Articles
- Page Path
- HOME > Restor Dent Endod > Volume 39(2); 2014 > Article
-
Research Article
Antibacterial properties of composite resins incorporating silver and zinc oxide nanoparticles on
Streptococcus mutans andLactobacillus - Shahin Kasraei1, Lida Sami2, Sareh Hendi3, Mohammad-Yousef AliKhani4, Loghman Rezaei-Soufi5, Zahra Khamverdi1
-
2014;39(2):-114.
DOI: https://doi.org/10.5395/rde.2014.39.2.109
Published online: March 21, 2014
1Dental Research Center, Department of Restorative Dentistry, Dental School, Hamadan University of Medical Sciences, Hamadan, Iran.
2Department of Oral Medicine, Dental School, Hamadan University of Medical Sciences, Hamadan, Iran.
3Department of Endodontic, Dental School, Hamadan University of Medical Sciences, Hamadan, Iran.
4Department of Microbiology, Dental School, Hamadan University of Medical Sciences, Hamadan, Iran.
5Department of Restorative Dentistry, Dental School, Hamadan University of Medical Sciences, Hamadan, Iran.
- Correspondence to Zahra Khamverdi, DDS, MS. Associate Professor, Dental Research Center, Department of Restorative Dentistry, Dental faculty, Hamadan University of Medical Sciences, Besat Blvd, Hamadan, Iran 65176-59114. TEL, +98-811-8381059; FAX, +98-811-8381085; zkhamverdi@yahoo.ca
©Copyights 2014. The Korean Academy of Conservative Dentistry.
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 5,862 Views
- 57 Download
- 209 Crossref
Abstract
-
Objectives Recurrent caries was partly ascribed to lack of antibacterial properties in composite resin. Silver and zinc nanoparticles are considered to be broad-spectrum antibacterial agents. The aim of the present study was to evaluate the antibacterial properties of composite resins containing 1% silver and zinc-oxide nanoparticles on Streptococcus mutans and Lactobacillus.
-
Materials and Methods Ninety discoid tablets containing 0%, 1% nano-silver and 1% nano zinc-oxide particles were prepared from flowable composite resin (n = 30). The antibacterial properties of composite resin discs were evaluated by direct contact test. Diluted solutions of Streptococcus mutans (PTCC 1683) and Lactobacillus (PTCC 1643) were prepared. 0.01 mL of each bacterial species was separately placed on the discs. The discs were transferred to liquid culture media and were incubated at 37℃ for 8 hr. 0.01 mL of each solution was cultured on blood agar and the colonies were counted. Data was analyzed with Kruskall-Wallis and Mann-Whitney U tests.
-
Results Composites containing nano zinc-oxide particles or silver nanoparticles exhibited higher antibacterial activity against Streptococcus mutans and Lactobacillus compared to the control group (p < 0.05). The effect of zinc-oxide on Streptococcus mutans was significantly higher than that of silver (p < 0.05). There were no significant differences in the antibacterial activity against Lactobacillus between composites containing silver nanoparticles and those containing zinc-oxide nanoparticles.
-
Conclusions Composite resins containing silver or zinc-oxide nanoparticles exhibited antibacterial activity against Streptococcus mutans and Lactobacillus.
Introduction
Materials and Methods
Results
Discussion
Conclusions
Acknowledgement
- 1. Opdam NJ, Bronkhorst EM, Roeters JM, Loomans BA. A retrospective clinical study on longevity of posterior composite and amalgam restorations. Dent Mater 2007;23:2-8.ArticlePubMed
- 2. Sadowsky SJ. An overview of treatment considerations for esthetic restorations: a review of the literature. J Prosthet Dent 2006;96:433-442.ArticlePubMed
- 3. Pereira-Cenci T, Cenci MS, Fedorowicz Z, Marchesan MA. Antibacterial agents in composite restorations for the prevention of dental caries. Cochrane Database Syst Rev 2009;(3):CD007819.ArticlePubMed
- 4. Papagiannoulis L, Kakaboura A, Eliades G. In vivo vs in vitro anticariogenic behavior of glass-ionomer and resin composite restorative materials. Dent Mater 2002;18:561-569.ArticlePubMed
- 5. Thomé T, Mayer MP, Imazato S, Geraldo-Martins VR, Marques MM. In vitro analysis of inhibitory effects of the antibacterial monomer MDPB-containing restorations on the progression of secondary root caries. J Dent 2009;37:705-711.ArticlePubMed
- 6. Kim S, Song M, Roh BD, Park SH, Park JW. Inhibition of Streptococcus mutans biofilm formation on composite resins containing ursolic acid. Restor Dent Endod 2013;38:65-72.ArticlePubMedPMC
- 7. Kim JS, Shin DH. Inhibitory effect on Streptococcus mutans and mechanical properties of the chitosan containing composite resin. Restor Dent Endod 2013;38:36-42.ArticlePubMedPMC
- 8. Hernández-Sierra JF, Ruiz F, Pena DC, Martínez-Gutiérrez F, Martínez AE, Guillén Ade J, Tapia-Pérez H, Castañón GM. The antimicrobial sensitivity of Streptococcus mutans to nanoparticles of silver, zinc oxide, and gold. Nanomedicine 2008;4:237-240.ArticlePubMed
- 9. Bürgers R, Eidt A, Frankenberger R, Rosentritt M, Schweikl H, Handel G, Hahnel S. The anti-adherence activity and bactericidal effect of microparticulate silver additives in composite resin materials. Arch Oral Biol 2009;54:595-601.ArticlePubMed
- 10. Melo MA, Cheng L, Zhang K, Weir MD, Rodrigues LK, Xu HH. Novel dental adhesives containing nanoparticles of silver and amorphous calcium phosphate. Dent Mater 2013;29:199-210.ArticlePubMed
- 11. Tavassoli Hojati S, Alaghemand H, Hamze F, Ahmadian Babaki F, Rajab-Nia R, Rezvani MB, Kaviani M, Atai M. Antibacterial, physical and mechanical properties of flowable resin composites containing zinc oxide nanoparticles. Dent Mater 2013;29:495-505.ArticlePubMed
- 12. Xu T, Xie CS. Tetrapod-like nano-particle ZnO/acrylic resin composite and its multi-function property. Prog Org Coat 2003;46:297-301.Article
- 13. Li LH, Deng JC, Deng HR, Liu ZL, Li XL. Preparation, characterization and antimicrobial activities of chitosan/Ag/ZnO blend films. Chem Eng J 2010;160:378-382.Article
- 14. Sheng J, Nguyen PT, Marquis RE. Multi-target antimicrobial actions of zinc against oral anaerobes. Arch Oral Biol 2005;50:747-757.ArticlePubMed
- 15. Cummins D. Zinc citrate/Triclosan: a new anti-plaque system for the control of plaque and the prevention of gingivitis: short-term clinical and mode of action studies. J Clin Periodontol 1991;18:455-461.ArticlePubMed
- 16. Pinto RJ, Marques PA, Neto CP, Trindade T, Daina S, Sadocco P. Antibacterial activity of nanocomposites of silver and bacterial or vegetable cellulosic fibers. Acta Biomater 2009;5:2279-2289.ArticlePubMed
- 17. Martinez-Gutierrez F, Olive PL, Banuelos A, Orrantia E, Nino N, Sanchez EM, Ruiz F, Bach H, Av-Gay Y. Synthesis, characterization, and evaluation of antimicrobial and cytotoxic effect of silver and titanium nanoparticles. Nanomedicine 2010;6:681-688.ArticlePubMed
- 18. Sondi I, Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J Colloid Interface Sci 2004;275:177-182.ArticlePubMed
- 19. Kim JS, Kuk E, Yu KN, Kim JH, Park SJ, Lee HJ, Kim SH, Park YK, Park YH, Hwang CY, Kim YK, Lee YS, Jeong DH, Cho MH. Antimicrobial effects of silver nanoparticles. Nanomedicine 2007;3:95-101.ArticlePubMed
- 20. Spacciapoli P, Buxton D, Rothstein D, Friden P. Antimicrobial activity of silver nitrate against periodontal pathogens. J Periodontal Res 2001;36:108-113.ArticlePubMedPDF
- 21. Sahoo SK, Parveen S, Panda JJ. The present and future of nanotechnology in human health care. Nanomedicine 2007;3:20-31.ArticlePubMed
- 22. Rai M, Yadav A, Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv 2009;27:76-83.ArticlePubMed
- 23. Li LH, Deng JC, Deng HR, Liu ZL, Xin L. Synthesis and characterization of chitosan/ZnO nanoparticle composite membranes. Carbohydr Res 2010;345:994-998.ArticlePubMed
- 24. Kumar R, Münstedt H. Silver ion release from antimicrobial polyamide/silver composites. Biomaterials 2005;26:2081-2088.ArticlePubMed
- 25. Svanberg M, Mjör IA, Orstavik D. Mutans streptococci in plaque from margins of amalgam, composite, and glass-ionomer restorations. J Dent Res 1990;69:861-864.ArticlePubMedPDF
- 26. Bundy KJ, Butler MF, Hochman RF. An investigation of the bacteriostatic properties of pure metals. J Biomed Mater Res 1980;14:653-663.ArticlePubMed
- 27. Applerot G, Perkas N, Amirian G, Girshevitz O, Gedanken A. Coating of glass with ZnO via ultrasonic irradiation and a study of its antibacterial properties. Appl Surf Sci 2009;256:S3-S8.Article
- 28. Imazato S. Antibacterial properties of resin composites and dentin bonding systems. Dent Mater 2003;19:449-457.ArticlePubMed
