Muhammet Karcı, Hayriye Esra Ülker, Nuray Günaydın
Acta Odontologica Turcica.2017;[Epub] CrossRef
Cytotoxicity and biocompatibility of Zirconia (Y-TZP) posts with various dental cements
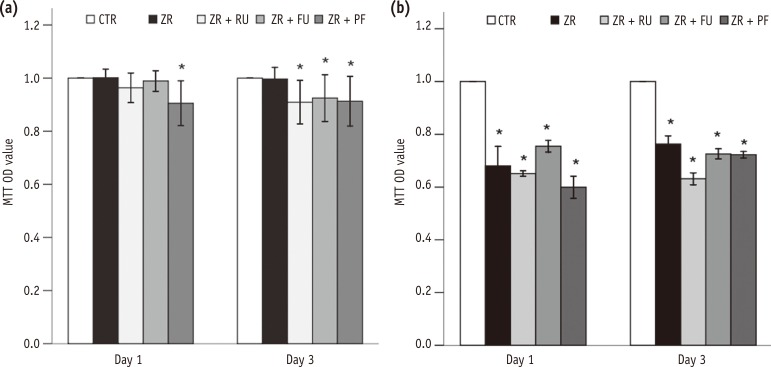
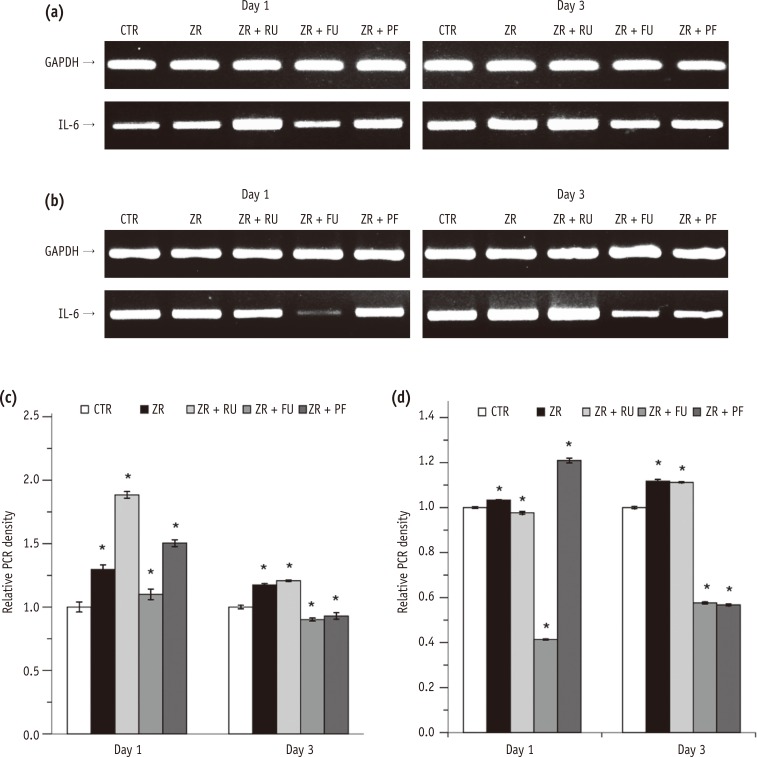
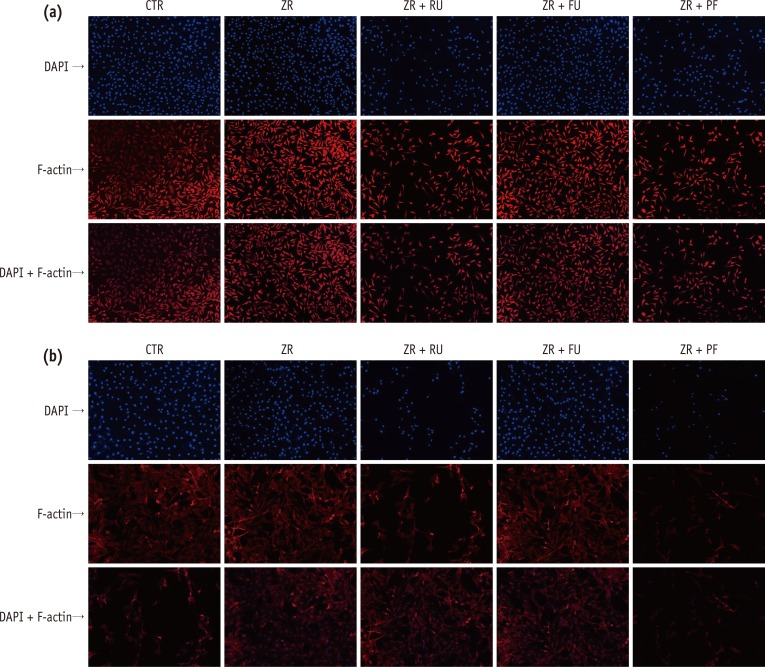
Figure 1 Effects of zirconia with or without an intermediate cement layer on the viability of (a) L929 and (b) MC3T3-E1 cells measured by MTT assays. Groups with the same lower case letters did not differ significantly on those days.*Significant difference from the negative control group at each time period, according to Tukey tests. Error bars mean ± 1.0 standard deviations.MTT, 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide; OD, optical density; CTR, Group 1, negative control; ZR, Group 2, positive control; ZR + RU, Group 3, zirconia with RelyX U200; ZR + FU, Group 4, zirconia with FujiCEM 2; ZR + PF, Group 5, zirconia with Panavia F 2.
