Articles
- Page Path
- HOME > Restor Dent Endod > Volume 40(3); 2015 > Article
- Research Article Evaluation of the effects of two novel irrigants on intraradicular dentine erosion, debris and smear layer removal
- Melahat Görduysus1,2, Selen Küçükkaya1, Nursel Pekel Bayramgil3, Mehmet Ömer Görduysus1,4
-
2015;40(3):-222.
DOI: https://doi.org/10.5395/rde.2015.40.3.216
Published online: July 3, 2015
1Department of Endodontics, Faculty of Dentistry, Hacettepe University, Ankara, Turkey.
2RAK College of Dental Science, RAK Medical and Health Science University, Ras Al Khaimah, UAE.
3Chemistry Department, Faculty of Science, Hacettepe University, Ankara, Turkey.
4Department of Endodontics, Sharjah University Dental College, Sharjah, UAE.
- Correspondence to Selen Küçükkaya, DDS, PhD. Research Assistant, Department of Endodontics, Hacettepe University Faculty of Dentistry, 06100, Sıhhiye, Ankara, Turkey. TEL, +90-312-305-2260; FAX, +90-312-310-4440; selenkkkaya@yahoo.com
©Copyrights 2015. The Korean Academy of Conservative Dentistry.
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,597 Views
- 6 Download
- 12 Crossref
Abstract
-
Objectives To evaluate the effects of copolymer of acrylic acid and maleic acid (Poly[AA-co-MA]) and calcium hypochlorite (Ca(OCl)2) on root canal dentin using scanning electron microscope (SEM).
-