- 29. Aydin Sevinç B, Hanley L. Antibacterial activity of dental composites containing zinc oxide nanoparticles. J Biomed Mater Res B Appl Biomater 2010;94:22-31.PubMedPMC
- 30. Beyth N, Houri-Haddad Y, Baraness-Hadar L, Yudovin-Farber I, Domb AJ, Weiss EI. Surface antimicrobial activity and biocompatibility of incorporated polyethylenimine nanoparticles. Biomaterials 2008;29:4157-4163.ArticlePubMed
- 31. Yoshida K, Tanagawa M, Matsumoto S, Yamada T, Atsuta M. Antibacterial activity of resin composites with silver-containing materials. Eur J Oral Sci 1999;107:290-296.ArticlePubMedPDF
- 32. Spencer CG, Campbell PM, Buschang PH, Cai J, Honeyman AL. Antimicrobial effects of zinc oxide in an orthodontic bonding agent. Angle Orthod 2009;79:317-322.ArticlePubMedPDF
- 33. Tanagawa M, Yoshida K, Matsumoto S, Yamada T, Atsuta M. Inhibitory effect of antibacterial resin composite against Streptococcus mutans. Caries Res 1999;33:366-371.ArticlePubMedPDF
- 34. Pandit S, Kim GR, Lee MH, Jeon JG. Evaluation of Streptococcus mutans biofilms formed on fluoride releasing and non fluoride releasing resin composites. J Dent 2011;39:780-787.ArticlePubMed
- 35. van Dijken JW, Kalfas S, Litra V, Oliveby A. Fluoride and mutans streptococci levels in plaque on aged restorations of resin-modified glass ionomer cement, compomer and resin composite. Caries Res 1997;31:379-383.ArticlePubMed
- 36. Wiegand A, Buchalla W, Attin T. Review on fluoride-releasing restorative materials-fluoride release and uptake characteristics, antibacterial activity and influence on caries formation. Dent Mater 2007;23:343-362.ArticlePubMed
- 37. Schmidlin PR, Schätzle M, Fischer J, Attin T. Bonding of brackets using a caries-protective adhesive patch. J Dent 2008;36:125-129.ArticlePubMed
REFERENCES
Tables & Figures
REFERENCES
Citations

- Fabricated modified compomer bearing CF/SBA-15 nanomaterials: Physicochemical and antibacterial properties
Fatma Nur Kızılay, Mustafa Aydınbelge, Sezer Demirbuğa, Kevser Kolçakoğlu, Nilay Ildız, Serkan Dayan
Dental Materials.2026; 42(3): 451. CrossRef - Physicochemical and antibacterial evaluation of novel nano α-TCP–AgNPs biocomposites for direct pulp-capping applications
Selviana Wulansari, Hendra Dian Adhita Dharsono, Nasrul Wathoni, Rosalina Tjandrawinata, Arief Cahyanto, Moehamad Orliando Roeslan
Frontiers in Oral Health.2026;[Epub] CrossRef - Dental plaque biofilm-targeting composite nanomaterials: advances and outlook
Jiaxuan Zhao, Shengyuan Huang, Yongjia Yang, Jilei Wang, Qianqian Guo, Yueming Xu, Bingyin Jiang, Jiang Lin
Biomaterials Science.2026; 14(4): 952. CrossRef - Next-Gen Restorative Materials to Revolutionise Smiles
John Yun Niu, Kelsey Xingyun Ge, Iris Xiaoxue Yin, Olivia Lili Zhang, Irene Shuping Zhao, Chun Hung Chu
Bioengineering.2026; 13(2): 143. CrossRef - Antibacterial activity and cytotoxicity of tricalcium silicate-based cements with different antibacterial additives
Reda Banon, Luc Martens, Peter De Coster, Jakob van Acker, Jerina Boelens, Elanagai Rathinam, Sivaprakash Rajasekharan
Scientific Reports.2026;[Epub] CrossRef - Clinical Roles of Nanoparticles in Orthodontic Bonding Materials
Maria Arampatzi, Ellas Spyratou, Iosif Sifakakis, Efstathios P. Efstathopoulos
Applied Sciences.2026; 16(4): 1996. CrossRef - The effect of photoinitiator systems on resin-based composite containing ZnO-nanoparticles
Abdulaziz Alayed, Nikolaos Silikas, David C. Watts
Dental Materials.2025; 41(2): 220. CrossRef - Synthesis and evaluation of antibacterial and antioxidant effects of propolis nanoparticles and cinnamon nanostructures in preventive dentistry: Experimental and theoretical approaches
Faeze Hamze, Mahnaz Amiri, Zeinab Sadat Islami, Tayebeh Shamspur, Razieh Razavi, Payam Khazaeli
Phytochemical Analysis.2025; 36(8): 2236. CrossRef - Synthesis of boron nitride@copper oxide‐based light‐curing resin composites: Investigating mechanical and antibacterial properties
Shuya Li, Dawei Liu, Zegang Shi, Wenyi Yu, Tingting Yang, Yufeng Bai, Tianlu He, Tai Peng
Polymer Composites.2025; 46(3): 2073. CrossRef - Long-lasting antimicrobial effect of multipurpose ZnO nanoparticle-loaded dental resins enhanced by blue light photodynamic therapy
Maria Luisa Leite, Patricia Comeau, Ala Zaghwan, Ya Shen, Adriana Pigozzo Manso
Dental Materials.2025; 41(3): 347. CrossRef - Use of Antimicrobial Nanoparticles for the Management of Dental Diseases
Iris Xiaoxue Yin, Anjaneyulu Udduttulla, Veena Wenqing Xu, Kitty Jieyi Chen, Monica Yuqing Zhang, Chun Hung Chu
Nanomaterials.2025; 15(3): 209. CrossRef - Assessment of cytotoxicity of clear aligners coated with zinc oxide nanoparticles
Indu Ravi, Vignesh Kailasam
Journal of Oral Biology and Craniofacial Research.2025; 15(2): 262. CrossRef - Emerging developments in plant-based metal nanomaterials for diverse versatile applications - A review
Garima Rana, Vivek Kumar Dhiman, Syed Kashif Ali, Ankush Chauhan, Majid S. Jabir, Suresh Ghotekar
Results in Chemistry.2025; 15: 102231. CrossRef - An in vitro comparative evaluation of silver and chitosan nanoparticles on shear bond strength of nanohybrid composite using different adhesion protocols
Roopadevi Garlapati, Nagesh Bolla, Mayana Aameena Banu, Anila Bandlapally Sreenivasa Guptha, Niharika Halder, Ram Chowdary Basam
Journal of Conservative Dentistry and Endodontics.2025; 28(6): 522. CrossRef - Assessment of the Antimicrobial Properties of Mesoporous Zinc Oxide Nanoparticles Against Streptococcus mutans: An In Vitro Investigation
Zahra Jowkar, Shima Askarzadeh, Seyed Ahmadreza Hamidi, Zahra Fattah, Ali Moaddeli, Hannah Wesley
International Journal of Dentistry.2025;[Epub] CrossRef - The role of theranostic nanoparticles in dental infectious diseases: A review
Mitra Rostami, Pouria Farahani, Moslem Karimzadeh, Samar Esmaelian, Abbas Fadel Hussein, Kamyar Nasiri, Hareth A. Alrikabi, Naghmeh Shenasa
Journal of Drug Delivery Science and Technology.2025; 112: 107223. CrossRef - Effect on hygroscopic characteristics of n‐ZnO additions to resin composite
Abdulaziz Alayed, Nikolaos Silikas, David C. Watts
European Journal of Oral Sciences.2025;[Epub] CrossRef - Applications of Nanoparticles in Endodontics as an Antibacterial Agent: A Mini-review
Mina Saliminasab
Advances in Applied NanoBio-Technologies.2025; 6(2): 46. CrossRef - Effect of Nanohydroxyapatite and Silver Nanoparticle Incorporation on the Flexural Strength of Resin Composites
Marzie Moradaian, Maryam Saadat, Shahab Agharezaei, Zahra Khorshidi Asl, Baisakhi Banerjee
BioMed Research International.2025;[Epub] CrossRef - An In Vitro Comparative Evaluation of Microleakage and Microhardness of Omnichroma and Silver Nanoparticles- incorporated Omnichroma
Mrithyunjay Satish Mendon, Mansi Jain, Suma Sogi, Gulbar Shah, Gagandeep Bhagat, Simran Gupta
International Journal of Clinical Pediatric Dentistry.2025; 18(10): 1181. CrossRef - EVOLUTION OF DENTAL IMPLANT AND IMPLANT SURFACE TREATMENTS- A NARRATIVE REVIEW
Wamiq Fareed, Hossam Mossa, Medhat Mohamed, Malik Almutairi, Rashed Alfehaid, Yousef Ahmad
BULLETIN OF STOMATOLOGY AND MAXILLOFACIAL SURGERY.2025; : 303. CrossRef - Comprehensive review on zinc oxide nanoparticle production and the associated antibacterial mechanisms and therapeutic potential
Aeshah M. Mohammed, Mohammed Mohammed, Jawad K. Oleiwi, Falah H. Ihmedee, Tijjani Adam, Bashir O. Betar, Subash C.B. Gopinath
Nano Trends.2025; 11: 100145. CrossRef - Evaluation of antibacterial efficacy and surface roughness of orthodontic brackets coated with silver–copper hybrid or zinc oxide nanoparticles: An in-vitro study
Aseem Sharma, Tanushree Sharma, Nambi. Rammohan. Shrinivaasan, Geetika Tomer, Nisha Gupta, Pramada Kishore, Prashant Babaji, Azhar Mohammed, Ananya Neralla
Journal of Orthodontic Science.2025;[Epub] CrossRef - In vitro evaluation of the antimicrobial efficacy, antibiofilm effect, and resistance to biodegradation of a novel composite against Streptococcus mutans
S. Pallavi, A. Devadathan, Lizymol Philipose Pampadykandathil, Vibha Chandrababu, N J Nagaraj, Arvind Kumar Alexander
Journal of Conservative Dentistry and Endodontics.2025; 28(10): 1001. CrossRef - Cymbopogon citratus essential oil infused zinc oxide nanoparticles for eco-friendly anticariogenic action
Preeti Pallavi, Saswat Aryan, Pragnya Paramita Sahoo, Adyasha Anapurba Sahoo, Sangeeta Raut
