Articles
- Page Path
- HOME > Restor Dent Endod > Volume 34(1); 2009 > Article
- Original Article Effect of pre-heating on some physical properties of composite resin
- Myoung Uk Jin, Sung Kyo Kim
-
2009;34(1):-37.
DOI: https://doi.org/10.5395/JKACD.2009.34.1.030
Published online: January 31, 2009
Department of Conservative Dentistry, School of Dentistry, Kyungpook National University, Daegu, Korea.
- Corresponding Author: Sung Kyo Kim. Department of Conservative Dentistry, School of Dentistry, Kyungpook National University, Samdeok-dong 2-ga, 188-1, Jung-gu, Daegu, 700-412, Korea. Tel:82-53-600-7621, Fax:82-53-426-8958, skykim@knu.ac.kr
• Received: December 10, 2008 • Revised: December 20, 2008 • Accepted: December 22, 2008
Copyright © 2009 The Korean Academy of Conservative Dentistry
- 2,320 Views
- 11 Download
- 3 Crossref
Abstract
-
The purpose of this study was to evaluate the effect of pre-heating on some physical properties of composite resin.Eighty extracted, noncarious human molars were used in the present study. Four different temperatures of composite resin were used: 4℃, 17℃, 48℃, and 56℃. The 4℃ and 17℃ values represented the refrigerator storage temperature and room temperature respectively. For 48℃ and 56℃, composite resin was heated to the temperatures. As physical properties of composite resin, shear bond strength, microhardness, and degree of conversion were measured. The data for each group were subjected to one-way ANOVAs followed by the Tukey's HSD test at 95% confidence level.Both in enamel and dentin, among composite resin of 4℃, 17℃, 48℃, and 56℃, the pre-heated composite resin up to 56℃ revealed the highest shear bond strength, and pre-heated composite resin to the higher temperature revealed higher shear bond strength.Microhardness value was also higher with composite resin of higher temperature.Degree of conversion was also higher with composite resin of the higher temperature.In this study, it seems that pre-heating composite resin up to the higher temperature may show higher shear bond strength, higher microhardness value, and higher degree of conversion. Therefore, when using composite resin in the clinic, preheating the composite resin could be recommended to have enhanced physical properties of it.
I. INTRODUCTION
The ultimate objective of composite resin restoration is an ideal restoration that is relatively easy to place, convenient to cure, long lasting, and esthetic. The parameters of effective practice have placed the additional burdens of technique and dental material awareness on the practitioner.
Polymerization of photo-activated restorative dimethacrylate-based materials leaves a significant proportion of methacrylate groups unreacted depending upon monomer and filler composition, filler particle size and type, interactions between monomers and filler initiator system, and light-curing procedure1-4). It exhibited incomplete degree of conversion, approximately 45% to 70%. The reason of incomplete conversion may be related to an increase in the viscosity of the rapid formed-highly cross-linked polymeric network which prevents the initiator from coming into contact with the carbon groups5). As the level of unreacted or residual monomer increases, the mechanical characteristics of the restoration may be decreased. Degree of conversion, independent of cure method, has a critical effect on the final mechanical properties and wear rate of composite6,7). A higher conversion ratio at a greater depth was found to increase the material modulus, resulting in less flexure and less potential for restoration fracture under loading8).
To increase the rate of polymerization, the resulting cross-linking density, and the ultimate degree of conversion of dimethacrylate-based monomers and resins, raising the temperature after initial curing was tried at which the photopolymerization occurs9). Heat curing has been another method to enhance some physical properties of resin. Some advantages of heat-curing composites are already well known for a long time in the manufacture of extraorally fabricated inlays and onlays10). Such studies found some mechanical properties to be enhanced with elevated temperature: those were improved diametral tensile strength, increased fracture toughness and increased hardness11-13). The main effect of post-cure heating was to relieve stresses formed during the gel stage of polymerization14). Such a homogenization of stress would then account for the immediate property enhancement of post-cure heated specimens over that of the light-cured material alone.
Among the methods to increase of the degree of conversion, pre-heating composite resin may be one of the most applicable method in clinic, and it will be necessary to investigate the properties of the directly- placed composite under varying thermal conditions. Therefore, the purpose of this study was to investigate the effect of pre-heating on some physical properties of composite resin.
