Articles
- Page Path
- HOME > Restor Dent Endod > Volume 38(1); 2013 > Article
- Research Article Effective application duration of sodium ascorbate antioxidant in reducing microleakage of bonded composite restoration in intracoronally-bleached teeth
- Jae-Young Park1, Tae-Yub Kwon2, Young-Kyung Kim1
-
2013;38(1):-47.
DOI: https://doi.org/10.5395/rde.2013.38.1.43
Published online: February 26, 2013
1Department of Conservative Dentistry, Kyungpook National University School of Dentistry, Daegu, Korea.
2Department of Dental Biomaterials, Kyungpook National University School of Dentistry, Daegu, Korea.
- Correspondence to Young Kyung Kim, DDS, PhD. Associate Professor, Department of Conservative Dentistry, Kyungpook National University School of Dentistry, 2-188-1 Samduk-dong, Jung-gu, Daegu, Korea 700-412. TEL, +82-53-600-7601; FAX, +82-53-426-8958; wisekim@knu.ac.kr
©Copyights 2013. The Korean Academy of Conservative Dentistry.
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,633 Views
- 7 Download
- 10 Crossref
Abstract
-
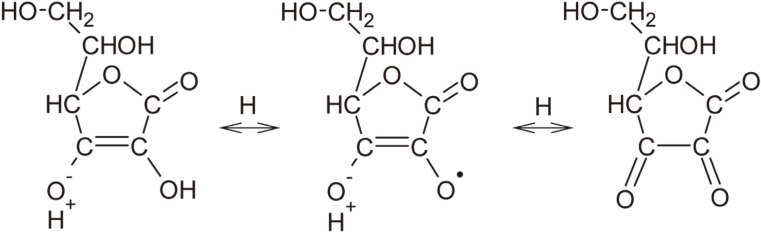
Objectives The aim of this study was to determine an appropriate application duration of sodium ascorbate (SA) antioxidant gel in reducing microleakage of bonded composite restoration in intracoronally-bleached teeth.
-
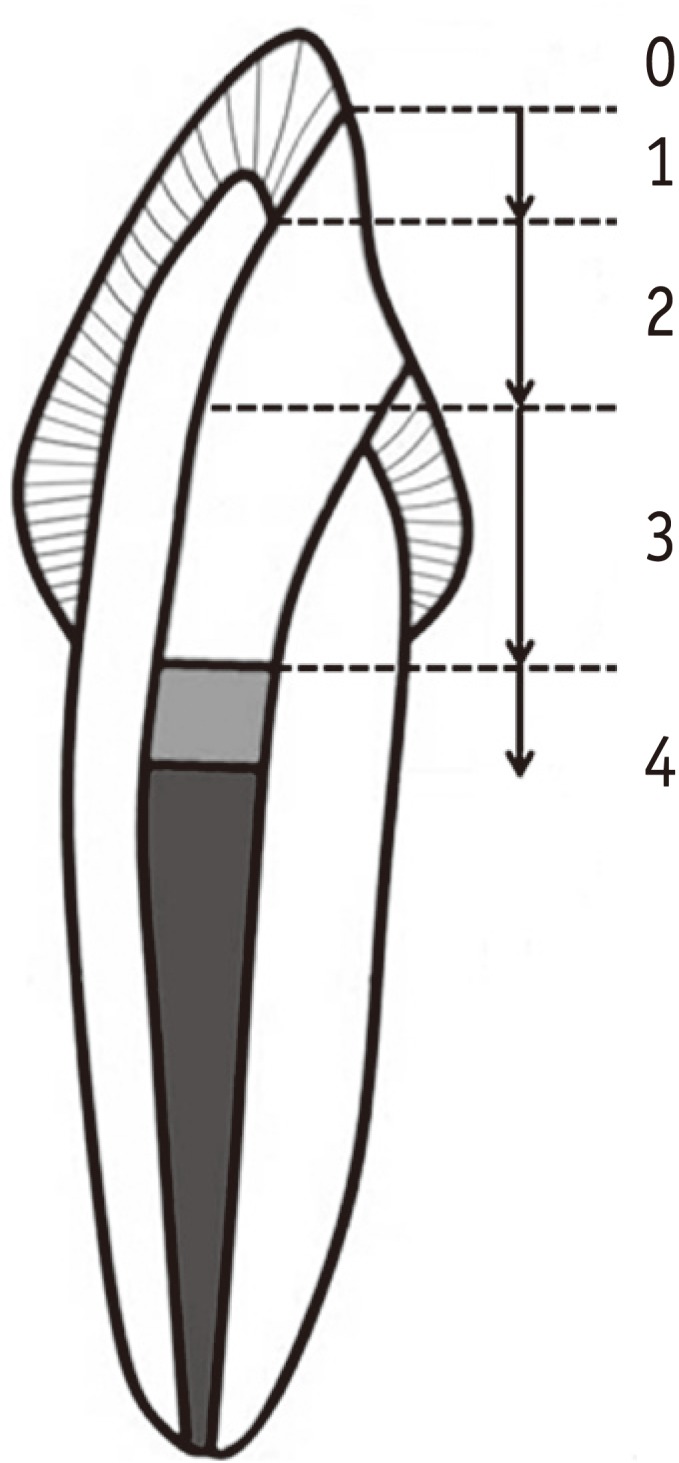
Materials and Methods Eighty endodontically-treated human incisors were randomly divided into eight groups: control, no bleaching; IB and DB, immediate and delayed bonding after bleaching, respectively; S10m, S60m, S24h, S3d and S7d, bleaching + SA gel for 10 min, 60 min, 24 hr, 3 day and 7 day, respectively. For bleaching, a mixture of 30% hydrogen peroxide and sodium perborate was applied for 7 day. All access cavities were restored