Articles
- Page Path
- HOME > Restor Dent Endod > Volume 38(1); 2013 > Article
-
Research Article
Inhibitory effect on
Streptococcus mutans and mechanical properties of the chitosan containing composite resin - Ji-Sun Kim, Dong-Hoon Shin
-
2013;38(1):-42.
DOI: https://doi.org/10.5395/rde.2013.38.1.36
Published online: February 26, 2013
Department of Conservative Dentistry, Dankook University College of Dentistry and Institute of Dental Science, Cheonan, Korea.
- Correspondence to Dong-Hoon Shin, DDS, PhD. Professor, Department of Conservative Dentistry, Dankook University College of Dentistry and Institute of Dental Science, San 7-1, Shinbu-dong, Dongnam-gu, Cheonan, Korea 330-716. TEL, +82-41-550-1965; FAX, +82-41-553-1258; donyshin@dankook.ac.kr
©Copyights 2013. The Korean Academy of Conservative Dentistry.
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,896 Views
- 14 Download
- 42 Crossref
Abstract
-
Objectives This study evaluated the antibacterial effect and mechanical properties of composite resins (LCR, MCR, HCR) incorporating chitosan with three different molecular weights (L, Low; M, Medium; H, High).
-
Materials and Methods Streptococcus (S). mutans 100 mL and each chitosan powder were inoculated in sterilized 10 mL Brain-Heart Infusion (BHI) solution, and was centrifuged for 12 hr. Absorbance of the supernatent was measured at OD660 to estimate the antibacterial activities of chitosan. After S. mutans was inoculated in the disc shaped chitosan-containing composite resins, the disc was cleansed with BHI and diluted with serial dilution method. S. mutans was spread on Mitis-salivarius bacitracin agar. After then, colony forming unit (CFU) was measured to verify the inhibitory effect on S. mutans biofilm. To ascertain the effect on the mechanical properties of composite resin, 3-point bending and Vickers hardness tests were done after 1 and 3 wk water storage, respectively. Using 2-way analysis of variance (ANOVA) and Scheffe test, statistical analysis was done with 95% significance level.
-
Results All chitosan powder showed inhibition effect against S. mutans. CFU number in chitosan-containing composite resins was smaller than that of control resin without chitosan. The chitosan containing composite resins did not show any significant difference in flexural strength and Vickers hardness in comparison with the control resin. However, the composite resin, MCR showed a slightly decreased flexural strength and the maximum load than those of control and the other composite resins HCR and LCR.
-
Conclusions LCR and HCR would be recommended as a feasible antibacterial restorative due to its antibacterial nature and mechanical properties.
Introduction
Materials and Methods
Results
Discussion
Conclusions
-
The current research was conducted by the Research Fund of Dankook University in 2011.
-
No potential conflict of interest relevant to this article was reported.
- 1. Kato K, Fukui K, Nakagaki H, Sato T, Takahashi N. Density profiles of total bacteria and S. mutans within dental plaque treated with stannous fluoride gel. Int Congr Ser 2005;1284:185-186.Article
- 2. Imazato S. Antibacterial properties of resin composites and dentin bonding systems. Dent Mater 2003;19:449-457.ArticlePubMed
- 3. Addy M, Thaw M. In vitro studies into the release of chlorhexidine acetate, prednisolone sodium phosphate, and prednisolone alcohol from cold cure denture base acrylic. J Biomed Mater Res 1982;16:145-157.ArticlePubMed
- 4. Wilson SJ, Wilson HJ. The release of chlorhexidine from modified dental acrylic resin. J Oral Rehabil 1993;20:311-319.ArticlePubMed