Figure 2 Effects of zirconia with or without an intermediate cement layer on IL-6 expression by (a) L929 and (b) MC3T3-E1 cells. The graphs in (c) and (d) show the densitometric quantification of protein expression of the bands shown in (a) and (b). The results are presented as fold increases relative to control. The graphs in e and f present ELISA results.*Significant difference from the negative control group at each time period, according to Tukey tests. Different lower case letters indicate significant between group differences.GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IL, interleukin-6; PCR, polymerase chain reaction; CTR, Group 1, negative control; ZR, Group 2, positive control; ZR + RU, Group 3, zirconia with RelyX U200; ZR + FU, Group 4, zirconia with FujiCEM 2; ZR + PF, Group 5, zirconia with Panavia F 2.0.
Figure 3 Fluorescent staining of (a) L929 and (b) MC3T3-E1 cells treated with medium only (CTR) or the supernatant of each test group (ZR, ZR + RU, ZR + FU, and ZR + PF). Rows represent cells stained with DAPI, rhodamine-conjugated phalloidin, and both after three days in culture. Cells exposed to FujiCEM 2 (ZR + FU) showed very similar cell densities with control groups in DAPI staining, and the cells were as undamaged as in the control group. In contrast, a few of the nuclei of the RelyX U200 (ZR + RU) and Panavia F 2.0 (ZR + PF) extract-treated cells remarkably decreased in cell numbers. Damaged cells showed flat and thin shapes.CTR, negative control; ZR, positive control; ZR + RU, zirconia with RelyX U200; ZR + FU, zirconia with FujiCEM 2; ZR + PF, zirconia with Panavia F 2.0, DAPI, 4',6-diamidino-2-phenylindole.
Figure 1
Figure 2
Figure 3
Cytotoxicity and biocompatibility of Zirconia (Y-TZP) posts with various dental cements
Type, composition, and batch number of tested cements
| Product | Type | Composition | Lot No. | Manufacturer |
|---|---|---|---|---|
| RelyX U200 | Dual polymerized self-adhesive resin cement | 55 - 65% glass powder | 535365 | 3M ESPE, St. Paul, MN, USA |
| 15 - 25% methacrylated phosphoric acid esters | ||||
| 10 - 20% TEGDMA | ||||
| 1 - 5% silane-treated silica | ||||
| 1 - 5% sodium persulfate | ||||
| FujiCEM 2 | Resin-modified glass-ionomer cement | 30 - 40% polyacrylic acid | 1307041 | GC Dental, Tokyo, Japan |
| 30 - 40% distilled water | ||||
| 2% silica powder | ||||
| 20% silicone dioxide | ||||
| 2 - 3% benzensulfonic acid sodium salt | ||||
| Panavia F 2.0 | Dual polymerized self-adhesive resin cement | Paste A: 10-MDP, silanated silica, hydrophobic aromatic and aliphatic dimethacrylate, hydrophilic dimethacrylate photoinitiator, and dibenzoyl peroxide | A: 00571A | Kuraray Medical Inc., Okayama, Japan |
| Paste B: silanated barium glass, sodium fluoride, sodium aromatic sulfinate, dimethacrylate monomer, and BPO | B: 00110B |
MDP, 10-methacryloxydecyl dihydrogen phosphate; TEGDMA, Triethyleneglycol Dimethacrylate; BPO, benzoyl peroxide.
Table 1 Type, composition, and batch number of tested cements
MDP, 10-methacryloxydecyl dihydrogen phosphate; TEGDMA, Triethyleneglycol Dimethacrylate; BPO, benzoyl peroxide.

 KACD
KACD
 ePub Link
ePub Link Cite
Cite

