Articles
- Page Path
- HOME > Restor Dent Endod > Volume 34(1); 2009 > Article
- Original Article THE DYNAMIC CHANGE OF ARTIFICIALLY DEMINERALIZED ENAMEL BY DEGREE OF SATURATION OF REMINERALIZATION SOLUTION AT pH 4.3
- Ji-Sook Yi, Bung-Duk Roh, Su-Jung Shin, Yoon Lee, Hyung-Kyu Gong, Chan-Young Lee,
-
J Korean Acad Conserv Dent 2009;34(1):-29.
DOI: https://doi.org/10.5395/JKACD.2009.34.1.20
Published online: January 14, 2009
Department of Conservative Dentistry, College of dentistry, Yonsei University
- Corresponding Author: Chan-Young Lee, College of Dentistry, Yonsei University 134 Shinchon-Dong, Seodaemun-Ku, Seoul, 120-752, Korea, Tel: 82-2-2228-8700 Fax: 82-2-313-7575, E-mail: chanyoungl@yuhs.ac
• Received: September 10, 2008 • Revised: October 23, 2008 • Accepted: November 20, 2008
Copyright © 2009 The Korean Academy of Conservative Dentistry
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,032 Views
- 3 Download
- 1 Crossref
Abstract
-
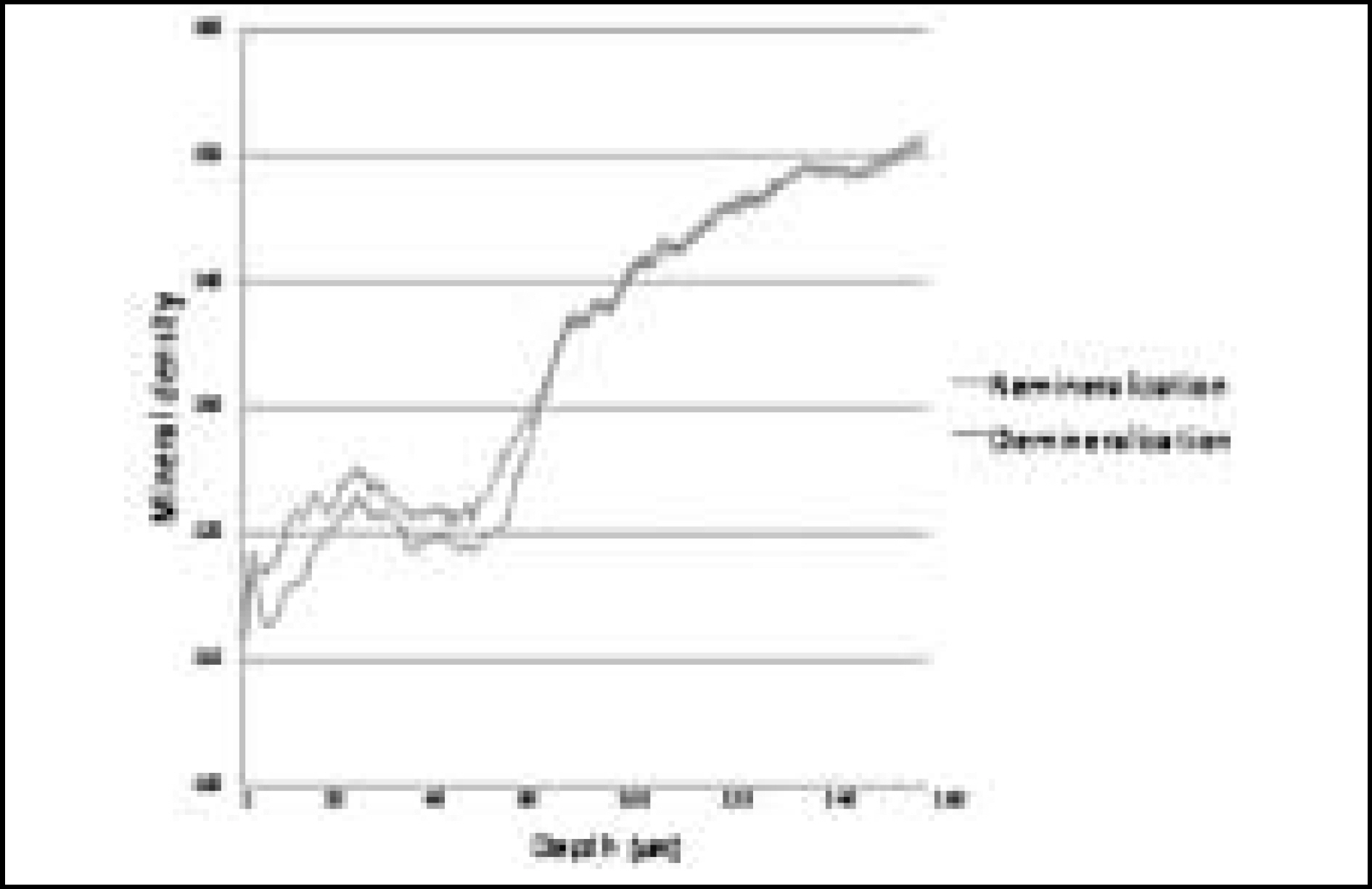
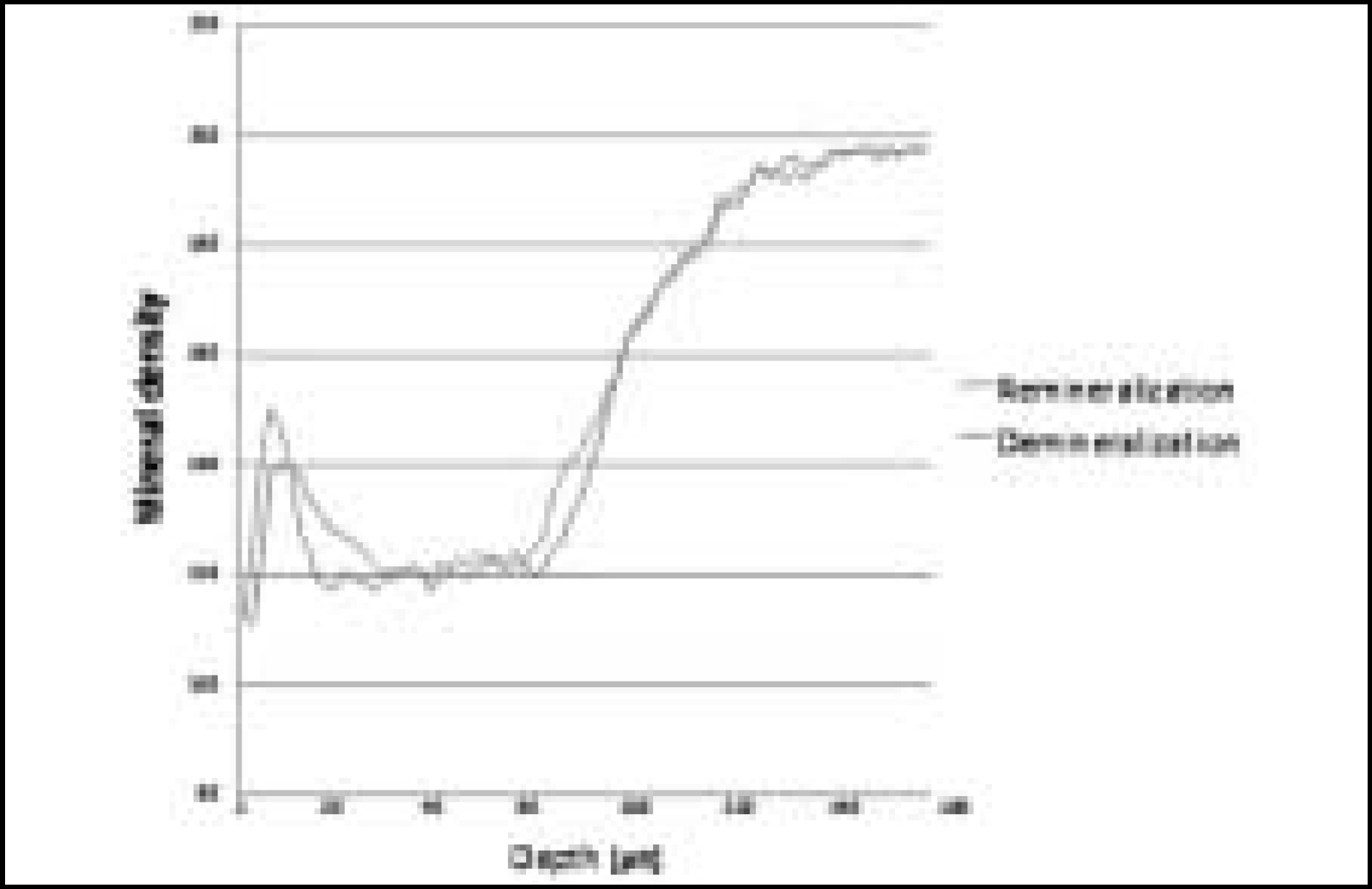
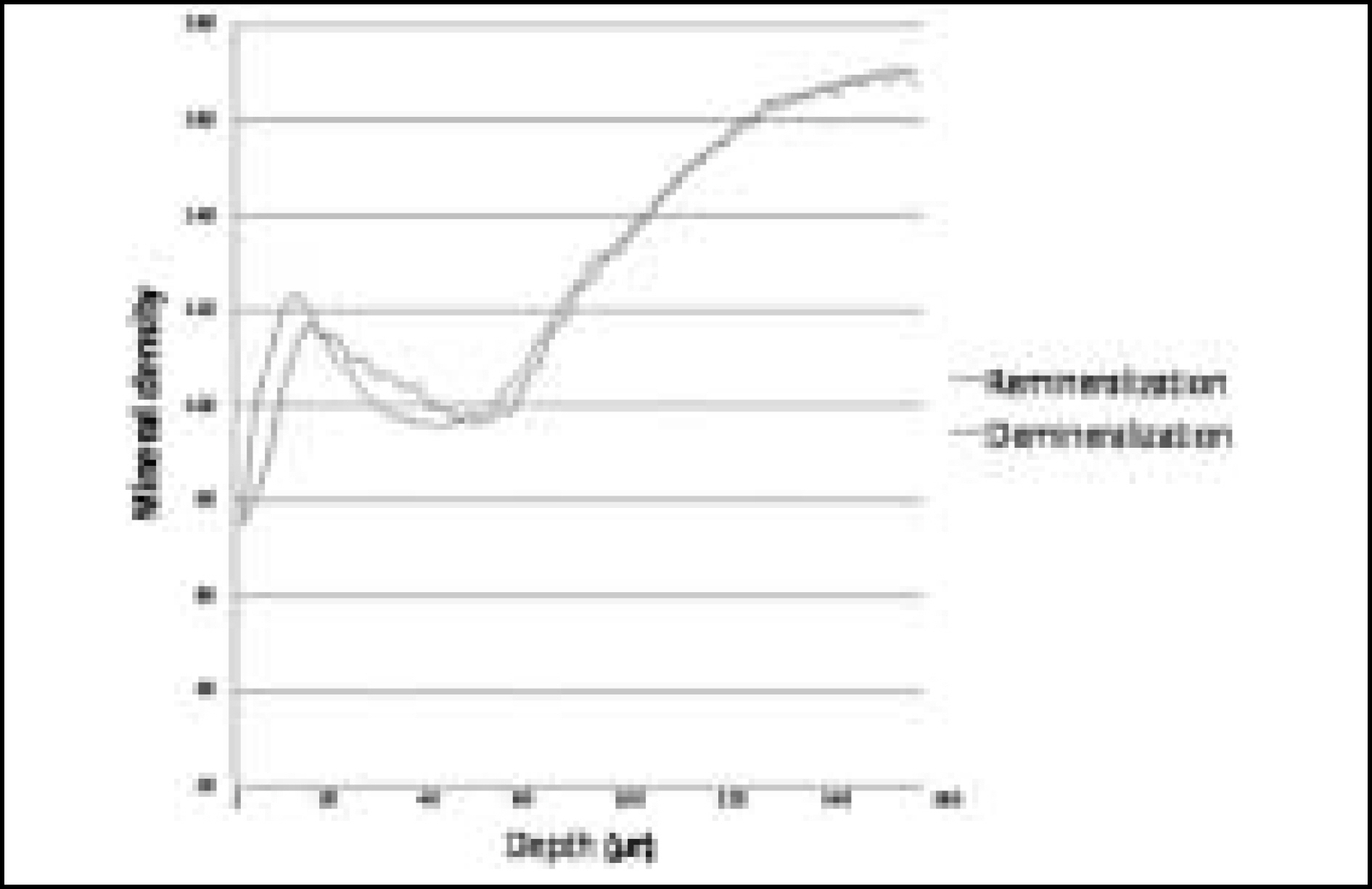
The purpose of this study is to observe and compare the dynamic change of artificially demineralized enamel by remineralization solutions of different degrees of saturation at pH 4.3.In this study, 30 enamel specimens were demineralized artificially by lactic acid buffered solution. Each of 10 specimens was immersed in pH 4.3 remineralization solution of three different degrees of saturation (0.22, 0.30, 0.35) for 10 days. After demineralization and remineralization, images were taken by a polarizing microscope (× 100). The density of lesion were determined from images taken after demineralization and