- 5. Jedrychowski JR, Caputo AA, Kerper S. Antibacterial and mechanical properties of restorative materials combined with chlorhexidines. J Oral Rehabil 1983;10:373-381.ArticlePubMed
- 6. Imazato S, Russell RR, McCabe JF. Antibacterial activity of MDPB polymer incorporated in dental resin. J Dent 1995;23:177-181.ArticlePubMed
- 7. Uragami T, Yamamoto S, Miyata T. Dehydration from alcohols by polyion complex cross-linked chitosan composite membranes during evapomeation. Biomacromolecules 2003;4:137-144.ArticlePubMed
- 8. Ikinci G, Senel S, Akincibay H, Kaş S, Erciş S, Wilson CG, Hincal AA. Effect of chitosan on a periodontal pathogen Porphyromonas gingivalis. Int J Pharm 2002;235:121-127.ArticlePubMed
- 9. Chung YC, Wang HL, Chen YM, Li SL. Effect of abiotic factors on the antibacterial activity of chitosan against waterborne pathogens. Bioresour Technol 2003;88:179-184.ArticlePubMed
- 10. Helander IM, Nurmiaho-Lassila EL, Ahvenainen R, Rhoades J, Roller S. Chitosan disrupts the barrier properties of the outer membranes of gram-negative bacteria. Int J Food Microbiol 2001;71:235-244.PubMed
- 11. Rabea EI, Badawy ME, Stevens CV, Smagghe G, Steurbaut W. Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules 2003;4:1457-1465.ArticlePubMed
- 12. Chung YC, Su YP, Chen CC, Jia G, Wang HL, Wu JC, Lin JG. Relationship between antibacterial activity of chitosan and surface characteristics of cell wall. Acta Pharmacol Sin 2004;25:932-936.PubMed
- 13. Decker EM, von Ohle C, Weiger R, Wiech I, Brecx M. A synergistic chlorhexidine/chitosan combination for improved antiplaque strategies. J Periodontal Res 2005;40:373-377.ArticlePubMed
- 14. Choi BK, Kim KY, Yoo YJ, Oh SJ, Choi JH, Kim CY. In vitro antimicrobial activity of a chitooligosaccharide mixture against Actinobacillus actinomycetemcomitans and Streptococcus mutans. Int J Antimicrob Agents 2001;18:553-557.ArticlePubMed
- 15. Fujiwara M, Hayashi Y, Ohara N. Inhibitory effect of water-soluble chitosan on growth of Streptococcus mutans. New Microbiol 2004;27:83-86.PubMed
- 16. Tarsi R, Muzzarelli RA, Guzmán CA, Pruzzo C. Inhibition of Streptococcus mutans adsorption to hydroxyapatite by low-molecular-weight chitosans. J Dent Res 1997;76:665-672.ArticlePubMedPDF
- 17. Tarsi R, Corbin B, Pruzzo C, Muzzarelli RA. Effect of low-molecular-weight chitosans on the adhesive properties of oral streptococci. Oral Microbiol Immunol 1998;13:217-224.ArticlePubMed
- 18. Miyazaki S, Nakayama A, Oda M, Takada M, Attwood D. Chitosan and sodium alginate based bioadhesive tablets for intraoral drug delivery. Biol Pharm Bull 1994;17:745-747.PubMed
- 19. Hernández-Lauzardo AN, Bautista-Baños S, Velázquezdel Valle MG, Méndez-Montealvo MG, Sánchez-Rivera MM, Bello-Pérez LA. Antifungal effects of chitosan with different molecular weights on in vitro development of Rhizopus stolonifer (Ehrenb.:Fr.) Vuill. Carbohydr Polym 2008;73:541-547.ArticlePubMed
- 20. Madihally SV, Matthew HW. Porous chitosan scaffolds for tissue engineering. Biomaterials 1999;20:1133-1142.ArticlePubMed
- 21. Bae K, Jun EJ, Lee SM, Paik DI, Kim JB. Effect of water-soluble reduced chitosan on Streptococcus mutans, plaque regrowth and biofilm vitality. Clin Oral Investig 2006;10:102-107.ArticlePubMedPDF
- 22. Kaş HS. Chitosan: properties, preparations and application to microparticulate systems. J Microencapsul 1997;14:689-711.ArticlePubMed
- 23. Sano H, Matsukubo T, Shibasaki K, Itoi H, Takaesu Y. Inhibition of adsorption of oral streptococci to saliva treated hydroxyapatite by chitin derivatives. Bull Tokyo Dent Coll 1991;32:9-17.PubMed
- 24. Tanzer JM. In: Slots J, Taubman M, editors. Microbiology of dental caries. Contemporary oral microbiology and immunology. 1992. St. Louis: Mosby; p. 377-424.