Clinical Oral Investigations.2025;[Epub] CrossRef - Computational Insights Into Antimicrobial Peptide‐Enhanced Dental Resin Composites: Targeting Porphyromonas gingivalis Heme‐Binding Proteins and Biofilms
Ravinder S. Saini, Doni Dermawan, Abdulkhaliq Ali F. Alshadidi, Rayan Ibrahim H. Binduhayyim, Rajesh Vyas, Fahad Hussain Alhamoudi, Sunil Kumar Vaddamanu, Mohamed Saheer Kuruniyan, Lujain Ibrahim N. Aldosari, Artak Heboyan
MicrobiologyOpen.2025;[Epub] CrossRef - The formation of cariogenic plaque to contemporary adhesive restorative materials: an in vitro study
Anna Lehrkinder, Olivia Rydholm, Anna Wänström, Keisuke Nakamura, Ulf Örtengren
Odontology.2024; 112(4): 1090. CrossRef - The Impact of Incorporating Five Different Boron Materials into a Dental Composite on Its Mechanical Properties
Mehmet Kutluhan Ucuk, Musa Kazim Ucuncu
Applied Sciences.2024; 14(3): 1054. CrossRef - Albumin nanoparticles are a promising drug delivery system in dentistry
Mohammad Kiarashi, Saman Yasamineh
BioMedical Engineering OnLine.2024;[Epub] CrossRef - The Effects of Three Antibacterial Nanoparticle Coatings on the Surface Characteristics of Stainless Steel
Ahmed Al-Mayali, Ammar Kadhum, Thair Alzubaydi
Metals.2024; 14(8): 853. CrossRef - Bioresponsive nanotechnology in pediatric dental drug delivery
Seyed Ebrahim Alavi, Lieba Malik, Raghad Matti, Farah Al-Najafi, Hasan Ebrahimi Shahmabadi, Lavanya A. Sharma
Journal of Drug Delivery Science and Technology.2024; 93: 105436. CrossRef - Visible light-activated curcumin-doped zinc oxide nanoparticles integrated into orthodontic adhesive on Micro-tensile bond strength, degree of conversion, and antibacterial effectiveness against Staphylococcus Aureus. An investigation using scanning elect
Abdullah A. Alnazeh, Muhammad Abdullah Kamran, Salem Almoammar, Mohammed Mohsen Al Jearah, Muhammad Qasim, Ibrahim Alshahrani
Journal of Photochemistry and Photobiology B: Biology.2024; 253: 112888. CrossRef - Antimicrobial activity of PMMA enriched with nano-clay loaded with metronidazole and chlorhexidine
Eduardo Buozi Moffa, Samuel Santana Malheiros, Larissa Tavares Sampaio Silva, Delcio Ildefonso Branco, Regis Cléo Fernandes Grassia Junior, William Cunha Brandt, Flavia Goncalves, Valentim Adelino Ricardo Barao, Letícia Cristina Cidreira Boaro
Brazilian Oral Research.2024;[Epub] CrossRef - Navigating Antibacterial Frontiers: A Panoramic Exploration of Antibacterial Landscapes, Resistance Mechanisms, and Emerging Therapeutic Strategies
Krittika Ralhan, Kavita A. Iyer, Leilani Lotti Diaz, Robert Bird, Ankush Maind, Qiongqiong Angela Zhou
ACS Infectious Diseases.2024; 10(5): 1483. CrossRef - Use of nanotechnology-based nanomaterial as a substitute for antibiotics in monogastric animals
Abdul Qadeer, Aamir Khan, Noor Muhammad Khan, Abdul Wajid, Kaleem Ullah, Sylvie Skalickova, Pompido Chilala, Petr Slama, Pavel Horky, Mohammed S. Alqahtani, Maha Awjan Alreshidi
Heliyon.2024; 10(11): e31728. CrossRef - Local and systemic adverse effects of nanoparticles incorporated in dental materials- a critical review
Harini Karunakaran, Jogikalmat Krithikadatta, Mukesh Doble
The Saudi Dental Journal.2024; 36(1): 158. CrossRef - Determining the cytotoxicity of the Minimum Inhibitory Concentration (MIC) of silver and zinc oxide nanoparticles in ESBL and carbapenemase producing Proteus mirabilis isolated from clinical samples in Shiraz, Southwest Iran
Farshad Kakian, Esmaeil Mirzaei, Afagh Moattari, Sara Takallu, Abdollah Bazargani
BMC Research Notes.2024;[Epub] CrossRef - New insights into nanotherapeutics for periodontitis: a triple concerto of antimicrobial activity, immunomodulation and periodontium regeneration
Jiaxin Li, Yuxiao Wang, Maomao Tang, Chengdong Zhang, Yachen Fei, Meng Li, Mengjie Li, Shuangying Gui, Jian Guo
Journal of Nanobiotechnology.2024;[Epub] CrossRef - The effect of silver and calcium fluoride nanoparticles on antibacterial activity of composite resin against Streptococcus mutans: An in vitro study
Mehdi Fathi, Zahra Hosseinali, Tina Molaei, Somayeh Hekmatfar
Dental Research Journal.2024;[Epub] CrossRef - Flowable resin-based composites modified with chlorhexidine-loaded mesoporous silica nanoparticles induce superior antibiofilm properties
Barsha Shrestha, Sultan Aati, Sheetal Maria Rajan, Amr Fawzy
Journal of Nanoparticle Research.2024;[Epub] CrossRef - A Temporary Acrylic Soft Denture Lining Material Enriched with Silver-Releasing Filler-Cytotoxicity, Mechanical and Antifungal Properties
Grzegorz Chladek, Igor Kalamarz, Wojciech Pakieła, Izabela Barszczewska-Rybarek, Zenon Czuba, Anna Mertas
Materials.2024; 17(4): 902. CrossRef - The Antibacterial Properties of a Reinforced Zinc Oxide Eugenol Combined with Cloisite 5A Nanoclay: An In-Vitro Study
Bahareh Nazemisalman, Shaghayegh Niaz, Shayan Darvish, Ayda Notash, Ali Ramazani, Ionut Luchian
Journal of Functional Biomaterials.2024; 15(7): 198. CrossRef - Assessing the physico-mechanical, anti-bacterial, and anti-demineralization properties of orthodontic resin composite containing different concentrations of photoactivated zinc oxide nanoparticles on Streptococcus mutans biofilm around ceramic and metal o
Yasamin Babaee Hemmati, Rashin Bahrami, Maryam Pourhajibagher
International Orthodontics.2024; 22(4): 100901. CrossRef - Biosynthesis of a Novel Composite Resin Incorporating Gamma Radiation Synthesized Pomegranate Extract–Coated Zinc Oxide Nanoparticles and In Vitro Assessment Against Streptococcus mutans Causing Dental Caries
Amany Badr El-Deen Abd El-Aziz, Mehreshan El-Mokadem, Hoda Hassan Abo-Ghalia, Zakaria Ahmed Mattar, Abdelrazq Ibrahim Sallam
BioNanoScience.2024; 14(5): 5017. CrossRef - Global trend and hotspot of resin materials for dental caries repair: a bibliometric analysis
Baodi Han, Lian Wang
Frontiers in Materials.2024;[Epub] CrossRef - Studying the Application of Nanoparticles in Orthodontics: A Review Study
Wojciech Dobrzynski, Maria Szymonowicz, Rafal J. Wiglusz, Zbigniew Rybak, Anna Zawadzka-Knefel, Mateusz Janecki, Adam Lubojanski, Karolina Kurek, Maciej Dobrzynski, Wojciech Zakrzewski
Annals of Dental Specialty.2024; 12(1): 57. CrossRef - Comparative evaluation of zinc oxide nanoparticles (ZnONPs): Photocatalysis, antibacterial, toxicity and genotoxicity
Olcay Gençyılmaz, Fahriye Zemheri Navruz, Sinan İnce, Abdulsattar Ali Abbas, Abdullah Hüssein Salim Salim
Journal of Photochemistry and Photobiology A: Chemistry.2024; 456: 115847. CrossRef - Advancements in Nanoparticle-Based Strategies for Enhanced Antibacterial Interventions
Madineh Moradialvand, Nastaran Asri, Mahtab Jahdkaran, Maryam Beladi, Hamidreza Houri
Cell Biochemistry and Biophysics.2024; 82(4): 3071. CrossRef - Performance evaluation of carbon quantum dots impregnated glass ionomer cement to avoid peri-implant disease
Febina Josephraj, Ashwin Kumar N, Vidyashree Nandini V, Sujatha S, Varshini Karthik
Biomedical Materials.2024; 19(3): 035040. CrossRef - Recent advances in nanomaterial-based biosensor for periodontitis detection
Mohammad Hosseini Hooshiar, Masoud Amiri Moghaddam, Mohammad Kiarashi, Athraa Y. Al-Hijazi, Abbas Fadel Hussein, Hareth A.Alrikabi, Sara Salari, Samar Esmaelian, Hassan Mesgari, Saman Yasamineh
Journal of Biological Engineering.2024;[Epub] CrossRef - Comparison of the antibacterial properties of Resin cements with and without the addition of nanoparticles: a systematic review
Ravinder Saini, Sunil Kumar Vaddamanu, Masroor Ahmed Kanji, Syed Altafuddin Quadri, Saeed Awod Bin Hassan, Sukumaran Anil, Deepti Shrivastava, Kumar Chandan Srivastava
BMC Oral Health.2024;[Epub] CrossRef - Nanotecnologia aplicada a Biomateriais em técnicas preventivas e restauradoras
Lucas Mateus Do Nascimento, Ricardo Felipe Ferreira Da Silva
Revista Sociedade Científica.2024; 7(1): 2326. CrossRef - Comparative Evaluation of Penetration of Various Nano-sized Intra-canal Medicaments: An In Vitro Confocal Laser Scanning Microscopic Study
Mounika Veeraiyan, Chikine Yashas Chandhar, Deepa Mastammanavar, Kantheti Kavya, Deepa Jarupula, Gangishetti Sairam
Journal of Pharmacy and Bioallied Sciences.2024; 16(Suppl 2): S1690. CrossRef - Preparation and characterization of nano silver antibacterial gel for gynecolog
Qiuqun Xiao, Jingnan Zhu, Tao Fang, Ruyi Peng, Jiayi Chen, Kailan Liu, Yanshi Ceng, Meng Yuan, Yunrui Hu
Ferroelectrics.2024; 618(13-14): 2249. CrossRef - Synthesis and characterization of mesoporous zinc oxide nanoparticles and evaluation of their biocompatibility in L929 fibroblasts