remineralization.During remineralization process, mineral deposition and mineral loss occurred at the same time. After remineralization, total mineral amount and width of surface lesion increased in all groups. The higher degree of saturation was, the more mineral deposition occurred in surface lesion and the amount of mineral deposition was not much in subsurface lesion. Total demineralized depth increased in all groups.
Figure 8.
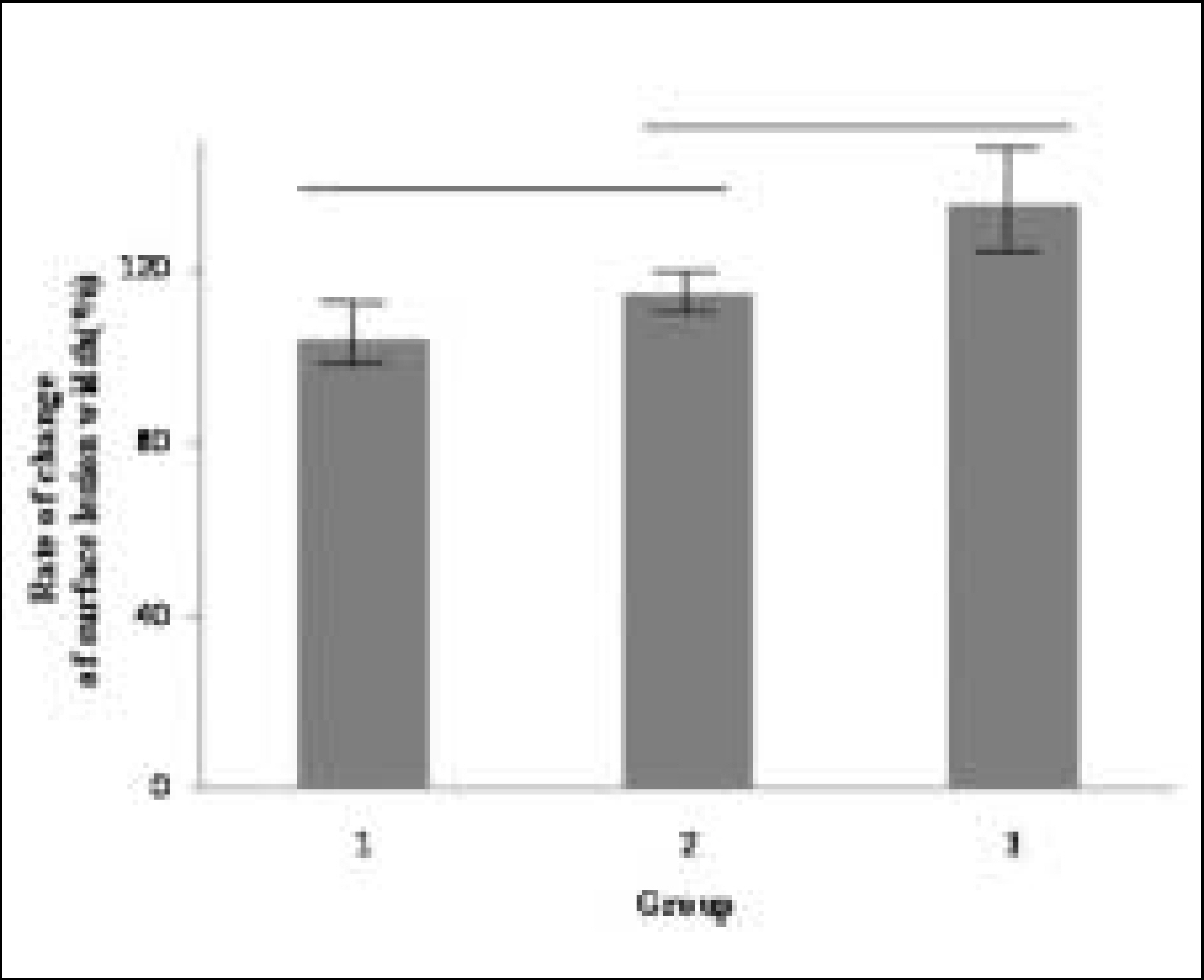
Rate of change of surface lesion width at Group 1, 2, 3 ((width of surface lesion after remineralization / width of surface lesion before remineralization) × 100(%)). Horizontal bars represent no statistically significant differences (P > .05).

Table 1.
Initial composition of demineralization solution
| Composition | Concentration |
|---|---|
| Lactic acid (mM) | 100 |