- 25. Slots J, Gibbons RJ. Attachment of Bacteroides melaninogenicus subsp. asaccharolyticus to oral surfaces and its possible role in colonization of the mouth and of periodontal pockets. Infect Immun 1978;19:254-264.ArticlePubMedPMCPDF
- 26. Wise MD, Dykema RW. The plaque-retaining capacity of four dental materials. J Prosthet Dent 1975;33:178-190.ArticlePubMed
- 27. Frank RM, Steuer P. Transmission electron microscopy of plaque accumulations in denture stomatitis. J Prosthet Dent 1985;53:115-124.ArticlePubMed
- 28. Skjörland KK. Plaque accumulation on different dental filling materials. Scand J Dent Res 1973;81:538-542.PubMed
- 29. Skjørland KK, Sønju T. Effect of sucrose rinses on bacterial colonization on amalgam and composite. Acta Odontol Scand 1982;40:193-196.ArticlePubMed
- 30. Sano H, Shibasaki K, Matsukubo T, Takaesu Y. Effect of chitosan rinsing on reduction of dental plaque formation. Bull Tokyo Dent Coll 2003;44:9-16.ArticlePubMed
- 31. Chen YM, Chung YC, Wang LW, Chen KT, Li SY. Antibacterial properties of chitosan in waterborne pathogen. J Environ Sci Health A Tox Hazard Subst Environ Eng 2002;37:1379-1390.ArticlePubMed
- 32. Busscher HJ, Engels E, Dijkstra RJ, van der Mei HC. Influence of a chitosan on oral bacterial adhesion and growth in vitro. Eur J Oral Sci 2008;116:493-495.ArticlePubMed
REFERENCES

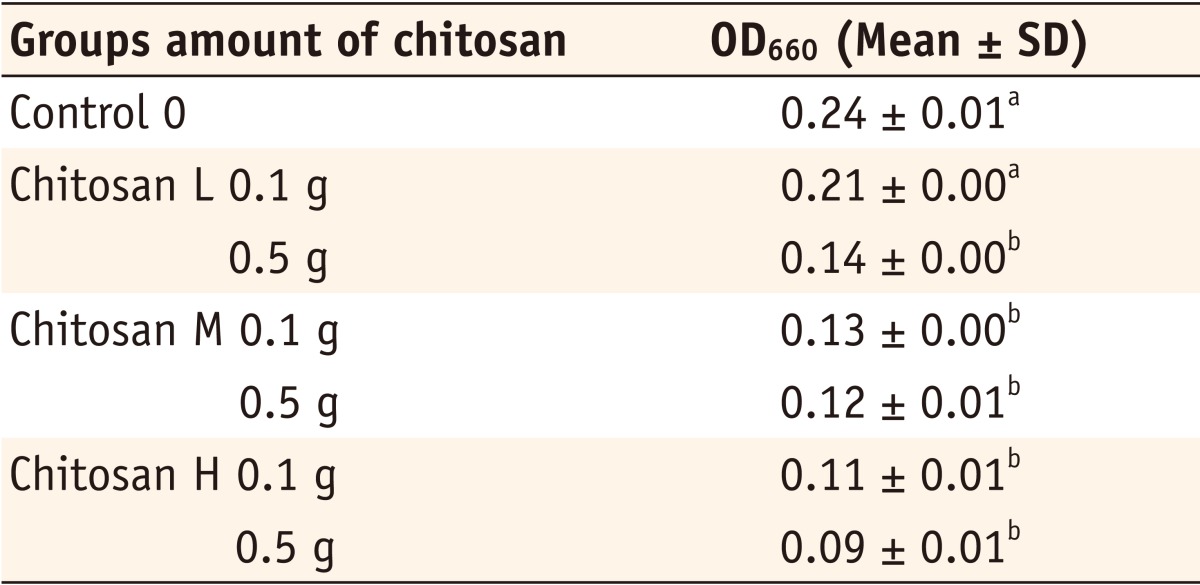
Chitosan L, low molecular weight chitosan powder; Chitosan M, medium molecular weight chitosan powder; Chitosan H, high molecular weight chitosan powder
*The same superscripts represent that there was no statistically significant difference (p > 0.05).
Chitosan LCR, composite resin containing low molecular weight chitosan; Chitosan MCR, composite resin containing medium molecular weight chitosan; Chitosan HCR, composite resin containing high molecular weight chitosan.
*The same superscripts represent that there was no statistically significant difference (p > 0.05).
CFU, colony forming unit.
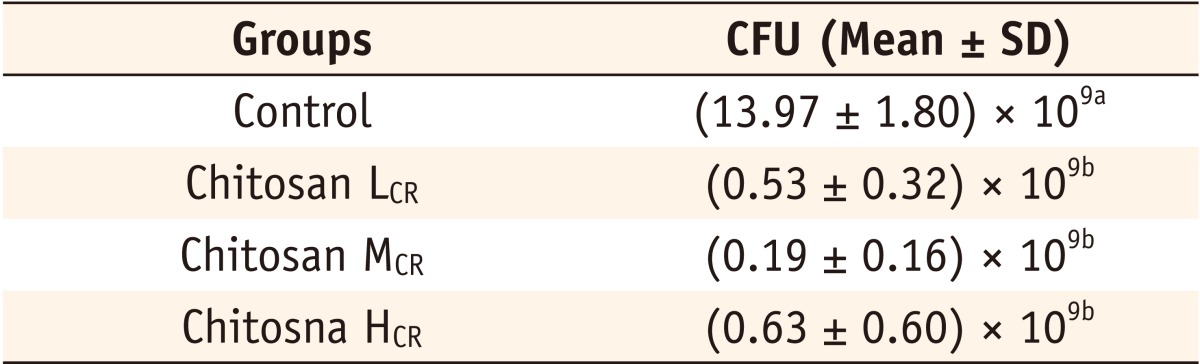
Chitosan LCR, composite resin containing low molecular weight chitosan; Chitosan MCR, composite resin containing medium molecular weight chitosan; Chitosan HCR, composite resin containing high molecular weight chitosan.