Zahra Jowkar, Ali Moaddeli, Fereshteh Shafiei, Tara Tadayon, Seyed Ahmadreza Hamidi
Clinical and Experimental Dental Research.2024;[Epub] CrossRef - Impact of Nanoparticles on Dental Composites: A Systematic Review and Meta-Analysis
Dhruv Ahuja, M. R. Akhila, Ashish Kumar Singh, Puneet Batra
Journal of International Oral Health.2024; 16(6): 439. CrossRef - Nanotechnology in Orthodontics: Current Applications and Future Perspectives
Wojciech Dobrzynski, Maria Szymonowicz, Rafal J. Wiglusz, Zbigniew Rybak, Anna Zawadzka-Knefel, Mateusz Janecki, Adam Lubojanski, Karolina Kurek, Maciej Dobrzynski, Wojciech Zakrzewski
Asian Journal of Periodontics and Orthodontics.2024; 4(1): 24. CrossRef - Inorganic Compounds as Remineralizing Fillers in Dental Restorative Materials: Narrative Review
Leena Ibraheem Bin-Jardan, Dalal Ibrahim Almadani, Leen Saleh Almutairi, Hadi A. Almoabid, Mohammed A. Alessa, Khalid S. Almulhim, Rasha N. AlSheikh, Yousif A. Al-Dulaijan, Maria S. Ibrahim, Afnan O. Al-Zain, Abdulrahman A. Balhaddad
International Journal of Molecular Sciences.2023; 24(9): 8295. CrossRef - Antimicrobial Efficacy of Various Nanoparticles on Addition to Orthodontic
Materials- A Systematic Review and Meta-Analysis
Crystal Runa Soans, Deesha Kumari, Shalin Shersha, Rahila Mansoor, M.S. Ravi
Nanoscience & Nanotechnology-Asia.2023;[Epub] CrossRef - Influence of inorganic nanoparticles on dental materials’ mechanical properties. A narrative review
Ghada Naguib, Abdulrahman A. Maghrabi, Abdulghani I. Mira, Hisham A. Mously, Maher Hajjaj, Mohamed T. Hamed
BMC Oral Health.2023;[Epub] CrossRef - Application of Nanomaterials in Restorative Dentistry
Rutvik Mandhalkar, Priyanka Paul, Amit Reche
Cureus.2023;[Epub] CrossRef - The germicidal effect, biosafety and mechanical properties of antibacterial resin composite in cavity filling
Jiamu Ren, Xinwei Guo
Heliyon.2023; 9(9): e19078. CrossRef - Influence of the Loading with Newly Green Silver Nanoparticles Synthesized Using Equisetum sylvaticum on the Antibacterial Activity and Surface Hardness of a Composite Resin
Ionuț Tărăboanță, Ana Flavia Burlec, Simona Stoleriu, Andreia Corciovă, Adrian Fifere, Denisa Batir-Marin, Monica Hăncianu, Cornelia Mircea, Irina Nica, Andra Claudia Tărăboanță-Gamen, Sorin Andrian
Journal of Functional Biomaterials.2023; 14(8): 402. CrossRef - Antimicrobial and Cytotoxic Activity of Ocimum tenuiflorum and Stevia rebaudiana-Mediated Silver Nanoparticles – An In vitro Study
Indumathy Pandiyan, Meignana Indiran Arumugham, Sri Sakthi Doraikannan, Pradeep Kumar Rathinavelu, Jayashri Prabakar, S. Rajeshkumar
Contemporary Clinical Dentistry.2023; 14(2): 109. CrossRef - Comparison of Antibacterial Properties of an Orthodontic Composite Containing Silver and Amor-phous Tricalcium Phosphate Nanoparticles against Streptococcus mutans: An In Vitro Study
Zahra Tavakolinejad, Mahmood Sheikh Fathollahi, farzaneh Mirzaei, Farzaneh Mirzaei, Elham Mirzaei
Journal of Research in Dental and Maxillofacial Sciences.2023; 8(4): 257. CrossRef - Effect of incorporating silica-hydroxyapatite-silver hybrid nanoparticles into the resin-modified glass ionomer on the adhesive remnant index score and shear bond strength of orthodontic metal brackets: An in vitro study
Nazila Biglar, Elahe Chaychi Raghimi, Somayeh Sadighian, Farzaneh Karamitanha, Elham Zajkani, Azin Nourian
International Orthodontics.2023; 21(3): 100761. CrossRef - Method development for the intraoral release of nanoparticles from dental restorative materials
Laura Kleinvogel, Gregor Wemken, Cosima Reidelbach, Manuel Garcia-Käufer, Kirstin Vach, Elmar Hellwig, Benedikt C. Spies, Olga Polydorou
Dental Materials.2023; 39(8): 693. CrossRef - Recent advancements in blended and reinforced polymeric systems as bioscaffolds
Jasmin Joseph, Ramesh Parameswaran, Unnikrishnan Gopalakrishna Panicker
International Journal of Polymeric Materials and Polymeric Biomaterials.2023; 72(11): 834. CrossRef - Antibacterial and Antibiofilm Efficacy of Copper-Doped Phosphate Glass on Pathogenic Bacteria
Sunaina Shetty, Priyadharshini Sekar, Raghavendra M. Shetty, Ensanya Ali Abou Neel
Molecules.2023; 28(7): 3179. CrossRef - Effect of Zinc Oxide Incorporation on the Antibacterial, Physicochemical, and Mechanical Properties of Pit and Fissure Sealants
Ji-Won Choi, Song-Yi Yang
Polymers.2023; 15(3): 529. CrossRef - Novel bioactive dental restorations to inhibit secondary caries in enamel and dentin under oral biofilms
Wen Zhou, Hong Chen, Michael D. Weir, Thomas W. Oates, Xuedong Zhou, Suping Wang, Lei Cheng, Hockin H.K. Xu
Journal of Dentistry.2023; 133: 104497. CrossRef - Antimicrobial properties of glass-ionomer cement incorporated with zinc oxide nanoparticles against mutans streptococci and lactobacilli under orthodontic bands: An in vivo split-mouth study
Maryam Shirazi, Fatemeh Fotoohi Qazvini, Saeed Mohamadrezaie
Dental Research Journal.2023;[Epub] CrossRef - Evaluation of the cell viability and antimicrobial effects of orthodontic bands coated with silver or zinc oxide nanoparticles: An in vitro study
Rashin Bahrami, Maryam Pourhajibagher, Alireza Badiei, Reza Masaeli, Behrad Tanbakuchi
Korean Journal of Orthodontics.2023; 53(1): 16. CrossRef - Evaluation and comparison of the effect of incorporating zinc oxide and titanium dioxide nanoparticles on the bond strength and microleakage of two orthodontic fixed retainer adhesives
Leila Jazi, Ahmad Sodagar, Sepehr Sobhani Kazemi, Amirhossein Mirhashemi
Journal of the World Federation of Orthodontists.2023; 12(1): 22. CrossRef - Bioactive Materials for Caries Management: A Literature Review
Olivia Lili Zhang, John Yun Niu, Iris Xiaoxue Yin, Ollie Yiru Yu, May Lei Mei, Chun Hung Chu
Dentistry Journal.2023; 11(3): 59. CrossRef - Antimicrobial efficacy of zinc oxide nanoparticle-coated aligners on Streptococcus mutans and Candida albicans
Prathima Anita, Haritha Pottipalli Sathyanarayana, Kennedy Kumar, Krishnapriya Ramanathan, Vignesh Kailasam
American Journal of Orthodontics and Dentofacial Orthopedics.2023; 163(3): 338. CrossRef - The Impact of Adhesive-Containing Nanoparticles of ZrO2and TiO2 on Antimicrobial Effectiveness, the Strength of Bonding, and the Extent of Microleakage in Dentin Affected by Caries
Fayez Hussain Niazi, Shadi El Bahra, Nisren Ansary, Zeeshan Qamar, Hajar Albahkaly, Badr Bamousa, Ahlam Smran, Ahmed Al Ahmari, Saleh Wael S. Al-Akki, Abdulaziz Samran
Journal of Biomaterials and Tissue Engineering.2023; 13(9): 946. CrossRef - Comparitive evaluation of antimicrobial effectiveness of silver oxide coatings on different types of ceramic brackets against Streptococcus mutans
S. V. Ramesh Goud, K. Raja Sigamani, Bhaskar, Kurinchi Kumaran, Mohammed Arafat, S.N Reddy Duvvuri
The Journal of Dental Panacea.2022; 4(2): 75. CrossRef - Evaluation of antibacterial and mechanical features of dental adhesives containing colloidal gold nanoparticles
Sara Dadkan, Mehrdad Khakbiz, Lida Ghazanfari, Meizi Chen, Ki-Bum Lee
Journal of Molecular Liquids.2022; 365: 119824. CrossRef - Preparation of electrospun silver/poly(vinyl alcohol) fibrous membranes and characterization of the effect of sterilization processes on the antibacterial activity
Wen-Cheng Chen, Chia-Ying Ko, Kai-Chi Chang, Chih-Hua Chen, Dan-Jae Lin
Journal of Industrial Textiles.2022; 51(4_suppl): 7205S. CrossRef - Nanomaterial-Based Zinc Ion Interference Therapy to Combat Bacterial Infections
Yongbin Wei, Jiaming Wang, Sixuan Wu, Ruixue Zhou, Kaixiang Zhang, Zhenzhong Zhang, Junjie Liu, Shangshang Qin, Jinjin Shi
Frontiers in Immunology.2022;[Epub] CrossRef - Innovative Investigation of Zinc Oxide Nanoparticles Used in Dentistry
Ajay Kumar Tiwari, Saket Jha, Abhimanyu Kumar Singh, Sheo Kumar Mishra, Ashok Kumar Pathak, Rudra Prakash Ojha, Raghvendra Singh Yadav, Anupam Dikshit
Crystals.2022; 12(8): 1063. CrossRef - Efficient Route for the Preparation of Composite Resin Incorporating Silver Nanoparticles with Enhanced Antibacterial Properties
Drake Beery, Mohammad Abdul Mottaleb, Mohammed J. Meziani, James Campbell, Isabella Pires Miranda, Michael Bellamy
Nanomaterials.2022; 12(3): 471. CrossRef - Antibacterial performance of composite containing quaternary ammonium silica (QASi) filler – A preliminary study
Michal Dekel-Steinkeller, Ervin I. Weiss, Trudi Lev-Dor Samovici, Itzhak Abramovitz
Journal of Dentistry.2022; 123: 104209. CrossRef - Therapeutic Applications of Antimicrobial Silver-Based Biomaterials in Dentistry