II. MATERIALS AND METHODS
Tetric Ceram,TM shade A3 (Ivoclar Vivadent, Schaan, Liechtenstein, Germany) was used as a composite resin.
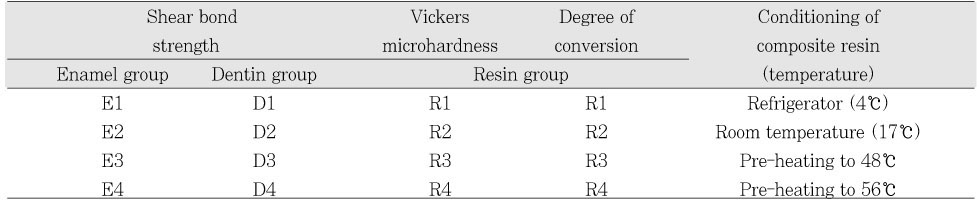
Four different temperatures of composite resin were used: 4℃, 17℃, 48℃, and 56℃. The 4℃ and 17℃ values represented the refrigerator storage temperature and room temperature respectively. For 48℃ and 56℃, composite resin was heated to the temperatures with Calset™ heating unit (AdDent, Inc., Danbury, CT, USA).
Eighty extracted, noncarious human molars stored in isotonic saline at 4℃ were used in the study. During the last 24 hours before the experiment, teeth were kept in distilled water. The teeth were embedded in auto-polymerizing acrylic resin (Orthodontic Resin, Dentsply/DeTray, Konstanz, Germany) molds so that the prepared enamel and dentin surface were 2 mm above the acrylic resin cylinders, and were placed in tap water to reduce the temperature rise from the exothermic polymerization reaction. After the acrylic resin mold had completely polymerized, the occlusal surfaces of the teeth were ground perpendicular to the long axis of the tooth with a water-cooled, precision low-speed diamond saw (Isomet, Buhler, Lake Bluff, IL, USA) to make enamel and dentin surfaces for bonding. The surfaces of enamel and dentin were hand finished with wet #240- and #600-grit silicon carbide abrasive papers using twenty 15 cm-long strokes for 15 seconds. After ultrasonic cleaning with distilled water for 3 minutes to remove the excess debris, these surfaces were washed and dried with oil-free compressed air (Hotman, Dentro, Tokyo, Japan).
The teeth were randomly divided into 8 groups of 10 teeth (Table 1). The enamel and dentin surfaces were etched with a 37% phosphoric acid gel (ETCH-37™, Bisco, Schaumburg, IL, USA) for 30 seconds and 15 seconds respectively, and were thoroughly rinsed for 5 seconds and blot dried with cotton pellets leaving a visibly moist surfaces. Two coats of acetone-based one-bottle adhesive system (One-Step, Bisco, Schaumburg, IL, USA) were applied on teeth surfaces with a microbrush (International Corp., Durgavan, Waterford, Ireland) and light cured for 15 seconds using LED-light curing unit (Bluephase™, Ivoclar Vivadent, Schaan, Liechtenstein, Germany).
Specimens were placed in mount jigs (Ultradent Product Inc., Jordan, Utah, USA). The jig was then lowered and secured to the teeth surface. Plastic mold with an internal ring of 2.3798 mm in diameter and height of 2.0 mm was placed against the tooth surface. Composite resin was packed into the mold and light cured for 40 seconds. The samples were carefully separated from the mold by lifting the jig while securing the sample with a rounded hand instrument to allow the bond to remain undisturbed. After twenty-four hours of water storage, the specimens in each group were tested in shear mode using a chisel-shaped rod in an Instron testing machine (Type 4411, Instron Corp., Canton, Massachusettes, USA) at a cross-head speed of 1 mm/min.
Teflon molds with a diameter of 6 mm and thickness of 2 mm were used to make composite resin specimens. Five disc-shaped specimens were made with composite resin (Tetric Ceram™, A3 shade, Ivoclar Vivadent, Schaan, Liechtenstein, Germany) for four different resin temperature groups. The molds were filled with composite resin and covered with clear Mylar strips lying on glass plate and light cured for 40 seconds using LED-light curing unit (Bluephase™, Ivoclar Vivadent, Schaan, Liechtenste in, Germany). The bottom surfaces of the specimens were ground and polished on silicon carbide abrasive papers of grit size #220, 600, 800, and 2000 sequentially. All specimens were stored in distilled water at room temperature for 24 hours before any further procedure.