using One-Step adhesive (Bisco Inc.) and then Aelite LS Packable composite (Bisco Inc.). The bonded specimens were subjected to 500 thermal cycles, immersed in 1% methylene blue for 8 hr, and longitudinally sectioned. Microleakage was assessed with a 0 - 4 scoring system and analyzed using nonparametric statistical methods (α = 0.05).
-
Results Group IB showed a significantly higher microleakge than the control group (p = 0.006) and group DB a statistically similar score to the control group (p > 0.999). Although groups S10m, S60m, and S24h exhibited significantly higher scores than group DB (p < 0.05), the microleakage in groups S3d and S7d was statistically similar to that in group DB (p = 0.771, p > 0.999).
-
Conclusions Application of SA gel for 3 day after nonvital bleaching was effective in reducing microleakage of composite restoration in intracoronally-bleached teeth.
Introduction
Materials and Methods
Results
Discussion
Conclusions
-
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science and Technology (2011-0028242).
-
No potential conflict of interest relevant to this article was reported.
- 1. Rueggeberg FA, Margeson DH. The effect of oxygen inhibition on an unfilled/filled composite system. J Dent Res 1990;69:1652-1658.ArticlePubMedPDF
- 2. Howell RA. The prognosis of bleached root-filled teeth. Int Endod J 1981;14:22-26.ArticlePubMed
- 3. Cavalli V, Reis AF, Giannini M, Ambrosano GM. The effect of elapsed time following bleaching on enamel bond strength of resin composite. Oper Dent 2001;26:597-602.PubMed
- 4. Khoroushi M, Feiz A, Ebadi M. Influence of intermediary filling material on microleakage of intracoronally bleached and restored teeth. Dent Res J (Isfahan) 2009;6:17-22.PubMedPMC