The numbers in parentheses were standard deviations.
*The same superscripts in the same column represent that there was no statistically significant difference (p > 0.05).
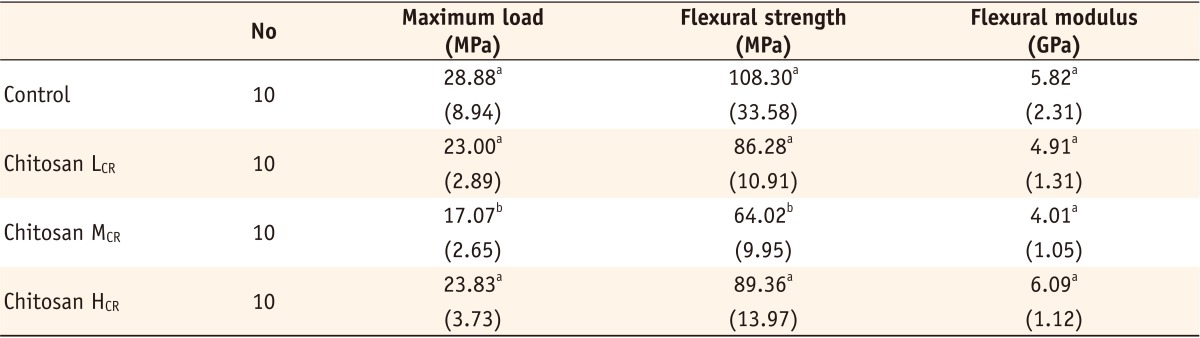
Chitosan LCR, composite resin containing low molecular weight chitosan; Chitosan MCR, composite resin containing medium molecular weight chitosan; Chitosan HCR, composite resin containing high molecular weight chitosan.
The numbers in parentheses were standard deviations.
*The same superscripts in the same column represent that there was no statistically significant difference (p > 0.05)
Tables & Figures
REFERENCES
Citations

- Dental Resin Composites Modified with Chitosan: A Systematic Review
Wojciech Dobrzyński, Paweł J. Piszko, Jan Kiryk, Sylwia Kiryk, Mateusz Michalak, Agnieszka Kotela, Julia Kensy, Witold Świenc, Natalia Grychowska, Jacek Matys, Maciej Dobrzyński
Marine Drugs.2025; 23(5): 199. CrossRef - Bactericidal Effects of Ultraviolet-C Light-Emitting Diode Prototype Device Through Thin Optical Fiber
Mi-Jeong Jeon, Yu-Sung Choi, Deog-Gyu Seo
Applied Sciences.2025; 15(8): 4504. CrossRef - Antibacterial activity, cytotoxicity, and microshear bond strength of an experimental adhesive system containing chitosan-based silver oxide particles
Hamideh Sadat Mohammadipour, Alireza Boruziniat, Seyedeh Azam Hoseini, Hosein Bagheri, Navid Ramezanian, Abbas Tanhaieian, Solmaz Pourgonabadi, Arsalan Shahri
Odontology.2025;[Epub] CrossRef - Synthesis and evaluation of a novel antibacterial nanocomposite resin restorative material
Rasha Abd El Rahman El Naggar, Manal A. ElEbiary, ElRefaie Kenawy, Gehan A. Elolimy
Tanta Dental Journal.2025; 22(3): 439. CrossRef - The impact of chitosan in experimental resin with different photoinitiator systems
Isaías Donizeti Silva, Letícia Cristina Cidreira Boaro, Bruno Vilela Muniz, Karina Cogo-Muller, Flávia Gonçalves, William Cunha Brandt
Journal of the Mechanical Behavior of Biomedical Materials.2024; 150: 106323. CrossRef - The effect of adding chitosan nanoparticles on different properties of the adhesive and high-filled composite resin
Mahan Masoumi, Sara Valizadeh, Ricardo M. Carvalho, Alireza Akbari Moghaddam, Safoura Ghodsi
International Journal of Adhesion and Adhesives.2024; 134: 103766. CrossRef - Prospective and applications of bacterial nanocellulose in dentistry
Yasmin Alimardani, Esmaeel Mirzakhani, Fereshteh Ansari, Hadi Pourjafar, Nadia Sadeghi
Cellulose.2024; 31(13): 7819. CrossRef - BNN/TiO2 nanocomposite system–modified dental flow resins and the mechanism of the enhancement of mechanical and antibacterial properties
Xinzi Kong, Qize Han, Axue Jiang, Yurui Wang, Ruizhi Li, Yuting Wang, Shengjie Xiao, Rong Wei, Yu Ma
Biomaterials Science.2023; 11(8): 2775. CrossRef - Influence of the Loading with Newly Green Silver Nanoparticles Synthesized Using Equisetum sylvaticum on the Antibacterial Activity and Surface Hardness of a Composite Resin
Ionuț Tărăboanță, Ana Flavia Burlec, Simona Stoleriu, Andreia Corciovă, Adrian Fifere, Denisa Batir-Marin, Monica Hăncianu, Cornelia Mircea, Irina Nica, Andra Claudia Tărăboanță-Gamen, Sorin Andrian