For the microhardness test, a Vickers microhardness was measured using Microhardness Tester FM™ (Future-Tech Corp., Kawasaki, Japan) with a 300 g load and a 10 seconds loading time. Two microhardness measurements per specimen were obtained on the bottom surface of composite resin. Each microhardness determination consisted of two evenly-spaced indentation measurements over the polished surface of each specimen. The resultant dimensions were measured as the lengths of the diagonals of the indentation marks with the aid of an optical microscope (300 ×) and expressed as a Vickers Hardness Number (VHN).
The degree of conversion was determined using a FT-IR spectrometer using ten experimental resin specimens of the four different temperatures.
The FT-IR spectra were recorded with 16 scans at a resolution of 4 cm-1. First, the spectrum of the unpolymerized resin was measured. After the resin specimen was irradiated for 40 seconds with a light curing unit, the spectrum of the polymerized resin was measured. According to the formula DC= [1-(cured/uncured)] × 100, the ratio between aliphatic (1640 cm-1) and aromatic (1610 cm-1) carbon double bonds stretching was used for calculating the degree of conversion15).
The data of shear bond strength, Vickers microhardness, and degree of conversion from each group were analyzed by one-way ANOVAs and Tukey's HSD comparison test to compare them between different temperature groups (α= 0.05).
III. RESULTS
The results of the shear bond strength tests to enamel are shown in Figure 1.
Among the temperatures of 4℃, 17℃, 48℃, and 56℃, pre-heated composite to 56℃ revealed the highest shear bond strength (P < .05). The higher temperature of pre-heated composite resin yielded the higher shear bond strength to enamel. There were statistically significant differences (P < .05) between all groups except between groups of 4℃ and 17℃.
The results of the shear bond strength tests to dentin are shown in Figure 2.
Among the temperatures of 4℃, 17℃, 48℃, and 56 ℃, pre-heated composite to 56℃ revealed the highest shear bond strength (P < .05). The higher temperature of pre-heated composite resin yielded the higher shear bond strength to dentin. There were statistically significant differences (P < .05) except between 4℃ and 17℃ groups (P < .05) and between 48℃ and 56℃ groups (P < .05).
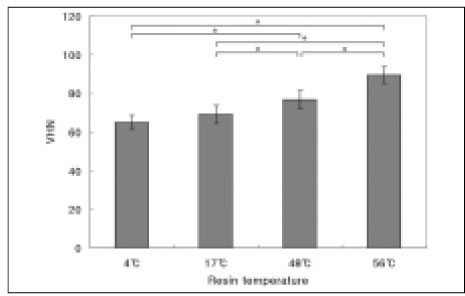
The results of the Vickers microhardness value are shown in Figure 3. The higher temperature of preheated composite resin yielded higher Vickers microhardness value. There were statistically significant differences between all groups except between 4℃ and 17℃ groups (P < .05). Among the experimental groups, pre-heated to 56℃ group yielded the highest value.
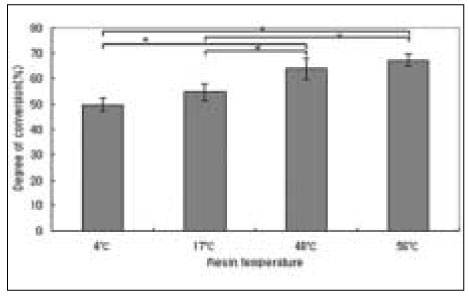
The results of degree of conversion are shown in Figure 4. The higher temperature of pre-heated composite resin yielded higher degree of conversion. There were statistically significant differences between all groups except between 4℃ and 17℃ groups (P < .05). Among the experimental groups, pre-heated to 56℃ group yielded the highest value.