Materials and Methods Twenty-four single-rooted teeth were instrumented and the apical and coronal thirds of each root were removed, leaving the 5 mm middle thirds, which were then separated into two pieces longitudinally. The specimens were randomly divided into six groups and subjected to each irrigant for 5 min as follows: G1, Ca(OCl)2; G2, Poly(AA-co-MA); G3, Ca(OCl)2 + Poly(AA-co-MA); G4, sodium hypochlorite (NaOCl); G5, ethylenediaminetetraacetic acid (EDTA); G6, NaOCl+EDTA. The specimens were prepared for SEM evaluation. Smear layer, debris and erosion scores were recorded by two blinded examiners. One image from G3 was analyzed with energy dispersive spectroscopy (EDS) on suspicion of precipitate formation. Data were analyzed using the Kruskal-Wallis and Dunn tests.
-
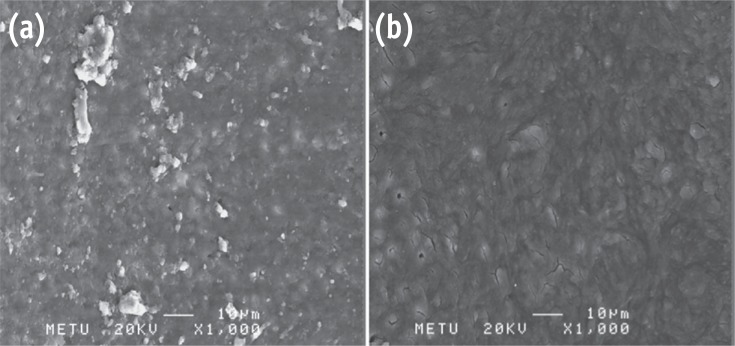
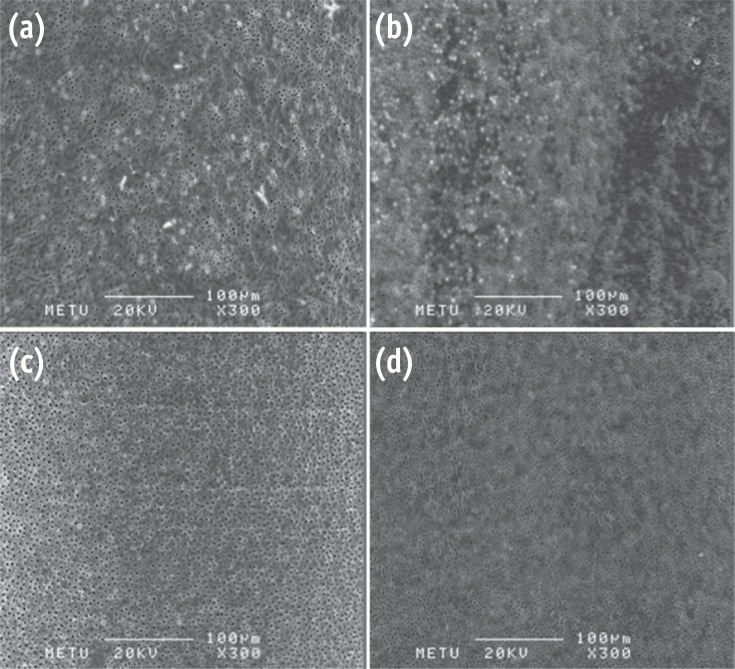
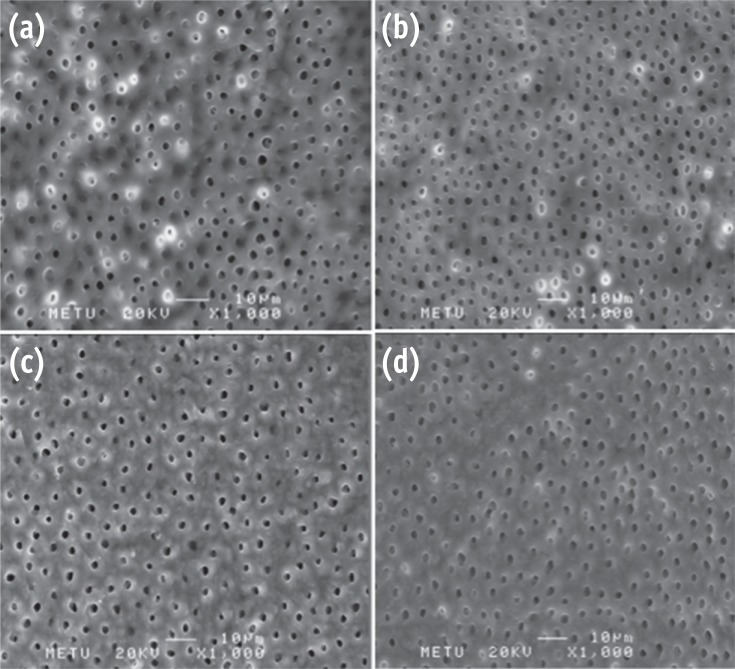
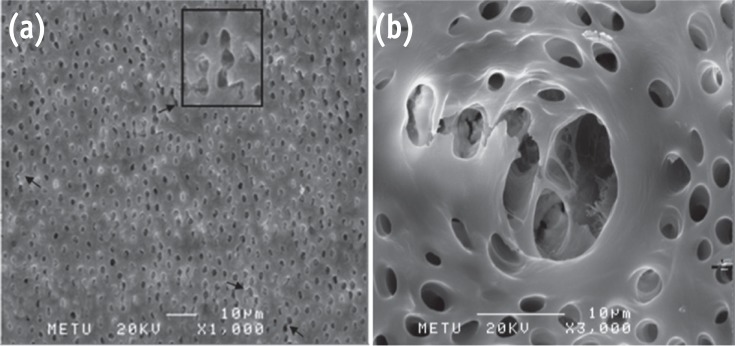
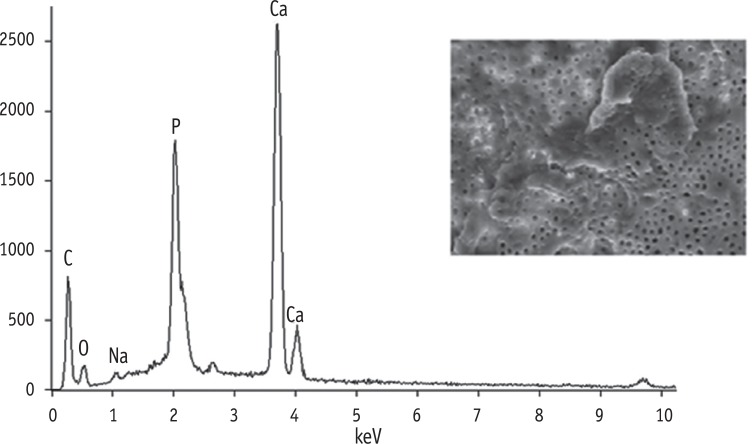
Results G1 and G4 showed the presence of debris and smear layer and they were statistically different from G2, G3, G5 and G6 where debris and smear layer were totally removed (p < 0.05). In G1 and G4, erosion evaluation could not be done because of debris and smear layer. G2, G3 and G5 showed no erosion, and there was no significant difference between them. G6 showed severe erosion and was statistically different from G2, G3 and G5 (p < 0.05). EDS microanalysis showed the presence of Na, P, and Ca elements on the surface.