Journal of Functional Biomaterials.2023; 14(8): 402. CrossRef - The Impact of Adding Chitosan Nanoparticles on Biofilm Formation, Cytotoxicity, and Certain Physical and Mechanical Aspects of Directly Printed Orthodontic Clear Aligners
Botan Barzan Taher, Tara Ali Rasheed
Nanomaterials.2023; 13(19): 2649. CrossRef - Synthesis of Submicrometric Chitosan Particles Loaded with Calcium Phosphate for Biomedical Applications
Diana Pereira Lopes, Selma Regina Muniz Freitas, Carina Baptiston Tanaka, Giovanne Delechiave, Lucia Nobuco Takamori Kikuchi, Roberto R. Braga, Jamie J. Kruzic, Maria Stella Moreira, Leticia Cristina Cidreira Boaro, Luiz Henrique Catalani, Flávia Gonçalve
AAPS PharmSciTech.2023;[Epub] CrossRef - Biodegradable Nonwoven Materials with Antipathogenic Layer
Longina Madej-Kiełbik, Karolina Gzyra-Jagieła, Jagoda Jóźwik-Pruska, Maria Wiśniewskia-Wrona, Marzena Dymel
Environments.2022; 9(7): 79. CrossRef - Comparative evaluation of eighth-generation bonding agent modified with 7% arginine and 0.12% chitosan for antibacterial property and microtensile bond strength
HimaliRajan Desai, SanjyotA Mulay, RonitR Shinde, PradeepK Shetty, SoumyaS Shetty
Journal of Conservative Dentistry.2022; 25(4): 440. CrossRef - Effects of the crosslinking of chitosan/DCPA particles in the antimicrobial and mechanical properties of dental restorative composites
Lucia Nobuco Takamori Kikuchi, Selma Regina Muniz Freitas, Aldo Ferreira Amorim, Giovanne Delechiave, Luiz Henrique Catalani, Roberto Ruggiero Braga, Maria Stella Moreira, Leticia Cristina Cidreira Boaro, Flávia Gonçalves
Dental Materials.2022; 38(9): 1482. CrossRef - Antimicrobial activity of lactoferrin-chitosan-gellan nanoparticles and their influence on strawberry preservation
Larissa G.R. Duarte, Carolina S.F. Picone
Food Research International.2022; 159: 111586. CrossRef - Polyphenol-Enriched Extract of Lacquer Sap Used as a Dentine Primer with Benefits of Improving Collagen Cross-Linking and Antibacterial Functions
Ying Zhao, Xi He, Han Wang, Huimin Wang, Zuosen Shi, Song Zhu, Zhanchen Cui
ACS Biomaterials Science & Engineering.2022; 8(9): 3741. CrossRef - Evaluation of the changes in physical properties and mineral content of enamel exposed to radiation after treating with remineralization agent
Merve Pelin Dur, Neslihan Celik, Nilgun Seven
Clinical Oral Investigations.2022; 26(9): 5673. CrossRef - Evaluation of Immediate and Delayed Microleakage of Class V Cavities Restored with Chitosan-incorporated Composite Resins: An In Vitro Study
Roopa R Nadig, Veena Pai, Arpita Deb
International Journal of Clinical Pediatric Dentistry.2021; 14(5): 621. CrossRef - Evaluation of Microleakage of Micro Hybrid Composite Resins versus Chitosan-Incorporated Composite Resins When Restored in Class V Cavities Using Total Etch and Self-Etch Adhesives
Arpita Deb, Veena Pai, Aesha Akhtar, Roopa R. Nadig
Contemporary Clinical Dentistry.2021; 12(4): 346. CrossRef - Nanomaterials Application in Orthodontics
Wojciech Zakrzewski, Maciej Dobrzynski, Wojciech Dobrzynski, Anna Zawadzka-Knefel, Mateusz Janecki, Karolina Kurek, Adam Lubojanski, Maria Szymonowicz, Zbigniew Rybak, Rafal J. Wiglusz