IV. DISCUSSION
This study demonstrated the influence of temperature on the shear bond strength, Vickers microhardness and degree of conversion of pre-heated composite resin. In the present study, higher temperature of pre-heated composite resin yielded higher shear bond strength, microhardness and degree of conversion. These result may be due to the fact that elevated temperature of composite increase the mobility of the radical and ensue additional polymerization16). Photoactivated polymerization of dimethacrylate-based materials is based on free-radical formation and leads to a strong, cross-linked network. The reaction kinetics of these multifunctional monomers used for dental restorations is a multifaceted process exhibiting complex features such as autoacceleration, autodeceleration, limited final conversion, cyclization, and radical trapping17) This complex behavior arises from the decrease in mobility of reaction media by network formation as polymerization proceeds, leading to the onset of autoacceleration from the very beginning of polymerization18). This phenomenon, also called the gel effect, corresponds to a sudden increase in reaction rate, despite the monomer's being consumed. It is generally accepted that autoacceleration occurs due to changes in the termination rate constant, and a consequent increase in the concentration of free-radicals.
It was shown that increasing the temperature of the composite resin with a warmer, Calset™ composite warmer (AdDent, Inc., Danbury, CT, USA) increased the flow of composites up to 68%19). When a composite resin becomes more flowable, the composite may have better adaptation to the tooth structure, which may decrease microleakage20). Because composite resin is a viscoelastic material, it may exhibit decreased viscosity and greater flowability with an increase in temperature19). A previous study revealed that the film thickness of a microhybrid composite was decreased by approximately 30% when the material was heated to 54℃. However, even though the flowability of a composite resin increases with heating, the degree of flow varies among brands and composite classifications, and the flowability of pre-heated composite cannot reach to that of a flowable composite material21).
It was reported that warming of composites to body temperature or somewhat higher level immediately before placement with heat-producing unit has been shown to improve composite properties and to reduce curing times22,23). Walker et al.24) reported that for some composite tested, a significant increase in flexural modulus was observed when specimens were made at simulated intraoral temperature and humidity levels with respect to specimens made at ambient temperature and humidity.
In this study, preheating the composite to the higher temperature induced higher degree of conversion. It was probable that by increasing temperature, greater segmental mobility of pendent groups is possible, resulting in a higher probability of free-radical collision with unreacted methacrylate unit25,26). The greater number of radicals might present in the gel state, the greater is the opportunity for them to react when heated and raised to a more mobile condition27).
If we take the amount of cross-linking into consideration, increasing the degree of conversion by increasing the temperature of the composite will be quite beneficial. Polymerization rate reaches its maximum value, and then the reaction proceeds with deceasing rate (autodeceleration), as propagation also becomes diffusion-controlled. Decreased reaction rate during autodeceleration is attributed to reduced mobility of both monomer and unreacted pendant double-bonds, and decreasing dissociation efficiency of photo-initiators in the viscous medium28). Upon continued reaction and cross-linking, mobility is reduced further, and the system becomes even more entangled and viscous until the reaction stops due to polymer vitrification. The onset of vitrification occurs when the increasing glass transition temperature of the reaction mixture reaches the polymerization temperature. The reaction rate will undergo a significant decrease after vitrification, and the reaction becomes very slow as it is controlled by the diffusion of the reactive species. The diffusion-controlled effect, which produces a slow-down of the polymerization process, will also determine the final degree of conversion. Therefore, increasing the degree of conversion would be quite beneficial, since the amount of cross-linking would increase, and the amount of leachable monomer would be decreased. As the temperature is increased, the reaction rate and final conversion achieved during the resin photopolymerization increased significantly in this study. Therefore, enhancement of strength and microhardness and degree of conversion could be partially attributed an increase in monomer conversion.
Another hypothesis for superior physical properties of pre-heated composite resin in the present study is that the photoinitiator efficiency, particularly in the case of the two-component camphoroquinon/ tertiary amine initiator system, might be slightly enhanced by the reduced resin viscosity. Thus, enhanced mobility at higher temperatures of both monomer and polymer could produce a significant effect of delaying the vitrification point to higher conversion28). It is possible that enough camphoroquinone molecules might be converted to the excited triplet state in pre-heated composite to allow adequate propagation of the polymerization reaction for these materials.
In composite resin flowability, filler particle content, shape, and size also may influence composite resin flow29). In general, filler loading level, irregular surface contour, and the type of filler size distribution may impact the ability of particles to easily slide past one another30). Heating would not directly affect the glassy particle itself, because, within the temperature range imparted at clinically relevant temperatures, the viscosity of the filler particle remains unchanged. Coatings on the filler particle could affect the ease with which a filler particle would move in the warmed resin fluid. Particles not silanated would be more difficult to move than those that are coated, as silanization imparts better resin wetting and, thus, ease of fluid movement around the particle31).