-
Conclusions Poly(AA-co-MA) is effective in removing the smear layer and debris without causing erosion either alone or with Ca(OCl)2.
Introduction
Materials and Methods
Results
Discussion
Conclusions
- 1. Schilder H. Cleaning and shaping the root canal. Dent Clin North Am 1974;18:269-296.ArticlePubMed
- 2. McComb D, Smith DC. A preliminary scanning electron microscopic study of root canals after endodontic procedures. J Endod 1975;1:238-242.ArticlePubMed
- 3. Peters OA, Schönenberger K, Laib A. Effects of four Ni-Ti preparation techniques on root canal geometry assessed by micro computed tomography. Int Endod J 2001;34:221-230.ArticlePubMedPDF
- 4. Goldman LB, Goldman M, Kronman JH, Lin PS. The efficacy of several irrigating solutions for endodontics: a scanning electron microscopic study. Oral Surg Oral Med Oral Pathol 1981;52:197-204.ArticlePubMed
- 5. McComb D, Smith DC, Beagrie GS. The results of in vivo endodontic chemomechanical instrumentation-a scanning electron microscopic study. J Br Endod Soc 1976;9:11-18.ArticlePubMed
- 6. Gwinnett AJ. Smear layer: morphological considerations. Oper Dent Suppl 1984;3:2-12.PubMed
- 7. Torabinejad M, Handysides R, Khademi AA, Bakland LK. Clinical implications of the smear layer in endodontics: a review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2002;94:658-666.ArticlePubMed
- 8. Zehnder M. Root canal irrigants. J Endod 2006;32:389-398.ArticlePubMed
- 9. Becking AG. Complications in the use of sodium hypochlorite during endodontic treatment. Report of three cases. Oral Surg Oral Med Oral Pathol 1991;71:346-348.PubMed
- 10. Cobankara FK, Ozkan HB, Terlemez A. Comparison of organic tissue dissolution capacities of sodium hypochlorite and chlorine dioxide. J Endod 2010;36:272-274.ArticlePubMed
- 11. Dutta A, Saunders WP. Comparative evaluation of calcium hypochlorite and sodium hypochlorite on soft-tissue dissolution. J Endod 2012;38:1395-1398.ArticlePubMed
- 12. Mader CL, Baumgartner JC, Peters DD. Scanning electron microscopic investigation of the smeared layer on root canal walls. J Endod 1984;10:477-483.ArticlePubMed
- 13. Sceiza MF, Daniel RL, Santos EM, Jaeger MM. Cytotoxic effects of 10% citric acid and EDTA-T used as root canal irrigants: an in vitro analysis. J Endod 2001;27:741-743.ArticlePubMed
- 14. Segura-Egea JJ, Jiménez-Rubio A, Rios-Santos JV, Velasco-Ortega E, Calvo-Gutierrez JR. In vitro inhibitory effect of EGTA on macrophage adhesion: endodontic implications. J Endod 2003;29:211-213.ArticlePubMed
- 15. Baumgartner JC, Mader CL. A scanning electron microscopic evaluation of four root canal irrigation regimens. J Endod 1987;13:147-157.ArticlePubMed
- 16. Calt S, Serper A. Smear layer removal by EGTA. J Endod 2000;26:459-461.ArticlePubMed
- 17. Popescu I, Suflet DM, Pelin IM, Chitanu GC. Biomedical applications of maleic anhydride copolymers. Revue Roumaine de Chimie 2011;56:173-188.