Nanomaterials.2021; 11(2): 337. CrossRef - Antibacterial Effect on Enterococcus Faecalis and Physical Properties of Chitosan Added Calcium Hydroxide Canal Filling Material
Sol Song, Yu-Jin Kim, Jung-Hwan Lee, Joonhaeng Lee, Jisun Shin, Jongbin Kim
THE JOURNAL OF THE KOREAN ACADEMY OF PEDTATRIC DENTISTRY.2021; 48(2): 198. CrossRef - Antibacterial and Bonding Properties of Universal Adhesive Dental Polymers Doped with Pyrogallol
Naji Kharouf, Ammar Eid, Louis Hardan, Rim Bourgi, Youri Arntz, Hamdi Jmal, Federico Foschi, Salvatore Sauro, Vincent Ball, Youssef Haikel, Davide Mancino
Polymers.2021; 13(10): 1538. CrossRef - Efficacy of chitosan-based chewing gum on reducing salivary S. mutans counts and salivary pH: a randomised clinical trial
Zahra Khamverdi, Fatemeh Farhadian, Salman Khazaei, Maryam Adabi
Acta Odontologica Scandinavica.2021; 79(4): 268. CrossRef - Effect of antiseptic gels in the microbiologic colonization of the suture threads after oral surgery
Samuel Rodríguez Zorrilla, Andrés Blanco Carrión, Abel García García, Pablo Galindo Moreno, Xabier Marichalar Mendía, Rafael Seoane Prado, Antonio J. Pérez Estévez, Mario Pérez-Sayáns
Scientific Reports.2020;[Epub] CrossRef - Evaluating antibacterial and surface mechanical properties of chitosan modified dental resin composites
Shahid Ali, Laila Sangi, Naresh Kumar, Bharat Kumar, Zohaib Khurshid, Muhammad S. Zafar
Technology and Health Care.2020; 28(2): 165. CrossRef - Comparison of antibacterial effects of orthodontic composites containing different nanoparticles on Streptococcus mutans at different times
Soghra Yassaei, Ali Nasr, Hengameh Zandi, Mohammad Nima Motallaei
Dental Press Journal of Orthodontics.2020; 25(2): 52. CrossRef - Development of novel dental restorative composites with dibasic calcium phosphate loaded chitosan fillers
Carina B. Tanaka, Diana P. Lopes, Lucia N.T. Kikuchi, Maria Stella Moreira, Luiz H. Catalani, Roberto R. Braga, Jamie J. Kruzic, Flávia Gonçalves
Dental Materials.2020; 36(4): 551. CrossRef - Effect of iodonium salt and chitosan on the physical and antibacterial properties of experimental infiltrants
Mariana Dias FLOR-RIBEIRO, Talita Signoreti GRAZIANO, Flávio Henrique Baggio AGUIAR, Rafael Nóbrega STIPP, Giselle Maria MARCHI
Brazilian Oral Research.2019;[Epub] CrossRef - Chitosan/Fluoride Nanoparticles for Preventing Dental Caries
Niousha Ebrahimi, Ali Asghar Soleimani, Jamal Rashidiani, Beheshteh Malekafzali, Fatemeh Abedini, Hossein Hosseinkhani
Current Dentistry.2019; 1(1): 61. CrossRef - Physical and chemical properties of model composites containing quaternary ammonium methacrylates
Marina Lermenn Vidal, Guilherme Ferreira Rego, Gil Mendes Viana, Lucio Mendes Cabral, Juliana Primo Basílio Souza, Nick Silikas, Luis Felipe Schneider, Larissa Maria Cavalcante
Dental Materials.2018; 34(1): 143. CrossRef - Chitosan—PRP nanosphere as a growth factors slow releasing device with superior antibacterial capability
Radyum Ikono, Etik Mardliyati, Iis Tentia Agustin, Muhammad Mufarrij Fuad Ulfi, Dimas Andrianto, Uswatun Hasanah, Boy Muchlis Bachtiar, Nofa Mardianingsih, Endang Winiati Bachtiar, Nurwenda Novan Maulana, Nurul Taufiqu Rochman, Li Xianqi, Hideaki Kagami,