- 5. Moosavi H, Moghaddas MJ, Ghoddusi J, Rajabi O. Effects of two antioxidants on the microleakage of resin-based composite restorations after nonvital bleaching. J Contemp Dent Pract 2010;11:E033-E040.Article
- 6. Türkün M, Türkün LS. Effect of nonvital bleaching with 10% carbamide peroxide on sealing ability of resin composite restorations. Int Endod J 2004;37:52-60.ArticlePubMed
- 7. May LG, Salvia AC, Souza RO, Michida SM, Valera MC, Takahashi FE, Bottino MA. Effect of sodium ascorbate and the time lapse before cementation after internal bleaching on bond strength between dentin and ceramic. J Prosthodont 2010;19:374-380.PubMed
- 8. Lai SC, Mak YF, Cheung GS, Osorio R, Toledano M, Carvalho RM, Tay FR, Pashley DH. Reversal of compromised bonding to oxidized etched dentin. J Dent Res 2001;80:1919-1924.ArticlePubMedPDF
- 9. Barkhordar RA, Kempler D, Plesh O. Effect of nonvital tooth bleaching on microleakage of resin composite restorations. Quintessence Int 1997;28:341-344.PubMed
- 10. Kimyai S, Valizadeh H. The effect of hydrogel and solution of sodium ascorbate on bond strength in bleached enamel. Oper Dent 2006;31:496-499.ArticlePubMedPDF
- 11. Teixeira EC, Hara AT, Turssi CP, Serra MC. Effect of non-vital tooth bleaching on microleakage of coronal access restorations. J Oral Rehabil 2003;30:1123-1127.ArticlePubMedPDF
- 12. Freire A, Souza EM, de Menezes Caldas DB, Rosa EA, Bordin CF, de Carvalho RM, Vieira S. Reaction kinetics of sodium ascorbate and dental bleaching gel. J Dent 2009;37:932-936.ArticlePubMed
- 13. Buettner GR. The pecking order of free radicals and antioxidants: lipid peroxidation, alpha-tocopherol, and ascorbate. Arch Biochem Biophys 1993;300:535-543.PubMed
- 14. May JM. Ascorbate function and metabolism in the human erythrocyte. Front Biosci 1998;3:d1-d10.ArticlePubMed