- 18. Paqué F, Barbakow F, Peters OA. Root canal preparation with Endo-Eze AET: changes in root canal shape assessed by micro-computed tomography. Int Endod J 2005;38:456-464.ArticlePubMed
- 19. Hülsmann M, Rümmelin C, Schäfers F. Root canal cleanliness after preparation with different endodontic handpieces and hand instruments: a comparative SEM investigation. J Endod 1997;23:301-306.ArticlePubMed
- 20. Torabinejad M, Khademi AA, Babagoli J, Cho Y, Johnson WB, Bozhilov K, Kim J, Shabahang S. A new solution for the removal of the smear layer. J Endod 2003;29:170-175.ArticlePubMed
- 21. Goldman M, Goldman LB, Cavaleri R, Bogis J, Lin PS. The efficacy of several endodontic irrigating solutions: a scanning electron microscopic study: Part 2. J Endod 1982;8:487-492.ArticlePubMed
- 22. Yamada RS, Armas A, Goldman M, Lin PS. A scanning electron microscopic comparison of a high volume final flush with several irrigating solutions: Part 3. J Endod 1983;9:137-142.ArticlePubMed
- 23. Niu W, Yoshioka T, Kobayashi C, Suda H. A scanning electron microscopic study of dentinal erosion by final irrigation with EDTA and NaOCl solutions. Int Endod J 2002;35:934-939.ArticlePubMed
- 24. Cruz-Filho AM, Sousa-Neto MD, Saquy PC, Pécora JD. Evaluation of the effect of EDTAC, CDTA, and EGTA on radicular dentin microhardness. J Endod 2001;27:183-184.ArticlePubMed
- 25. Seidberg BH, Schilder H. An evaluation of EDTA in endodontics. Oral Surg Oral Med Oral Pathol 1974;37:609-620.ArticlePubMed
- 26. De-Deus G, Reis C, Paciornik S. Critical appraisal of published smear layer-removal studies: methodological issues. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2011;112:531-543.ArticlePubMed
- 27. Peters OA, Peters CI, Schönenberger K, Barbakow F. ProTaper rotary root canal preparation: effects of canal anatomy on final shape analysed by micro CT. Int Endod J 2003;36:86-92.ArticlePubMedPDF
- 28. Taha NA, Ozawa T, Messer HH. Comparison of three techniques for preparing oval-shaped root canals. J Endod 2010;36:532-535.ArticlePubMed
- 29. Do Prado M, Simão RA, Gomes BP. Evaluation of different irrigation protocols concerning the formation of chemical smear layer. Microsc Res Tech 2013;76:196-200.ArticlePubMed
- 30. Luessen HL, Borchard G, De Boer AG, Junginger HE, Kolter K, Schehlmann V, Sanner A. Use of (meth) acrylic acid/maleic acid copolymers for improving mucosal permeability. USA. US patent 6004575 A. 1999 12 21.
REFERENCES
Photomicrographs showing the presence of debris and smear layer. (a) G1; (b) G4 (Bar = 10 µm; original magnification, ×1,000).
Photomicrographs showing the smear-free surfaces. (a) G2; (b) G3; (c) G5; (d) G6 (Bar = 100 µm; original magnification, ×300).
Representative photomicrographs for erosion evaluation. No erosion is present in (a) G2; (b) G3; (c) G5. Moderate erosion can be seen in (d) G6 (Bar = 10 µm; original magnification, ×1,000).
Representative photomicrographs of G6 showing severe erosion. (a) Bar = 10 µm, original magnification, ×1,000; (b) Bar = 10 µm, original magnification, ×3,000.