Biomedical Physics & Engineering Express.2018; 4(4): 045026. CrossRef - The Antibacterial Effect of Two Cavity Disinfectants against One of Cariogenic Pathogen: An In vitro Comparative Study
Hanaa M. Elgamily, Hoda S. El-Sayed, Ali Abdelnabi
Contemporary Clinical Dentistry.2018; 9(3): 457. CrossRef - Chitosan-Properties and Applications in Dentistry
Kmiec M
Advances in Tissue Engineering & Regenerative Medicine: Open Access.2017;[Epub] CrossRef - Analysis of the shelf life of chitosan stored in different types of packaging, using colorimetry and dentin microhardness
Antonio Miranda da Cruz-Filho, Angelo Rafael de Vito Bordin, Luis Eduardo Souza-Flamini, Débora Fernandes da Costa Guedes, Paulo César Saquy, Ricardo Gariba Silva, Jesus Djalma Pécora
Restorative Dentistry & Endodontics.2017; 42(2): 87. CrossRef - Restorative materials containing antimicrobial agents: is there evidence for their antimicrobial and anticaries effects? A systematic review
GS do Amaral, T Negrini, M Maltz, RA Arthur
Australian Dental Journal.2016; 61(1): 6. CrossRef - Antibacterial capacity of cavity disinfectants against Streptococcus mutans and their effects on shear bond strength of a self-etch adhesive
Han-Sol CHA, Dong-Hoon SHIN
Dental Materials Journal.2016; 35(1): 147. CrossRef - Antibacterial Effect and Physical-Mechanical Properties of Temporary Restorative Material Containing Antibacterial Agents
Amanda Mahammad Mushashe, Carla Castiglia Gonzaga, Paulo Henrique Tomazinho, Leonardo Fernandes da Cunha, Denise Piotto Leonardi, Janes Francio Pissaia, Gisele Maria Correr
International Scholarly Research Notices.2015; 2015: 1. CrossRef - Antimicrobial properties of conventional restorative filling materials and advances in antimicrobial properties of composite resins and glass ionomer cements—A literature review
Cher Farrugia, Josette Camilleri
Dental Materials.2015; 31(4): e89. CrossRef - Antibacterial effect of self-etching adhesive systems onStreptococcus mutans
Seung-Ryong Kim, Dong-Hoon Shin
Restorative Dentistry & Endodontics.2014; 39(1): 32. CrossRef - Dental materials with antibiofilm properties
Zhejun Wang, Ya Shen, Markus Haapasalo
Dental Materials.2014; 30(2): e1. CrossRef - Antibacterial properties of composite resins incorporating silver and zinc oxide nanoparticles onStreptococcus mutansandLactobacillus
Shahin Kasraei, Lida Sami, Sareh Hendi, Mohammad-Yousef AliKhani, Loghman Rezaei-Soufi, Zahra Khamverdi
Restorative Dentistry & Endodontics.2014; 39(2): 109. CrossRef - Antibakterielle fyllinger - hvor står vi i dag?
Nils Jacobsen
Den norske tannlegeforenings Tidende.2014; 124(8): 616. CrossRef

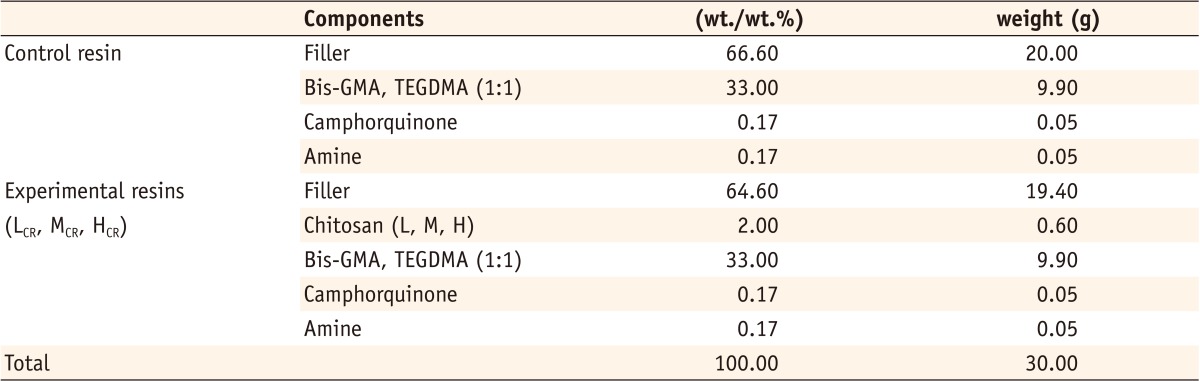
Figure 1
Compositions of experimental and control resins
Bacterial growth (OD660) of 0.1 g and 0.5 g chitosan powder (L, M, H)

Chitosan L, low molecular weight chitosan powder; Chitosan M, medium molecular weight chitosan powder; Chitosan H, high molecular weight chitosan powder
*The same superscripts represent that there was no statistically significant difference (p > 0.05).