- 15. Deutsch JC. Ascorbic acid oxidation by hydrogen peroxide. Anal Biochem 1998;255:1-7.ArticlePubMed
- 16. Rose RC, Bode AM. Biology of free radical scavengers: an evaluation of ascorbate. FASEB J 1993;7:1135-1142.ArticlePubMedPDF
- 17. Rose RC. Transport of ascorbic acid and other water-soluble vitamins. Biochim Biophys Acta 1988;947:335-366.ArticlePubMed
- 18. Vosoughhosseini S, Lotfi M, Shahmoradi K, Saghiri MA, Zand V, Mehdipour M, Ranjkesh B, Mokhtari H, Salemmilani A, Doosti S. Microleakage comparison of glass-ionomer and white mineral trioxide aggregate used as a coronal barrier in nonvital bleaching. Med Oral Patol Oral Cir Bucal 2011;16:e1017-e1021.ArticlePubMed
REFERENCES
Tables & Figures
REFERENCES
Citations

-
Effect of Herbal Antioxidant on Push-out Bond Strength of Resin-based Composite to Dentin after Intracoronal Bleaching: An
in vitro
Study
Parinitha MS, Akshay G, Vidya G. Doddawad, Ashwini Tumkur Shivakumar, Sowmya Halasabalu Kalgeri
Journal of Pharmacology and Pharmacotherapeutics.2025; 16(4): 439. CrossRef - Evaluation of the effect of the application of Quercus cerris extract and the use of fluoride bonding material on the bonding strength of orthodontic brackets after tooth bleaching with hydrogen peroxide
Ezgi Ay, Derya Dursun
PeerJ.2025; 13: e19335. CrossRef - Photon-Induced Photoacoustic Streaming Activation of the Postbleaching Antioxidant Application Rapidly Improves Bonding to Pulp Chamber Dentin
Nasibe Aycan Yilmaz, Hicran Dönmez Özkan
Photobiomodulation, Photomedicine, and Laser Surgery.2021; 39(4): 289. CrossRef - Hypericum perforatum L.: A Potent Antioxidant Source for the Treatment of Oxidized Dentin: An Experimental In Vitro Study
Nasibe Aycan Yilmaz, Rukiye Yavaser, Arife Alev Karagozler