Protocols for irrigation
| Group | Irrigant |
|---|---|
| G1 (n = 8) | 7% Ca(OCl)2 |
| G2 (n = 8) | 25% Poly(AA-co-MA) |
| G3# (n = 8) | 7% Ca(OCl)2 + 25% Poly(AA-co-MA) |
| G4 (n = 8) | 2.5% NaOCl |
| G5 (n = 8) | 17% EDTA |
| G6# (n = 8) | 2.5% NaOCl + 17% EDTA |
The scores and scales of the presence of debris, smear layer and degree of erosion of dentinal tubules
| Score | Definition |
|---|---|
| The presence of debris evaluated with a scale of five scores19 | |
| 1 | Clean root canal wall and only a few small debris particles |
| 2 | A few small agglomerations of debris |
| 3 | Many agglomerations of debris covering less than 50% of the root canal wall |
| 4 | More than 50% of the root canal walls were covered with debris |
| 5 | Complete or nearly complete root canal wall coverage with debris |
| The presence of smear layer evaluated with a scale of five scores19 | |
| 1 | No smear layer, and all dentinal tubules were open |
| 2 | A small amount of smear layer, and some dentinal tubules were open |
| 3 | Homogeneous smear layer covering the root canal wall, and only a few dentinal tubules open |
| 4 | Complete root canal wall covered by a homogeneous smear layer, and no open dentinal tubules were observed |
| 5 | Heavy, homogeneous smear layer covering the complete root canal wall |
| The degree of erosion of the dentinal tubules evaluated with a scale of three scores20 | |
| 1 | No erosion (all tubules were normal in appearance and size) |
| 2 | Moderate erosion (peritubular dentin was eroded) |
| 3 | Severe erosion (intertubular dentin was destroyed, and tubules were connected) |
Tables & Figures
REFERENCES
Citations

- Effect of Heat and Irrigation Agitation on the Smear Layer Removal Ability of Calcium Hypochlorite: A Scanning Electron Microscope Study
Damla Erkal, Kürşat Er
Microscopy Research and Technique.2026; 89(1): 65. CrossRef - Effect of sodium and calcium hypochlorite with or without surfactant on the adhesion of epoxy resin-based endodontic sealer
Guilherme Pauletto, Natália Franco Brum, Israel Bangel Carlotto, Lucas Saldanha da Rosa, Carlos Alexandre Souza Bier
Brazilian Journal of Oral Sciences.2025; 24: e254080. CrossRef - Bonding and Cleaning Effects of Irrigation Protocols Using Calcium Hypochlorite on the Post-space Radicular Dentin
JF Besegato, GR Bravo, JF Zaniboni, LG Belizário, ENM de Almeida, MB Gelio, WG Escalante-Otárola, MC Kuga
Operative Dentistry.2024; 49(6): E1. CrossRef - Effect of sodium hypochlorite and calcium hypochlorite on the apical sealing ability of endodontic sealers