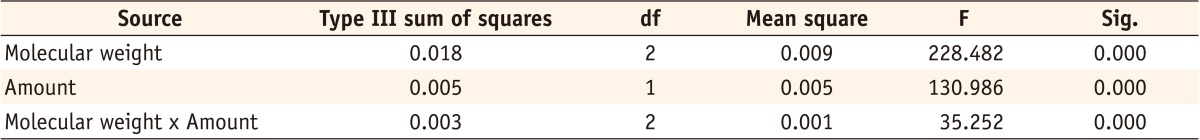
Statistical analysis (two-way ANOVA) of Streptococcus mutans growth explained by two factors of molecular weight and amount of incorporated chitosan

*R Squared = 0.989 (Adjusted R Squared = 0.985)
The number of the colonies formed by Streptococcus mutans on the Mitis-salivarius bacitracin agar plate in each group

Chitosan LCR, composite resin containing low molecular weight chitosan; Chitosan MCR, composite resin containing medium molecular weight chitosan; Chitosan HCR, composite resin containing high molecular weight chitosan.
*The same superscripts represent that there was no statistically significant difference (p > 0.05).
CFU, colony forming unit.
Maximum load, flexural strength, flexural modulus of control resin and experimental resins incorporating chitosan

Chitosan LCR, composite resin containing low molecular weight chitosan; Chitosan MCR, composite resin containing medium molecular weight chitosan; Chitosan HCR, composite resin containing high molecular weight chitosan.
The numbers in parentheses were standard deviations.
*The same superscripts in the same column represent that there was no statistically significant difference (p > 0.05).
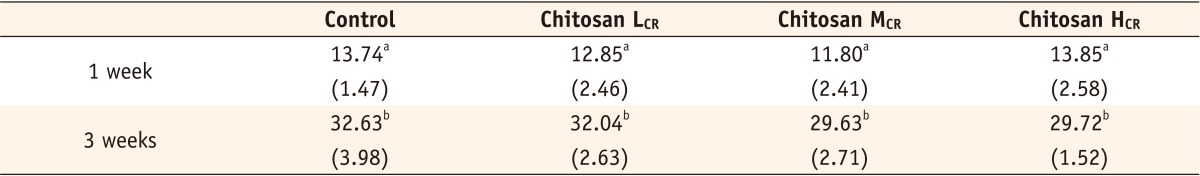
Vickers hardness (Unit: kgf/cm2; n = 10) of control resin and experimental resins incorporating chitosan (L, M, H) after 1 or 3 weeks

Chitosan LCR, composite resin containing low molecular weight chitosan; Chitosan MCR, composite resin containing medium molecular weight chitosan; Chitosan HCR, composite resin containing high molecular weight chitosan.
The numbers in parentheses were standard deviations.
*The same superscripts in the same column represent that there was no statistically significant difference (p > 0.05)
Chitosan L, low molecular weight chitosan powder; Chitosan M, medium molecular weight chitosan powder; Chitosan H, high molecular weight chitosan powder *The same superscripts represent that there was no statistically significant difference (
*R Squared = 0.989 (Adjusted R Squared = 0.985)
Chitosan LCR, composite resin containing low molecular weight chitosan; Chitosan MCR, composite resin containing medium molecular weight chitosan; Chitosan HCR, composite resin containing high molecular weight chitosan. *The same superscripts represent that there was no statistically significant difference ( CFU, colony forming unit.
Chitosan LCR, composite resin containing low molecular weight chitosan; Chitosan MCR, composite resin containing medium molecular weight chitosan; Chitosan HCR, composite resin containing high molecular weight chitosan. The numbers in parentheses were standard deviations. *The same superscripts in the same column represent that there was no statistically significant difference (
Chitosan LCR, composite resin containing low molecular weight chitosan; Chitosan MCR, composite resin containing medium molecular weight chitosan; Chitosan HCR, composite resin containing high molecular weight chitosan. The numbers in parentheses were standard deviations. *The same superscripts in the same column represent that there was no statistically significant difference (

 KACD
KACD

 ePub Link
ePub Link Cite
Cite