Qiyu Wang, Yu Zhang, Qiang Li, Li Chen, Hui Liu, Meng Ding, Heng Dong, Yongbin Mou
International Journal of Nanomedicine.2022; Volume 17: 443. CrossRef - In-vitro Comparative Assessment of Antibacterial and Anti-adherent Effect of Two Types of Surface Modificants on Stainless Steel Orthodontic Brackets Against Streptococcus mutans
Mrunmaye Math, Alok G. Shah, Parag Gangurde, Anita G. Karandikar, Anjali Gheware, Bhagyashree S. Jadhav
Journal of Indian Orthodontic Society.2022; 56(3): 282. CrossRef - Dental Composites with Magnesium Doped Zinc Oxide Nanoparticles Prevent Secondary Caries in the Alloxan-Induced Diabetic Model
Tahreem Tanweer, Nosheen Fatima Rana, Iqra Saleem, Iqra Shafique, Sultan M. Alshahrani, Hanadi A. Almukhlifi, Amenah S. Alotaibi, Sohad Abdulkaleg Alshareef, Farid Menaa
International Journal of Molecular Sciences.2022; 23(24): 15926. CrossRef - Effect of Different Concentrations of Silver Nanoparticles on the Quality of the Chemical Bond of Glass Ionomer Cement Dentine in Primary Teeth
Faisal Mohammed Abed, Sunil Babu Kotha, Haneen AlShukairi, Fatmah Nasser Almotawah, Rwan Abdulali Alabdulaly, Sreekanth Kumar Mallineni
Frontiers in Bioengineering and Biotechnology.2022;[Epub] CrossRef - Antibacterial Activity of Dental Composite with Ciprofloxacin Loaded Silver Nanoparticles
Wafa Arif, Nosheen Rana, Iqra Saleem, Tahreem Tanweer, Muhammad Khan, Sohad Alshareef, Huda Sheikh, Fatima Alaryani, Manal AL-Kattan, Hanan Alatawi, Farid Menaa, Aroosa Nadeem
Molecules.2022; 27(21): 7182. CrossRef - Experimental composite containing silicon dioxide-coated silver nanoparticles for orthodontic bonding: Antimicrobial activity and shear bond strength
Rogéria Christina de Oliveira AGUIAR, Larissa Pereira NUNES, Eduardo Silva BATISTA, Marina Mariante VIANA, Marcela Charantola RODRIGUES, Bruno BUENO-SILVA, Marina Guimarães ROSCOE
Dental Press Journal of Orthodontics.2022;[Epub] CrossRef - DEAE-Dextran Coated AgNPs: A Highly Blendable Nanofiller Enhances Compressive Strength of Dental Resin Composites
Shabia Azhar, Nosheen Fatima Rana, Amer Sohail Kashif, Tahreem Tanweer, Iqra Shafique, Farid Menaa
Polymers.2022; 14(15): 3143. CrossRef - Synthesis of a Novel, Biocompatible and Bacteriostatic Borosiloxane Composition with Silver Oxide Nanoparticles
Denis N. Chausov, Veronika V. Smirnova, Dmitriy E. Burmistrov, Ruslan M. Sarimov, Alexander D. Kurilov, Maxim E. Astashev, Oleg V. Uvarov, Mikhail V. Dubinin, Valery A. Kozlov, Maria V. Vedunova, Maksim B. Rebezov, Anastasia A. Semenova, Andrey B. Lisitsy
Materials.2022; 15(2): 527. CrossRef - Mechanical Properties, Biocompatibility and Anti-Bacterial Adhesion Property Evaluation of Silicone-Containing Resin Composite with Different Formulae
Muzi Liao, Hui Tong, Xiangya Huang, Fang Liu, Jingwei He, Sui Mai
Journal of Renewable Materials.2022; 10(12): 3201. CrossRef - Current trends and future perspectives on dental nanomaterials – An overview of nanotechnology strategies in dentistry
Vidhya Rekha Umapathy, Prabhu Manickam Natarajan, C. SumathiJones, Bhuminathan Swamikannu, W.M.S. Johnson, V. Alagarsamy, Ashequr Rahman Milon
Journal of King Saud University - Science.2022; 34(7): 102231. CrossRef - Boron nitride nanosheets modified with zinc oxide nanoparticles as novel fillers of dental resin composite
Ameenah Saad Alansy, Thekra Ali Saeed, Reem Al-Attab, Yuqing Guo, Yanwei Yang, Bin Liu, Zengjie Fan
Dental Materials.2022; 38(10): e266. CrossRef - Zinc Oxide Nanoparticles: A Review on Its Applications in Dentistry
C Pushpalatha, Jithya Suresh, VS Gayathri, SV Sowmya, Dominic Augustine, Ahmed Alamoudi, Bassam Zidane, Nassreen Hassan Mohammad Albar, Shankargouda Patil
Frontiers in Bioengineering and Biotechnology.2022;[Epub] CrossRef - Evaluation of Mechanical Properties and Antibacterial Activity of Nano Titania-Enriched Alkasite Restorative Material: An In Vitro Study
Neven S. Aref, Reham M. Abdallah
The Open Dentistry Journal.2022;[Epub] CrossRef - Exploring the Physicochemical, Mechanical, and Photocatalytic Antibacterial Properties of a Methacrylate-Based Dental Material Loaded with ZnO Nanoparticles
Patricia Comeau, Julia Burgess, Niknaz Malekafzali, Maria Luisa Leite, Aidan Lee, Adriana Manso
Materials.2022; 15(14): 5075. CrossRef - Mechanical characterization and adhesive properties of a dental adhesive modified with a polymer antibiotic conjugate
Camila Sabatini, Russell J. Aguilar, Ziwen Zhang, Steven Makowka, Abhishek Kumar, Megan M. Jones, Michelle B. Visser, Mark Swihart, Chong Cheng
Journal of the Mechanical Behavior of Biomedical Materials.2022; 129: 105153. CrossRef - Development of Direct Immobilization Technique of Ag Nanoparticles on Resin Substrates Imparting High Antibacterial and Antiviral Activities
Satoshi Seino, Yuji Ohkubo, Tomonari Magara, Hiroki Enomoto, Eri Nakajima, Tomoki Nishida, Yasuo Imoto, Takashi Nakagawa
Nanomaterials.2022; 12(17): 3046. CrossRef - Nanotechnology interventions as a putative tool for the treatment of dental afflictions
Pooja Jain, Uzma Farooq, Nazia Hassan, Mohammed Albratty, Md. Shamsher Alam, Hafiz A. Makeen, Mohd. Aamir Mirza, Zeenat Iqbal
Nanotechnology Reviews.2022; 11(1): 1935. CrossRef - Impact of Different Antibacterial Substances in Dental Composite Materials: A Comprehensive Review
Badr Soliman AlHussain, Abdulrahman Abdulaziz AlFayez, Abdullah Abdulrahman AlDuhaymi, Essam Abdulaziz AlMulhim, Mohammad Yahya Assiri, Shahzeb Hasan Ansari
International Journal of Dental Research and Allied Sciences.2022; 2(1): 1. CrossRef - Chlorhexidine in operative dentistry - A review
TanviSanjay Satpute, SanjyotA Mulay
Journal of the International Clinical Dental Research Organization.2021; 13(2): 80. CrossRef - Nanotechnology-based materials as emerging trends for dental applications
Tejas Barot, Deepak Rawtani, Pratik Kulkarni
REVIEWS ON ADVANCED MATERIALS SCIENCE.2021; 60(1): 173. CrossRef - A Brief Review on the Evolution of Metallic Dental Implants: History, Design, and Application
Sumanth Ratna Kandavalli, Qingge Wang, Mahmoud Ebrahimi, Ceren Gode, Faramarz Djavanroodi, Shokouh Attarilar, Shifeng Liu
Frontiers in Materials.2021;[Epub] CrossRef - Inhibition of Streptococcus mutans, antioxidant property and cytotoxicity of novel nano-zinc oxide varnish
Manali Deb Barma, Indumathy Muthupandiyan, Srinivasan Raj Samuel, Bennett T. Amaechi
Archives of Oral Biology.2021; 126: 105132. CrossRef - Nanomaterials Application in Orthodontics
Wojciech Zakrzewski, Maciej Dobrzynski, Wojciech Dobrzynski, Anna Zawadzka-Knefel, Mateusz Janecki, Karolina Kurek, Adam Lubojanski, Maria Szymonowicz, Zbigniew Rybak, Rafal J. Wiglusz
Nanomaterials.2021; 11(2): 337. CrossRef - Mechanical properties of new denture base material modified with gold nanoparticles
Adamović Tijana, Veselinović Valentina, Trtić Nataša, Hadži-Mihailović Miloš, Gotovac Atlagić Suzana, Balaban Milica, Hattori Yoshiyuki, Sugiyama Hironori, Andrej Ivanič, Rudolf Rebeka
Journal of Prosthodontic Research.2021; 65(2): 155. CrossRef - Dynamics of different ion release from denture-base acrylic resins and their mechanical properties after the addition of bioactive materials
Zbigniew Raszewski
The Saudi Dental Journal.2021; 33(8): 1071. CrossRef - Genotoxic assay of silver and zinc oxide nanoparticles synthesized by leaf extract of Garcinia livingstonei T. Anderson: A comparative study
AzharuddinB Daphedar, SiddappaB Kakkalameli, Govindappa Melappa, TarikereChandrashekharappa Taranath, Chandrashekar Srinivasa, Chandan Shivamallu, Asad Syed, Najat Marraiki, AbdallahM Elgorban, Ravindra Veerapur, SharangoudaS Patil, ShivaPrasad Kollur
Pharmacognosy Magazine.2021; 17(5): 114. CrossRef - Nanostructures as Targeted Therapeutics for Combating Oral Bacterial Diseases
Shima Afrasiabi, Nasim Chiniforush, Hamid Reza Barikani, Alireza Partoazar, Ramin Goudarzi
Biomedicines.2021; 9(10): 1435. CrossRef - Well-Orientation Strategy Biosynthesis of Cefuroxime-Silver Nanoantibiotic for Reinforced Biodentine™ and Its Dental Application against Streptococcus mutans
Sanaa M. F. Gad El-Rab, Amal A. Ashour, Sakeenabi Basha, Amal Ahmed Alyamani, Nayef H. Felemban, Enas Tawfik Enan
Molecules.2021; 26(22): 6832. CrossRef - Application of Selected Nanomaterials and Ozone in Modern Clinical Dentistry
Adam Lubojanski, Maciej Dobrzynski, Nicole Nowak, Justyna Rewak-Soroczynska, Klaudia Sztyler, Wojciech Zakrzewski, Wojciech Dobrzynski, Maria Szymonowicz, Zbigniew Rybak, Katarzyna Wiglusz, Rafal J. Wiglusz