In the past, heavily filled materials, particularly packable materials, have had difficulty in achieving good marginal adaptation26). Therefore, improved flowability of pre-heated microhybrid or packable resin may result in more accurate adaptation to the marginal area or sharp line angle of cavity. As each increment of composite is placed and cured at its ideal polymerization temperature, curing at the elevated temperature might have provided improved physical and mechanical properties.
In the present study, with preheating the composite, the microhardness of the composite was related with the degree of conversion measured by the FT-IR spectra. The finding of the present study supports that the microhardness may be a good indicator of conversion of double bonds32,33). Asmussen et al.12) also showed that, for a given resin composite, mechanical properties were a good indicator of degree of conversion.
From the results of the present study, it seems that pre-heating of composite resin up to the higher temperature may induce higher shear bond strength, higher microhardness value, and higher degree of conversion as well. Therefore, when using composite resin in the clinic, pre-heating the composite resin could be recommended to have enhanced physical properties of it. Further study is needed to understand the effect of temperature on the other properties such as volume change and internal stress release of the pre-heated composite resin.
V. CONCLUSION
In the present study, both in enamel and dentin, among the composite resin of 4℃, 17℃, 48℃, and 56℃, the pre-heated composite resin up to 56℃ revealed the highest shear bond strength, and preheated composite resin to the higher temperature revealed the higher shear bond strength.
Microhardness value and the degree of conversion also were shown to be higher with composite resin of higher temperature.
Within the limitations of the present study, it seems that pre-heating of composite resin up to the higher temperature may induce higher shear bond strength, higher microhardness value, and higher degree of conversion as well. Therefore, when using composite resin in the clinic, pre-heating the composite resin could be recommended to have enhanced physical properties of it.
Further studies will be needed to assess the effect of pre-heating on other properties of composite resin.
- 1. Freedman G, Goldstep F. Ultraconservative resin restorations. J Can Dent Assoc. 1999;65: 579-581.PubMed
- 2. Cook D, Beech R, Tyas J. Resin-based restorative materials- a review. Aust Dent J. 1984;29: 291-295.ArticlePubMed
- 3. Asmussen E, Peutzfeldt A. Influence of selected components on crosslink density in polymer structures. Eur J Oral Sci. 2004;109: 282-285.ArticlePDF
- 4. Dickens S, Stansburry J, Choi K. Photopolymerization kinetics of methacrylate dental resin. Macromolecules. 2003;36: 6043-6053.Article
- 5. Ferracane L. Correlation between hardness and degree of conversion during the setting reaction of unfilled dental restorative resins. Dent Mater. 1985;1: 11-14.ArticlePubMed
- 6. Condon J, Ferracane J. In vitro wear of composite with varied cure, filler level, and filler treatment. J Dent Res. 1997;76(7):1405-1411.ArticlePubMedPDF
- 7. Freiberg R, Ferracane J. Evaluation of cure, properties and wear resistance of artglass dental composite. Am J Dent. 1998;11: 214-218.PubMed
- 8. Trujillo M, Stansbury W. Thermal effects on composite photopolymerization monitored by real-time NIR. J Dent Res. 2003;82(special issue A):Abstract 0819.
- 9. Draughn R. Effects of temperature on mechanical properties of composite dental restorative materials. J Biomed mater Res. 1981;15: 489-495.ArticlePubMed
- 10. Covey A, Tahaney R, Davenport M. Mechanical properties of heat-treated composite restorative materials. J Prosthet Dent. 1992;68: 458-461.PubMed
- 11. Ferracane L, Condon R. Post-cure heat treatments for composites: Properties and fractography. Dent Mater. 1992;8: 290-295.ArticlePubMed
- 12. Asmussen E. Restorative resins: Hardness and strength vs. quantity of remaining double bonds. Scand J Dent Res. 1982;90: 484-489.ArticlePubMed
- 13. Davidson L, Duysters E, DeLange C. Structural changes in composite surface material after dry polishing. J Oral Rehabil. 1981;8: 431-439.ArticlePubMed
- 14. de Gee AJ, Pallav P, Werner A, Davidson CL. Annealing as a mechanism of increasing wear resistance of composites. Dent Mater. 1990;6: 266-270.ArticlePubMed
- 15. Ferracane JL, Greener EH. Fourier transform infrared analysis of degree of polymerization in unfilled resin-Methods comparison. J Dent Res. 1984;63: 1093-1095.ArticlePubMedPDF
- 16. Cook W, Simon G, Burchill P. Curing kinetics and thermal properties of vinyl ester resins. J Appl Polym Sci. 1997;64: 769-781.Article
- 17. Daronch M, Rueggerberg F, DeGoes M, Giudici R. Polymerization kinetics of pre-heated composite. J Dent Res. 2006;85(1):38-43.ArticlePubMedPDF
- 18. Andrzejewska E. Photopoloymerization kinetics of multifunctional monomers. Prog Polym Sci. 2001;26: 605-665.