Israel Bangel Carlotto, Natália Franco Brum, Guilherme Pauletto, Lucas Saldanha da Rosa, Carlos Alexandre Souza Bier
Brazilian Journal of Oral Sciences.2024; 23: e242700. CrossRef - Comparative evaluation of antifungal activity of Sodium Hypochlorite, Calcium Hypochlorite and modified Salt Solution associated with passive ultrasonic irrigation against Candida albicans - An In-Vitro study
Helen Thomas, D. N. Nirupama, Mohan Thomas Nainan, D. N. Naveen, C. Y. Ranjini, R. Vijay
Journal of Conservative Dentistry and Endodontics.2024; 27(2): 159. CrossRef - Effect of calcium hypochlorite as an irrigant alternative in the removal of methylene blue after photodynamic therapy under the post-space adhesive interface
João Felipe Besegato, Joatan Lucas de Sousa Gomes Costa, Joissi Ferrari Zaniboni, Giovanna Righetti Bravo, Jéssika Mayhara Pereira Morais, Wilfredo Gustavo Escalante-Otárola, Milton Carlos Kuga
Laser Physics.2023; 33(5): 055601. CrossRef - Effectiveness of Different Irrigation Techniques on Post Space Smear Layer Removal: SEM Evaluation
Alfredo Iandolo, Massimo Pisano, Dina Abdellatif, Alessandra Amato, Francesco Giordano, Alessio Buonavoglia, Giuseppe Sangiovanni, Mario Caggiano
Prosthesis.2023; 5(2): 539. CrossRef - The dynamic interplay of dietary acid pH and concentration during early-stage human enamel and dentine erosion
J. Pattem, J. Field, P. J. Waterhouse, M. J. German
Frontiers in Dental Medicine.2022;[Epub] CrossRef - Effect of EDTA, sodium, and calcium hypochlorite on the inorganic component of root canal dentin: A SEM analysis
Luana Roleto Cardoso, Flávia Emi Razera Baldasso, Débora Delai, Francisco Montagner, Patrícia Maria Poli Kopper
Microscopy Research and Technique.2019; 82(2): 128. CrossRef - Comparative Analysis of Manual, Rotary and Reciprocal Systems on Primary Teeth Root Canals: An In Vitro Scanning Electron Microscopy Study
İpek ARSLAN, Sema AYDINOĞLU, Ozgul BAYGIN, Tamer TÜZÜNER, Murat ŞİRİN
Cumhuriyet Dental Journal.2019; 22(3): 299. CrossRef - Regenerating a monoblock to obturate root canalsvia a mineralising strategy
Le Zhang, Quan-Li Li, Ying Cao, Yun Wang
Scientific Reports.2018;[Epub] CrossRef - Antibacterial Efficacy of Calcium Hypochlorite with Vibringe Sonic Irrigation System onEnterococcus faecalis: An In Vitro Study
Aysin Dumani, Hatice Korkmaz Guvenmez, Sehnaz Yilmaz, Oguz Yoldas, Zeliha Gonca Bek Kurklu
BioMed Research International.2016; 2016: 1. CrossRef