Nanomaterials.2021; 11(2): 259. CrossRef - Nanomaterials as drug delivery systems with antibacterial properties: current trends and future priorities
Khatereh Khorsandi, Reza Hosseinzadeh, Homa Sadat Esfahani, Saeedeh Keyvani-Ghamsari, Saeed Ur Rahman
Expert Review of Anti-infective Therapy.2021; 19(10): 1299. CrossRef - Synthesis and antibacterial activity of polymer–antibiotic conjugates incorporated into a resin-based dental adhesive
Ziwen Zhang, Megan M. Jones, Camila Sabatini, Stephen T. Vanyo, Ming Yang, Abhishek Kumar, Yancheng Jiang, Mark T. Swihart, Michelle B. Visser, Chong Cheng
Biomaterials Science.2021; 9(6): 2043. CrossRef - Effect Of Various Antibacterial Materials In Dental Composites: A Systematic Review
Abdulrahman Abdulaziz Alfayez, Abdullah Abdulrahman Alduhaymi, Essam Abdulaziz Almulhim, Mohammad Yahya Assiri, Shahzeb Hasan Ansari
Annals of Dental Specialty.2021; 9(3): 39. CrossRef - Low-temperature flow-synthesis-assisted urethane-grafted zinc oxide-based dental composites: physical, mechanical, and antibacterial responses
Jaffar Hussain Bukhari, Abdul Samad Khan, Kashif Ijaz, Shahreen Zahid, Aqif Anwar Chaudhry, Muhammad Kaleem
Journal of Materials Science: Materials in Medicine.2021;[Epub] CrossRef - Review of nano‐technology applications in resin‐based restorative materials
Natalia Almeida Bastos, Sandro Basso Bitencourt, Emerson Alves Martins, Grace Mendonca De Souza
Journal of Esthetic and Restorative Dentistry.2021; 33(4): 567. CrossRef - Minimally Invasive Therapies for the Management of Dental Caries—A Literature Review
Hetal Desai, Cameron Stewart, Yoav Finer
Dentistry Journal.2021; 9(12): 147. CrossRef - In-vitro Cytotoxicity Evaluation of Green Synthesized Gold Nanoparticles and Its Indigenous Mouthwash
Lichi. A. Solanki, KK Shantha Sundari, S Rajeshkumar
Journal of Pure and Applied Microbiology.2021; 15(2): 735. CrossRef - Antibacterial response of oral microcosm biofilm to nano-zinc oxide in adhesive resin
Isadora Martini Garcia, AbdulRahman A. Balhaddad, Maria S. Ibrahim, Michael D. Weir, Hockin H.K. Xu, Fabrício Mezzomo Collares, Mary Anne S. Melo
Dental Materials.2021; 37(3): e182. CrossRef - Toxic mechanisms of Roth801, Canals, microparticles and nanoparticles of ZnO on MG-63 osteoblasts
Mei-Chi Chang, Chia-Mei Tang, Yu-Heng Lin, Hsin-Cheng Liu, Tong-Mei Wang, Wen-Chien Lan, Ru-Hsiu Cheng, Yan-Ru Lin, Hsiao-Hua Chang, Jiiang-Huei Jeng
Materials Science and Engineering: C.2021; 119: 111635. CrossRef - Initial Mechanical Stabilization of Conventional Glass Ionomer Cements with Different Active Principles
Caroline Santos Ribeiro, Mayra Manoella Perez, Pablo Lenin Benitez-Sellan, Renata de Oliveira Guaré, Eduardo Bresciani, Michele Baffi Diniz
Pesquisa Brasileira em Odontopediatria e Clínica Integrada.2021;[Epub] CrossRef - Zinc oxide nanoparticles inhibit bacterial biofilm formation via altering cell membrane permeability
Tanvir Kaur, Chayanika Putatunda, Ashish Vyas, Gaurav Kumar
Preparative Biochemistry & Biotechnology.2021; 51(4): 309. CrossRef - Biocompatibility, mechanical, and bonding properties of a dental adhesive modified with antibacterial monomer and cross-linker
Hoda Moussa, Megan M. Jones, Ningbo Huo, Runsheng Zhang, Mayuresh Keskar, Michelle B. Visser, Mark T. Swihart, Chong Cheng, Camila Sabatini
Clinical Oral Investigations.2021; 25(5): 2877. CrossRef - The Benefits of Smart Nanoparticles in Dental Applications
Silvia Vasiliu, Stefania Racovita, Ionela Aurica Gugoasa, Maria-Andreea Lungan, Marcel Popa, Jacques Desbrieres
International Journal of Molecular Sciences.2021; 22(5): 2585. CrossRef - Microshear Bond Strength of Nanoparticle-Incorporated Conventional and Resin-Modified Glass Ionomer to Caries-Affected Dentin
Zahra Fattah, Zahra Jowkar, Safoora Rezaeian, Lucas da Fonseca Roberti Garcia
International Journal of Dentistry.2021; 2021: 1. CrossRef - An Insight in to Various Metallic Oxide Nanoparticles as Antimicrobials and Their Applications in Dentistry
Hema Kanathila, Ashwin M. Pangi, Suvidha Patil, Shilpa Shirlal, Rahul Jaiswal
Journal of Evolution of Medical and Dental Sciences.2021; 10(33): 2803. CrossRef - Innovative Strategies in Minimally Invasive Dentistry for Caries Management: A Literature Synthesis
Mariana Vega, Hugo Fuentes, Valeria Cordero, Luis Silva, Regina Cabrera
International Journal of Dental Research and Allied Sciences.2021; 1(2): 66. CrossRef - Nanoparticle technology and its implications in endodontics: a review
Natasha Raura, Anirudh Garg, Arpit Arora, M. Roma
Biomaterials Research.2020;[Epub] CrossRef Use of Silver Nanomaterials for Caries Prevention: A Concise Review
Iris Xiaoxue Yin, Irene Shuping Zhao, May Lei Mei, Quanli Li, Ollie Yiru Yu, Chun Hung Chu
International Journal of Nanomedicine.2020; Volume 15: 3181. CrossRef- Study on a novel antibacterial light-cured resin composite containing nano-MgO
Zhongyuan Wu, Haiping Xu, Wei Xie, Meimei Wang, Cunjin Wang, Cheng Gao, Fang Gu, Jie Liu, Jing Fu
Colloids and Surfaces B: Biointerfaces.2020; 188: 110774. CrossRef - Effect of Addition of Nano-TiO2, Nano-SiO2, and a Combination of Both, on Antimicrobial Activity of an Orthodontic Composite
Abbas Bahador, Mohammad J Kharazifard, Nazanin Kiomarsi, Paniz Zamani, Sedighe S Hashemikamangar, Maryam Pourhajibagher
The Journal of Contemporary Dental Practice.2020; 21(8): 857. CrossRef - Biomimetic Aspects of Oral and Dentofacial Regeneration
Akshaya Upadhyay, Sangeeth Pillai, Parisa Khayambashi, Hisham Sabri, Kyungjun T. Lee, Maryam Tarar, Stephanie Zhou, Ingrid Harb, Simon D. Tran
Biomimetics.2020; 5(4): 51. CrossRef Dental Materials Incorporated with Nanometals and Their Effect on the Bacterial Growth of Staphylococcus aureus
Rabeah Yousef Rawashdeh, Reyad Sawafta, Hanan I Malkawi
International Journal of Nanomedicine.2020; Volume 15: 4325. CrossRef- Does the Addition of Zinc Oxide Nanoparticles Improve the Antibacterial Properties of Direct Dental Composite Resins? A Systematic Review
Divya Arun, Dulanja Adikari Mudiyanselage, Rumana Gulam Mohamed, Michael Liddell, Nur Mohammad Monsur Hassan, Dileep Sharma
Materials.2020; 14(1): 40. CrossRef - Effect of zinc oxide nanoparticles on broilers’ performance and health status
Usama T. Mahmoud, Hosnia S. Abdel-Mohsein, Manal A. M. Mahmoud, Omar A. Amen, Rasha I. M. Hassan, Ashraf M. Abd-El-Malek, Sohair M. M. Rageb, Hanan S. A. Waly, Aly A. Othman, Mohamed A. Osman
Tropical Animal Health and Production.2020; 52(4): 2043. CrossRef - The potential anti‐infective applications of metal oxide nanoparticles: A systematic review
Yasmin Abo‐zeid, Gareth R. Williams
WIREs Nanomedicine and Nanobiotechnology.2020;[Epub] CrossRef - ZnO Modulates Swine Gut Microbiota and Improves Growth Performance of Nursery Pigs When Combined with Peptide Cocktail
Xiaoyuan Wei, Tsungcheng Tsai, Joshua Knapp, Kristopher Bottoms, Feilong Deng, Robert Story, Charles Maxwell, Jiangchao Zhao
Microorganisms.2020; 8(2): 146. CrossRef - Biogenic synthesis and antimicrobial activity of silica-coated silver nanoparticles for esthetic dental applications
Marcela Charantola Rodrigues, Wallace Rosado Rolim, Marina Mariante Viana, Thaís Rodrigues Souza, Flavia Gonçalves, Caio Junji Tanaka, Bruno Bueno-Silva, Amedea Barozzi Seabra
Journal of Dentistry.2020; 96: 103327. CrossRef - Advances of Anti-Caries Nanomaterials
Hui Chen, Lisha Gu, Binyou Liao, Xuedong Zhou, Lei Cheng, Biao Ren
Molecules.2020; 25(21): 5047. CrossRef - Antibacterial Properties of Nanoparticles in Dental Restorative Materials. A Systematic Review and Meta-Analysis
Elena Ferrando-Magraner, Carlos Bellot-Arcís, Vanessa Paredes-Gallardo, José Manuel Almerich-Silla, Verónica García-Sanz, Mercedes Fernández-Alonso, José María Montiel-Company
Medicina.2020; 56(2): 55. CrossRef - Polymeric and inorganic nanoscopical antimicrobial fillers in dentistry
Pooyan Makvandi, Jun Ting Gu, Ehsan Nazarzadeh Zare, Behnaz Ashtari, Arash Moeini, Franklin R. Tay, Li-na Niu
Acta Biomaterialia.2020; 101: 69. CrossRef - The effect of incorporation Nano Cinnamon powder on the shear bond of the orthodontic composite (an in vitro study)
Saba N. Yaseen, Amer A. Taqa, Ali R. Al-Khatib
Journal of Oral Biology and Craniofacial Research.2020; 10(2): 128. CrossRef - Synthesis of an anti-cariogenic experimental dental composite containing novel drug-decorated copper particles