- 19. Nicholls J. Polymerization shrinkage of densely-filled resin composite. Oper Dent. 2001;26(5):498-504.PubMed
- 20. Kim KO. Microleakage at the cervical margin of class II composite restorations with different intermediate layer treatments. J Korean Acad Conserv Dent. 2003;28: 467-474.Article
- 21. Holmes R, Ruerggeberg F. Composite film thickness at various temperatures. J Dent Res. 2004;83: (Abstract No. 3265).PMC
- 22. Freedman G, Leinfelder K. Seventh-generation adhesive system. Dent Today. 2002;21: 106-111.
- 23. Bortolotto T, Krejci I. The effect of temperature on hardness of a light-curing composite. J Dent Res. 2003;82(special issue):Abstract 0119.
- 24. Walker M, Reem Haj-Ali, Williams K. Influence of environmental condition on dental composite flexural properties. Dent Mater. 2006;22: 1002-1007.PubMed
- 25. Daronch M, Rueggerberg F, DeGoes M. Monomer conversion of pre-heated composite. J Dent Res. 2005;84(7):663-667.ArticlePubMedPDF
- 26. Opdam J, Roeters J, Joosten M, Veeke O. Porosities and voids in class I restorations placed by six operators using a packable or syringable composite. Dent Mater. 2002;18: 58-63.ArticlePubMed
- 27. Bagis H, Rueggerberg F. Effect of post-cure temperature and heat duration on monomer conversion of photo-activated dental resin composite. Dent Mater. 1997;13: 228-232.ArticlePubMed
- 28. Sideridou I, Tserki V. Effect of chemical structure on degree of conversion on light-cured dimethacrylate-based dental resins. Biomaterials. 2002;23: 1819-1829.ArticlePubMed
- 29. Chung JH, Roh BD. In vitro micro-shear bond strength of five composite resins to dentin with five different dentin adhesives. J Korean Acad Conserv Dent. 2004;29: 353-364.Article
- 30. Han SH, Kim ES, Cho YG. Enamel adhesion of lightand chemical cured composites coupled by two step self-etch adhesives. J Korean Acad Conserv Dent. 2007;32: 169-179.Article
- 31. Sun Y, Zhang Z, Wong CP. Study on mono-dispersed nano-sized silica by surface modification for underfill application. J Colloid interface Sci. 2005;292: 436-444.PubMed
- 32. Taira M, Khan M, Ohmoto K. Curing performances of four experimental Bis-GMA based binary monomer mixtures for dental visible-light cured composite resin inlays. J Mater Sci Lett. 1994;13: 1229-1231.ArticlePDF
- 33. Kurachi C, Tuboy A, Magalhaes S. Hardness evaluation of a dental composite polymerized with experimental LED-based devices. Dent Mater. 2001;17: 309-315.ArticlePubMed
REFERENCES
Figure 1
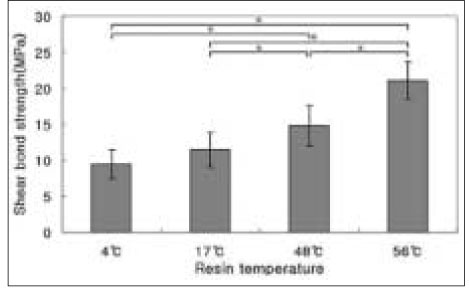
Shear bond strength of pre-heated composite resin to enamel. Mean ± S. D., n =10.