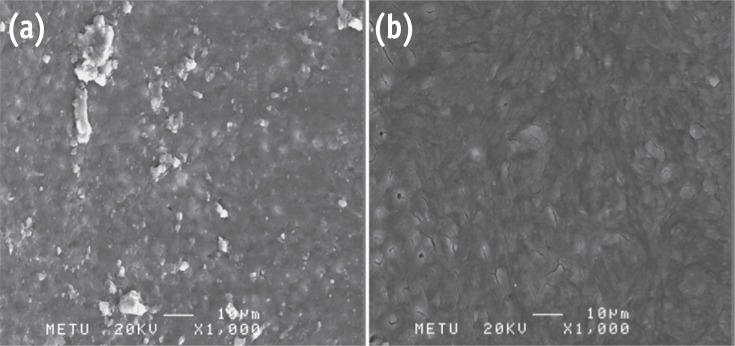

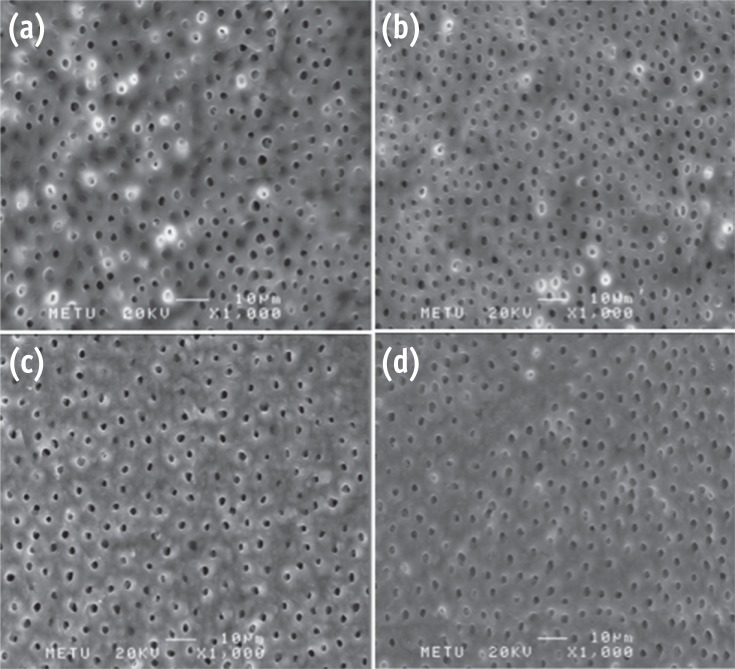
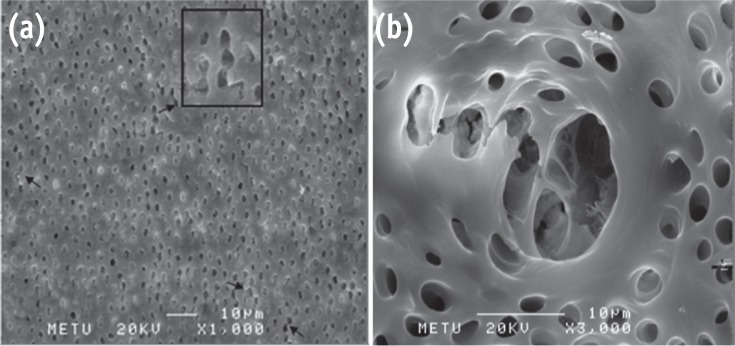
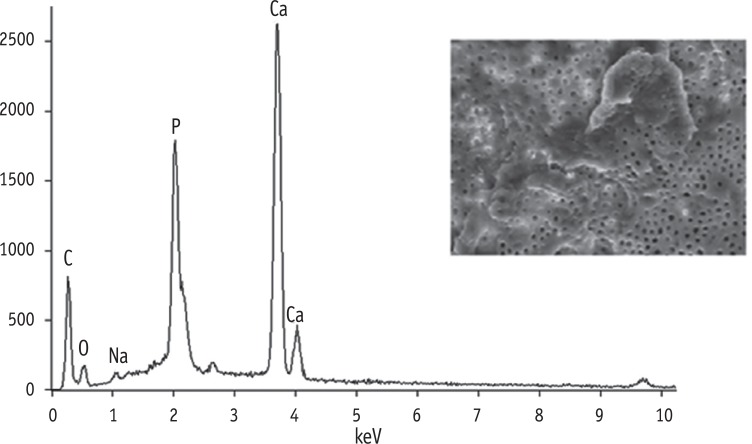
Figure 1
Figure 2
Figure 3
Figure 4
Figure 5
Protocols for irrigation
| Group | Irrigant |
|---|---|
| G1 (n = 8) | 7% Ca(OCl)2 |
| G2 (n = 8) | 25% Poly(AA-co-MA) |
| G3# (n = 8) | 7% Ca(OCl)2 + 25% Poly(AA-co-MA) |
| G4 (n = 8) | 2.5% NaOCl |
| G5 (n = 8) | 17% EDTA |
| G6# (n = 8) | 2.5% NaOCl + 17% EDTA |
#Represents combination groups.
EDTA, Ethylenediaminetetraacetic acid.
The scores and scales of the presence of debris, smear layer and degree of erosion of dentinal tubules
| Score | Definition |
|---|---|
| The presence of debris evaluated with a scale of five scores | |
| 1 | Clean root canal wall and only a few small debris particles |
| 2 | A few small agglomerations of debris |
| 3 | Many agglomerations of debris covering less than 50% of the root canal wall |
| 4 | More than 50% of the root canal walls were covered with debris |
| 5 | Complete or nearly complete root canal wall coverage with debris |
| The presence of smear layer evaluated with a scale of five scores | |
| 1 | No smear layer, and all dentinal tubules were open |
| 2 | A small amount of smear layer, and some dentinal tubules were open |
| 3 | Homogeneous smear layer covering the root canal wall, and only a few dentinal tubules open |
| 4 | Complete root canal wall covered by a homogeneous smear layer, and no open dentinal tubules were observed |
| 5 | Heavy, homogeneous smear layer covering the complete root canal wall |
| The degree of erosion of the dentinal tubules evaluated with a scale of three scores | |
| 1 | No erosion (all tubules were normal in appearance and size) |
| 2 | Moderate erosion (peritubular dentin was eroded) |
| 3 | Severe erosion (intertubular dentin was destroyed, and tubules were connected) |
The smear layer, debris and erosion scores of each group
| Group | Smear layer | Debris | Erosion |
|---|---|---|---|
| G1 | 4.5 ± 0.534 | 4.625 ± 0.517 | N/A |
| G2 | 1.125 ± 0.353 | 1.125 ± 0.353 | 1 |
| G3 | 1.125 ± 0.353 | 1 | 1 |
| G4 | 4.625 ± 0.517 | 4.5 ± 0.534 | N/A |
| G5 | 1.125 ± 0.353 | 1.125 ± 0.353 | 1 |
| G6 | 1.125 ± 0.353 | 1 | 2.75 ± 0.462 |
The values are expressed as mean ± SD. N/A, Not Applicable.
#Represents combination groups. EDTA, Ethylenediaminetetraacetic acid.
The values are expressed as mean ± SD. N/A, Not Applicable.

 KACD
KACD
 ePub Link
ePub Link Cite
Cite