Journal of Advanced Oral Research.2021; 12(1): 57. CrossRef - Influence of a short‐time antioxidant application on the dentin bond strength after intracoronal bleaching
Muhammet Karadas, Sezer Demirbuga
Microscopy Research and Technique.2019; 82(10): 1720. CrossRef - Composite resin shear bond strength on bleached dentin increased by 35% sodium ascorbate application
Tunjung Nugraheni, N Nuryono, Siti Sunarintyas, Ema Mulyawati
Dental Journal (Majalah Kedokteran Gigi).2017; 50(4): 178. CrossRef - Antioxidant therapy enhances pulpal healing in bleached teeth
Adriano Fonseca Lima, Marcelo Rocha Marques, Diana Gabriela Soares, Josimeri Hebling, Giselle Maria Marchi, Carlos Alberto de Souza Costa
Restorative Dentistry & Endodontics.2016; 41(1): 44. CrossRef - Influence of Ethanol Pretreatment on the Bonding of Resin Composite to Bleached Dentin
Ga-Eun Son, Tae-Yub Kwon, Young Kyung Kim
Korean Journal of Dental Materials.2015; 42(4): 279. CrossRef - Effect of 35% Sodium Ascorbate Treatment on Microtensile Bond Strength after Nonvital Bleaching
Jason R. Hansen, Kenneth J. Frick, Mary P. Walker
Journal of Endodontics.2014; 40(10): 1668. CrossRef - Pull-out bond strength of a self-adhesive resin cement to NaOCl-treated root dentin: effect of antioxidizing agents
Maryam Khoroushi, Marzieh Kachuei
Restorative Dentistry & Endodontics.2014; 39(2): 95. CrossRef
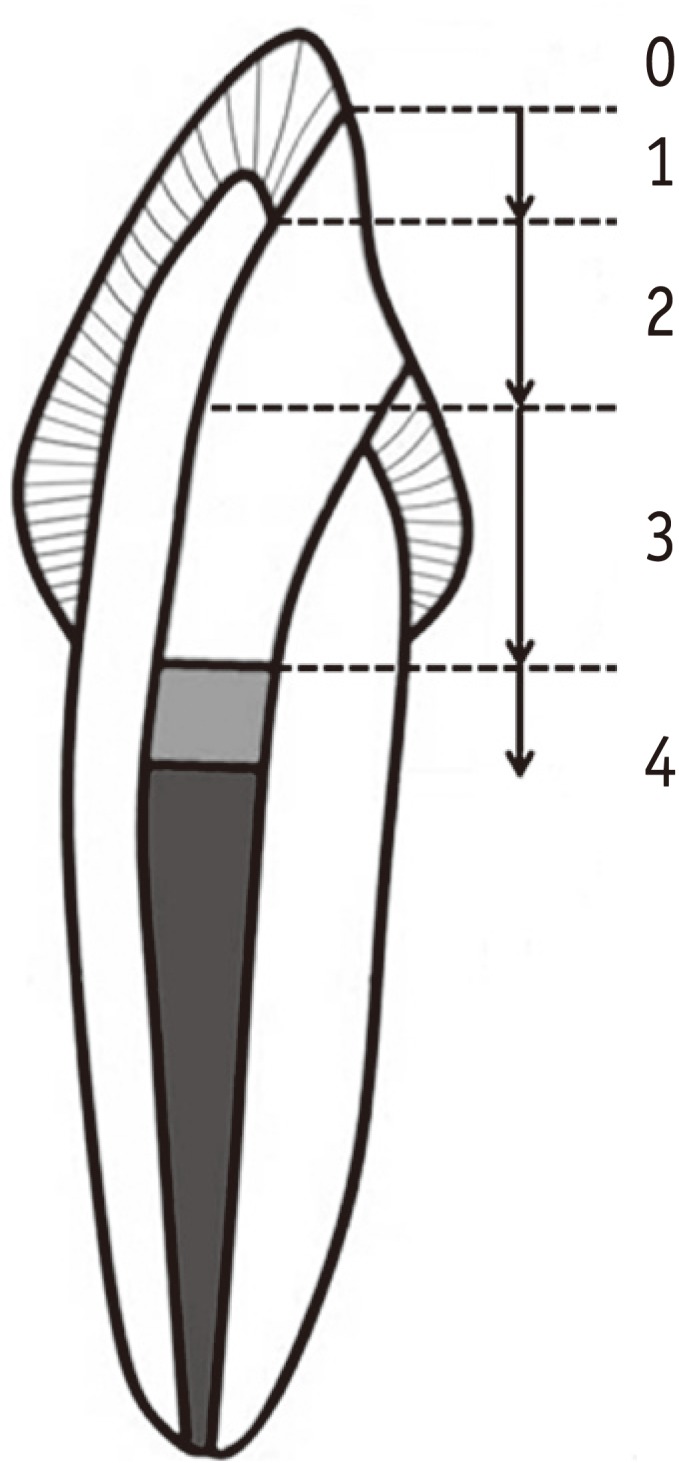
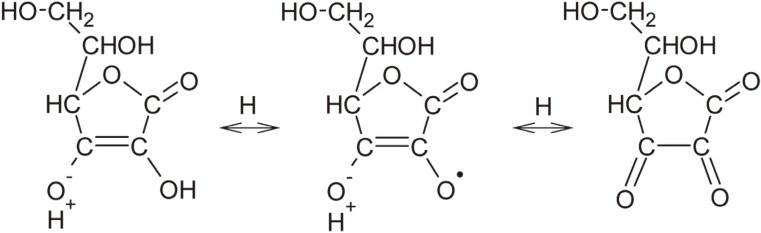
Figure 1
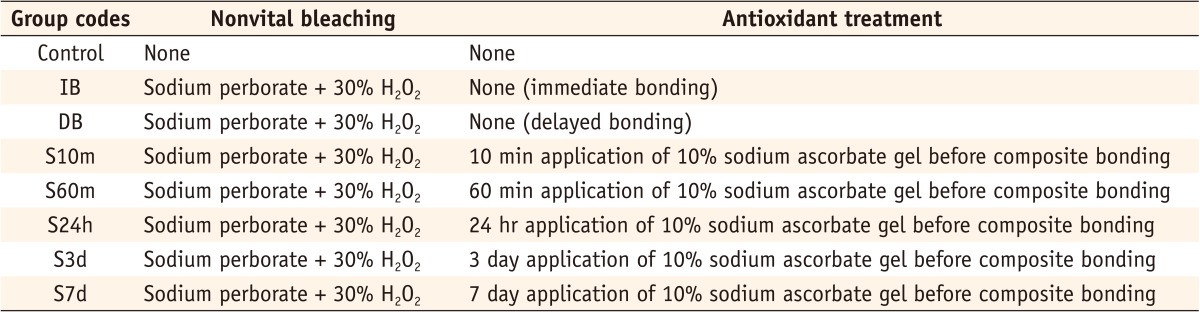
Figure 2
Group codes according to bleaching and antioxidant treatment
Microleakage score results
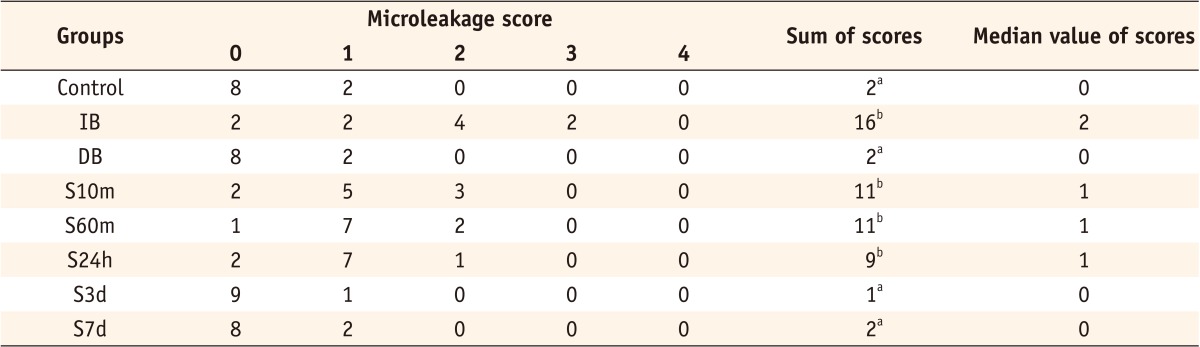
The same superscripts are not significantly different (p > 0.05).
The same superscripts are not significantly different (

 KACD
KACD


 ePub Link
ePub Link Cite
Cite