*significantly different at P < .05.

Figure 2
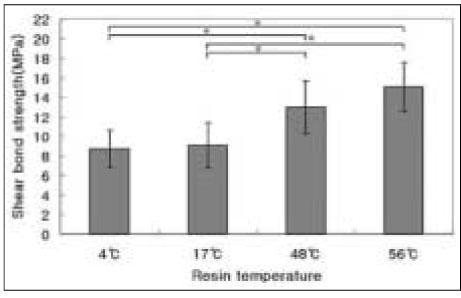
Shear bond strength of pre-heated composite to dentin. Mean ± S. D., n =10.
*significantly different at P < .05.

Tables & Figures
REFERENCES
Citations
Citations to this article as recorded by 

- Effect of Thermocycling on the Microhardness of Pre-Heated and Non-Heated Zirconium Composite Resin
P. Saloni, Kavitha Sankaran, S. Balaji Ganesh, S. Jayalakshmi, V. Vishnu Priya, R. Gayathri
Journal of International Oral Health.2025; 17(4): 304. CrossRef - Evaluation of Shear Bond Strength of Lithium Disilicate Veneers Using Pre-heated Resin Composite With Two Conventional Resin Cements: An In Vitro Study
Ghalia Akyle, Hassan Achour
Cureus.2024;[Epub] CrossRef - The different effects of preheating and heat treatment on the surface microhardness of nanohybrid resin composite
Brelian Elok Septyarini, Irfan Dwiandhono, Dian N. Agus Imam
Dental Journal.2020; 53(1): 6. CrossRef
Effect of pre-heating on some physical properties of composite resin
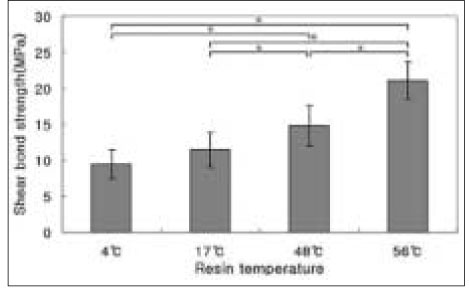
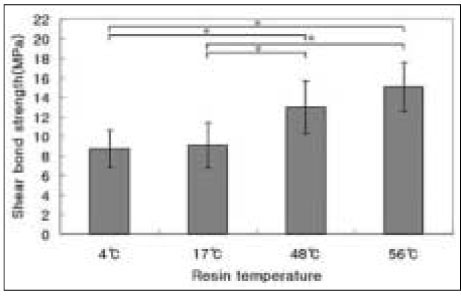
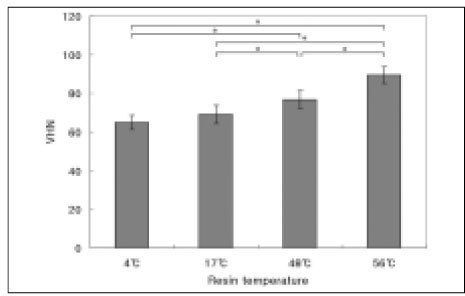
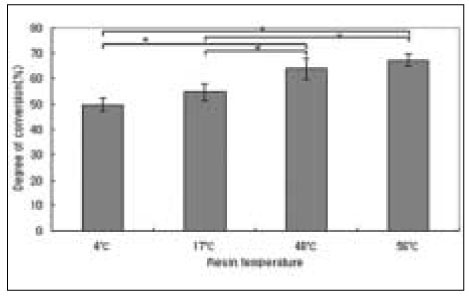
Figure 1
Shear bond strength of pre-heated composite resin to enamel. Mean ± S. D., n =10.
*significantly different at P < .05.
Figure 2
Shear bond strength of pre-heated composite to dentin. Mean ± S. D., n =10.
*significantly different at P < .05.
Figure 3
Vickers microhardness value. Mean ± S. D., n =10.
*significantly different at P < .05.
Figure 4
Degree of conversion of composite resin. Mean ± S. D., n =10.
*significantly different at P < .05.
Figure 1
Figure 2
Figure 3
Figure 4
Effect of pre-heating on some physical properties of composite resin
Summary of experimental design
Table 1
Summary of experimental design

 KACD
KACD
 ePub Link
ePub Link Cite
Cite