Mehwish Pasha, Nawshad Muhammad, Maleeha Nayyer, Jaffar Hussain Bokhari, Hina Ashraf, Sher Zaman Safi, Muhammad Kaleem
Materials Science and Engineering: C.2020; 114: 111040. CrossRef The Effects of Silver, Zinc Oxide, and Titanium Dioxide Nanoparticles Used as Dentin Pretreatments on the Microshear Bond Strength of a Conventional Glass Ionomer Cement to Dentin
Zahra Jowkar, Zahra Fattah, Saeedreza Ghanbarian, Fereshteh Shafiei
International Journal of Nanomedicine.2020; Volume 15: 4755. CrossRef- Metal‐Based Nanomaterials in Biomedical Applications: Antimicrobial Activity and Cytotoxicity Aspects
Pooyan Makvandi, Chen‐yu Wang, Ehsan Nazarzadeh Zare, Assunta Borzacchiello, Li‐na Niu, Franklin R. Tay
Advanced Functional Materials.2020;[Epub] CrossRef - Metal Oxide Nanoparticles as Biomedical Materials
Maria P. Nikolova, Murthy S. Chavali
Biomimetics.2020; 5(2): 27. CrossRef - Comparison of antibacterial effects of orthodontic composites containing different nanoparticles on Streptococcus mutans at different times
Soghra Yassaei, Ali Nasr, Hengameh Zandi, Mohammad Nima Motallaei
Dental Press Journal of Orthodontics.2020; 25(2): 52. CrossRef - Nanoparticles as Anti-Microbial, Anti-Inflammatory, and Remineralizing Agents in Oral Care Cosmetics: A Review of the Current Situation
Florence Carrouel, Stephane Viennot, Livia Ottolenghi, Cedric Gaillard, Denis Bourgeois
Nanomaterials.2020; 10(1): 140. CrossRef - Nanometals in Dentistry: Applications and Toxicological Implications—a Systematic Review
Rupali Agnihotri, Sumit Gaur, Sacharia Albin
Biological Trace Element Research.2020; 197(1): 70. CrossRef - Ex vivo investigation on internal tunnel approach/internal resin infiltration and external nanosilver-modified resin infiltration of proximal caries exceeding into dentin
Andrej M. Kielbassa, Marlene R. Leimer, Jens Hartmann, Stephan Harm, Markus Pasztorek, Ina B. Ulrich, Yogendra Kumar Mishra
PLOS ONE.2020; 15(1): e0228249. CrossRef - Nanocomposites based on low density polyethylene filled with carbon nanotubes prepared by high energy ball milling and their potential antibacterial activity
Erika Benigno, Miguel A Lorente, Dania Olmos, Gustavo González‐Gaitano, Javier González‐Benito
Polymer International.2019; 68(6): 1155. CrossRef - Titanium dioxide and modified titanium dioxide by silver nanoparticles as an anti biofilm filler content for composite resins
Hércules Bezerra Dias, Maria Inês Basso Bernardi, Taís Maria Bauab, Antônio Carlos Hernandes, Alessandra Nara de Souza Rastelli
Dental Materials.2019; 35(2): e36. CrossRef - Synthesis, characterization and application of Ag doped ZnO nanoparticles in a composite resin
Hércules Bezerra Dias, Maria Inês Basso Bernardi, Valéria Spolon Marangoni, Adilson César de Abreu Bernardi, Alessandra Nara de Souza Rastelli, Antônio Carlos Hernandes
Materials Science and Engineering: C.2019; 96: 391. CrossRef - The effect of silver nanoparticles incorporation in the self-etch adhesive system on its antibacterial activity and degree of conversion: an in-vitro study
Heba F. Mohammed, Mona I. Riad
F1000Research.2019; 8: 244. CrossRef - Influence of silver nanoparticles on resin-dentin bond strength durability in a self-etch and an etch-and-rinse adhesive system
Zahra Jowkar, Fereshteh Shafiei, Elham Asadmanesh, Fatemeh Koohpeima
Restorative Dentistry & Endodontics.2019;[Epub] CrossRef - Development and status of resin composite as dental restorative materials
Xinxuan Zhou, Xiaoyu Huang, Mingyun Li, Xian Peng, Suping Wang, Xuedong Zhou, Lei Cheng
Journal of Applied Polymer Science.2019;[Epub] CrossRef - Influence of silver nanoparticle solution on the mechanical properties of resin cements and intrarradicular dentin
Thaís Yumi Umeda Suzuki, Juno Gallego, Wirley Gonçalves Assunção, André Luiz Fraga Briso, Paulo Henrique dos Santos, Chun-Pin Lin
PLOS ONE.2019; 14(6): e0217750. CrossRef - Physical properties and cytotoxicity of antimicrobial dental resin adhesives containing dimethacrylate oligomers of Ciprofloxacin and Metronidazole
Yasaman Delaviz, Timothy W. Liu, Ashley R. Deonarain, Yoav Finer, Babak Shokati, J. Paul Santerre
Dental Materials.2019; 35(2): 229. CrossRef - Application of Antimicrobial Nanoparticles in Dentistry
Wenjing Song, Shaohua Ge
Molecules.2019; 24(6): 1033. CrossRef - Evaluation of antibacterial and antifungal properties of a tissue conditioner used in complete dentures after incorporation of ZnO‒Ag nanoparticles
Seyed Amin Mousavi, Reza Ghotaslou, Abolfazl Akbarzadeh, Niloufar Azima, Ali Aeinfar, Azin Khorramdel
Journal of Dental Research, Dental Clinics, Dental Prospects.2019; 13(1): 11. CrossRef - Antibacterial and Mechanical Properties of Pit and Fissure Sealants Containing Zinc Oxide and Calcium Fluoride Nanoparticles
Dara Lakshmi Swetha, C. Vinay, K. S. Uloopi, Kakarla Sri RojaRamya, Rayala Chandrasekhar
Contemporary Clinical Dentistry.2019; 10(3): 477. CrossRef - Addition of antibacterial agent effect on color stability of composites after immersion of different beverages
Makbule T. Tuncdemir, Nilgun Gulbahce
Journal of Esthetic and Restorative Dentistry.2019; 31(5): 508. CrossRef - Toward dental caries: Exploring nanoparticle-based platforms and calcium phosphate compounds for dental restorative materials
Abdulrahman A. Balhaddad, Anmar A. Kansara, Denise Hidan, Michael D. Weir, Hockin H.K. Xu, Mary Anne S. Melo
Bioactive Materials.2019; 4: 43. CrossRef - Antibacterial effects of polymeric PolymP-n Active nanoparticles. An in vitro biofilm study
M.C. Sánchez, M. Toledano-Osorio, J. Bueno, E. Figuero, M. Toledano, A.L. Medina-Castillo, R. Osorio, D. Herrera, M. Sanz
Dental Materials.2019; 35(1): 156. CrossRef - Potencial antimicrobiano de diferentes retentores intrarradiculares frente a Enterococcus faecalis: uma avaliação in vitro
Nicole Hoffmann FINGER, Marília PAULUS, Alexandra Flávia GAZZONI
Revista de Odontologia da UNESP.2019;[Epub] CrossRef - Nanomedicine for anticancer and antimicrobial treatment: an overview
Shatavari Kulshrestha, Asad U. Khan
IET Nanobiotechnology.2018; 12(8): 1009. CrossRef - Effect of Silver Nanoparticles, Zinc Oxide Nanoparticles and Titanium Dioxide Nanoparticles on Microshear Bond Strength to Enamel and Dentin
Fatemeh Koohpeima, Zahra Jowkar, Nazbanoo Farpour, Mohammad J Mokhtari, Fereshteh Shafiei
The Journal of Contemporary Dental Practice.2018; 19(11): 1405. CrossRef - Studies on the Curing Efficiency and Mechanical Properties of Bis-GMA and TEGDMA Nanocomposites Containing Silver Nanoparticles
Izabela Barszczewska-Rybarek, Grzegorz Chladek
International Journal of Molecular Sciences.2018; 19(12): 3937. CrossRef - Properties of Experimental Dental Composites Containing Antibacterial Silver-Releasing Filler
Robert Stencel, Jacek Kasperski, Wojciech Pakieła, Anna Mertas, Elżbieta Bobela, Izabela Barszczewska-Rybarek, Grzegorz Chladek
Materials.2018; 11(6): 1031. CrossRef - Evaluation of antibacterial properties on polysulfone composite membranes using synthesized biogenic silver nanoparticles with Ulva compressa (L.) Kütz. and Cladophora glomerata (L.) Kütz. extracts
Fozia T. Minhas, Gulsin Arslan, I. Hilal Gubbuk, Cengiz Akkoz, Betul Yılmaz Ozturk, Baran Asıkkutlu, Ugur Arslan, Mustafa Ersoz
International Journal of Biological Macromolecules.2018; 107: 157. CrossRef - Human In Situ Study of the effect of Bis(2-Methacryloyloxyethyl) Dimethylammonium Bromide Immobilized in Dental Composite on Controlling Mature Cariogenic Biofilm
Mary Anne S. Melo, Michael D. Weir, Vanara F. Passos, Juliana P. M. Rolim, Christopher D. Lynch, Lidiany K. A. Rodrigues, Hockin H. K. Xu
International Journal of Molecular Sciences.2018; 19(11): 3443. CrossRef - A systematic review on antibacterial activity of zinc against Streptococcus mutans
Manal Mohamed Almoudi, Alaa Sabah Hussein, Mohamed Ibrahim Abu Hassan, Nurhayati Mohamad Zain
The Saudi Dental Journal.2018; 30(4): 283. CrossRef - Antibacterial activity against Streptococcus mutans and diametrical tensile strength of an interim cement modified with zinc oxide nanoparticles and terpenes: An in vitro study
Verónica Andrade, Alejandra Martínez, Ninón Rojas, Helia Bello-Toledo, Paulo Flores, Gabriela Sánchez-Sanhueza, Alfonso Catalán
The Journal of Prosthetic Dentistry.2018; 119(5): 862.e1. CrossRef - Developing a New Generation of Therapeutic Dental Polymers to Inhibit Oral Biofilms and Protect Teeth
Ke Zhang, Bashayer Baras, Christopher Lynch, Michael Weir, Mary Melo, Yuncong Li, Mark Reynolds, Yuxing Bai, Lin Wang, Suping Wang, Hockin Xu
Materials.2018; 11(9): 1747. CrossRef - Do quaternary ammonium monomers induce drug resistance in cariogenic, endodontic and periodontal bacterial species?
Suping Wang, Haohao Wang, Biao Ren, Hao Li, Michael D. Weir, Xuedong Zhou, Thomas W. Oates, Lei Cheng, Hockin H.K. Xu
Dental Materials.2017; 33(10): 1127. CrossRef - Antimicrobial nanomaterials against biofilms: an alternative strategy
Chunhua Liu, Jing Guo, Xiaoqing Yan, Yongbing Tang, Asit Mazumder, Shikai Wu, Yan Liang
Environmental Reviews.2017; 25(2): 225. CrossRef - Metal oxide nanoparticles as antimicrobial agents: a promise for the future
Azhwar Raghunath, Ekambaram Perumal
International Journal of Antimicrobial Agents.2017; 49(2): 137. CrossRef - Antimicrobial Polymers in the Nano-World
Marta Álvarez-Paino, Alexandra Muñoz-Bonilla, Marta Fernández-García
Nanomaterials.2017; 7(2): 48. CrossRef - Biosynthesis of Silver Nanoparticles on Orthodontic Elastomeric Modules: Evaluation of Mechanical and Antibacterial Properties
Alma Hernández-Gómora, Edith Lara-Carrillo, Julio Robles-Navarro, Rogelio Scougall-Vilchis, Susana Hernández-López, Carlo Medina-Solís, Raúl Morales-Luckie
Molecules.2017; 22(9): 1407. CrossRef - Zinc Oxide Nanoparticles Influence Microflora in Ileal Digesta and Correlate Well with Blood Metabolites
Yanni Feng, Lingjiang Min, Weidong Zhang, Jing Liu, Zhumei Hou, Meiqiang Chu, Lan Li, Wei Shen, Yong Zhao, Hongfu Zhang
Frontiers in Microbiology.2017;[Epub] CrossRef - Antibacterial activity of glass ionomer cement modified by zinc oxide nanoparticles
Patrícia Petromilli Nordi Sasso Garcia, Mariana Florian Bell Cardia, Renata Serignoli Francisconi, Lívia Nordi Dovigo, Denise Madalena Palomari Spolidório, Alessandra Nara de Souza Rastelli, Ana Carolina Botta
Microscopy Research and Technique.2017; 80(5): 456. CrossRef - Effects on cytotoxicity and antibacterial properties of the incorporations of silver nanoparticles into the surface coating of dental alloys
Xiao-ting Shen, Yan-zhen Zhang, Fang Xiao, Jing Zhu, Xiao-dong Zheng
Journal of Zhejiang University-SCIENCE B.2017; 18(7): 615. CrossRef - Nanosilver coated orthodontic brackets:in vivoantibacterial properties and ion release
Gamze Metin-Gürsoy, Lale Taner, Gülçin Akca
The European Journal of Orthodontics.2017; 39(1): 9. CrossRef - Prenatal Exposure to Nanosized Zinc Oxide in Rats: Neurotoxicity and Postnatal Impaired Learning and Memory Ability
Feng Xiaoli, Wu Junrong, Lai Xuan, Zhang Yanli, Wei Limin, Liu Jia, Shao Longquan
Nanomedicine.2017; 12(7): 777. CrossRef - Heat-Polymerized Resin Containing Dimethylaminododecyl Methacrylate Inhibits Candida albicans Biofilm
Hui Chen, Qi Han, Xuedong Zhou, Keke Zhang, Suping Wang, Hockin Xu, Michael Weir, Mingye Feng, Mingyun Li, Xian Peng, Biao Ren, Lei Cheng
Materials.2017; 10(4): 431. CrossRef - Zinc oxide 3D microstructures as an antimicrobial filler content for composite resins
Hércules Bezerra Dias, Maria Inês Basso Bernardi, Matheus Aparecido dos Santos Ramos, Tamara Carolina Trevisan, Taís Maria Bauab, Antônio Carlos Hernandes, Alessandra Nara de Souza Rastelli
Microscopy Research and Technique.2017; 80(6): 634. CrossRef - Polymer-Ceramic Bionanocomposites for Dental Application
Jung-Hwan Lee, Hae-Won Kim, Seog-Jin Seo
Journal of Nanomaterials.2016; 2016: 1. CrossRef - Biocompatibility of nanosilver-coated orthodontic brackets: an in vivo study
Gamze Metin-Gürsoy, Lale Taner, Emre Barış
Progress in Orthodontics.2016;[Epub] CrossRef - Restorative materials containing antimicrobial agents: is there evidence for their antimicrobial and anticaries effects? A systematic review
GS do Amaral, T Negrini, M Maltz, RA Arthur
Australian Dental Journal.2016; 61(1): 6. CrossRef - Evaluation of Bond Strength and Microleakage of a Novel Metal-titanate Antibacterial Agent
S Deng, KH Chung, DCN Chan, C Spiekerman
Operative Dentistry.2016; 41(3): E48. CrossRef - Investigation of optical, structural, morphological and antimicrobial properties of carboxymethyl cellulose capped Ag-ZnO nanocomposites prepared by chemical and mechanical methods
Magdalena-Valentina Lungu, Eugeniu Vasile, Mariana Lucaci, Delia Pătroi, Natalia Mihăilescu, Florentina Grigore, Virgil Marinescu, Alexandra Brătulescu, Sorina Mitrea, Arcadie Sobetkii, Arcadii A. Sobetkii, Marcela Popa, Mariana-Carmen Chifiriuc
Materials Characterization.2016; 120: 69. CrossRef - Substantivity of Ag–Ca–Si mesoporous nanoparticles on dentin and its ability to inhibit Enterococcus faecalis
Wei Fan, Yujie Wu, Tengjiao Ma, Yanyun Li, Bing Fan
Journal of Materials Science: Materials in Medicine.2016;[Epub] CrossRef - Nanomaterials for Tissue Engineering In Dentistry
Manila Chieruzzi, Stefano Pagano, Silvia Moretti, Roberto Pinna, Egle Milia, Luigi Torre, Stefano Eramo
Nanomaterials.2016; 6(7): 134. CrossRef - Alternative Antimicrobial Approach: Nano-Antimicrobial Materials
Nurit Beyth, Yael Houri-Haddad, Avi Domb, Wahid Khan, Ronen Hazan
Evidence-Based Complementary and Alternative Medicine.2015; 2015: 1. CrossRef - Advances in Dental Materials through Nanotechnology: Facts, Perspectives and Toxicological Aspects
Gislaine C. Padovani, Victor P. Feitosa, Salvatore Sauro, Franklin R. Tay, Gabriela Durán, Amauri J. Paula, Nelson Durán
Trends in Biotechnology.2015; 33(11): 621. CrossRef - Antimicrobial properties of conventional restorative filling materials and advances in antimicrobial properties of composite resins and glass ionomer cements—A literature review
Cher Farrugia, Josette Camilleri
Dental Materials.2015; 31(4): e89. CrossRef - Biofilm and Dental Biomaterials
Marit Øilo, Vidar Bakken
Materials.2015; 8(6): 2887. CrossRef - Antibiofilm efficacy of silver nanoparticles as a vehicle for calcium hydroxide medicament against Enterococcus faecalis
Farzaneh Afkhami, Seyyed Jalal Pourhashemi, Mona Sadegh, Yasaman Salehi, Mohammad Javad Kharrazi Fard
Journal of Dentistry.2015; 43(12): 1573. CrossRef - Physical Properties of an Ag-Doped Bioactive Flowable Composite Resin
Hiba Kattan, Xanthippi Chatzistavrou, James Boynton, Joseph Dennison, Peter Yaman, Petros Papagerakis
Materials.2015; 8(8): 4668. CrossRef - Effect of sterilization techniques prior to antimicrobial testing on physical properties of dental restorative materials
Cher Farrugia, Glenn Cassar, Vasilis Valdramidis, Josette Camilleri
Journal of Dentistry.2015; 43(6): 703. CrossRef - The effects of gold coated and uncoated zinc oxide nanohexagons on the photophysicochemical properties of the low symmetry zinc phthalocyanine
Sarah D'Souza, Racheal Ogbodu, Tebello Nyokong
Journal of Molecular Structure.2015; 1099: 551. CrossRef - In Vitro Activity of Curcumin and Silver Nanoparticles Against Blastocystis hominis
Mona Abdel-Fattah Ahmed, Khadiga Ahmed Ismail, Sabah Abd-El-Ghany Ahmed, Ayman Nabil Ibrahim, Yousry Mahmoud Gohar
Infectious Diseases in Clinical Practice.2015; 23(3): 135. CrossRef - Development and characterization of novel ZnO-loaded electrospun membranes for periodontal regeneration
Eliseu A. Münchow, Maria Tereza P. Albuquerque, Bianca Zero, Krzysztof Kamocki, Evandro Piva, Richard L. Gregory, Marco C. Bottino
Dental Materials.2015; 31(9): 1038. CrossRef - Review of Nanomaterials in Dentistry: Interactions with the Oral Microenvironment, Clinical Applications, Hazards, and Benefits
Alexandros Besinis, Tracy De Peralta, Christopher J. Tredwin, Richard D. Handy
ACS Nano.2015; 9(3): 2255. CrossRef - Study on the Bactriostasis of Nano-Silver against Penicillium
Lu Qiu, Mei Hua Xie, Jia Yan Lv, Shu Guo Fan, Jian Hui Gao
Advanced Materials Research.2014; 1051: 62. CrossRef - Antibacterial Effects of Silver Nanoparticles Produced by Satureja hortensis Extract on Isolated Bacillus cereus from Soil of Sistan Plain
Ebrahim Shirmohammadi, Saeide Saeidi, Taher Mohasseli, Ali Rahimian Boogar
International Journal of Infection.2014;[Epub] CrossRef - Strategies to Improve Biocompatibility of Dental Materials
Gottfried Schmalz
Current Oral Health Reports.2014; 1(4): 222. CrossRef
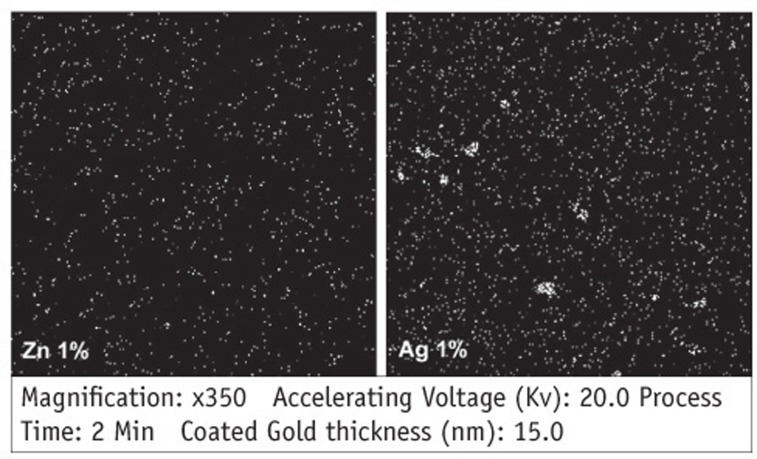
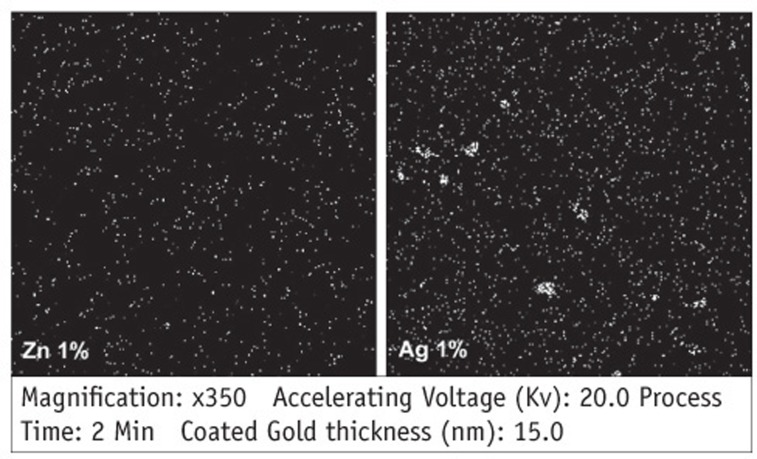
Figure 1
Comparing mean numbers of bacterial colonies (CFU) on the surface of blood agar plates in each group (n = 15)
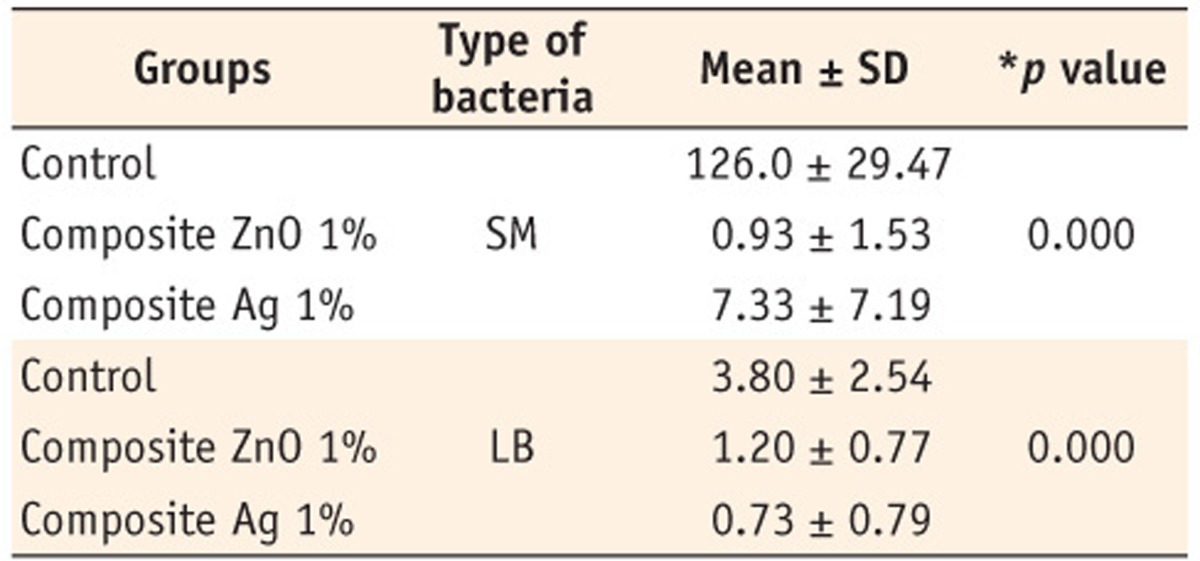
*Kruskal-Wallis test, SM, Streptococcus mutans; LB, Lactobacillus; SD, standard deviation.
*Kruskal-Wallis test, SM,

 KACD
KACD

 ePub Link
ePub Link Cite
Cite