| Calcium (mM) | 15.5 |
| Phosphate (mM) | 8.5 |
| Sodium azide (mM) | 3.08 |
| pH | 4.3 |
Table 2.
Initial composition of remineralization solution
Table 3.
Rate of change of quantitative value during demineralization & remineralization at enamel
| Condition | Demineralized | Surface lesion | Mineral |
|---|---|---|---|
|
Depth (%) |
Width (%) |
Change (%) |
|
| Group | (Mean ±S.D.) | (Mean ±S.D.) ( | (Mean ±S.D.) |
| 1 | 115.1 ±11.4 % |
 * *
|
109.8 ±3.8 % |
| 2 | 112.6 ±7.9 % | 101.9 ±2.2 % | |
| 3 | 111.4 ±6.5 % | 102.7 ±4.7 % |
- 1. Featherstone JDB, Duncan JF, Cutress TW. A Mechanism for dental caries based on chemical process and diffusion phenomena during in-vitro caries simulation on human tooth enamel. Arch Oral Biol 24:101-112. 1979.PubMed
- 2. Margolis HC, Moreno EC. Physicochemical perspectives on the cariostatic mechanisms of systemic and topical fluorides. J Dent Res 69:606-613. 1990.ArticlePubMedPDF
- 3. Head JA. A study of saliva and its action on tooth enamel in reference to its hardening and softening. J Am Med Assoc 59:2118-2122. 1912.Article
- 4. Anderson BG. Clinical study of arresting dental caries. J Dent Res 17:443-452. 1938.ArticlePDF
- 5. Backer , Dirks O. Posterupted changes in dental enamel. J Dent Res 45:503-511. 1966.ArticlePDF
- 6. ten Cate JM, Jongebloed WL, Arends J. Remineralization of artificial enamel lesions in vitro. IV. Influence of fluorides and diphophates on short and long term remineralization. Caries Res 15:60-69. 1981.ArticlePubMed
- 7. Margolis HC, Murphy BJ, Moreno EC. Development of carious-like lesions in partially saturated lactate buffers. Caries Res 19:36-45. 1985.ArticlePubMed
- 8. Margolis HC, Moreno EC. Kinetic and thermodynamic aspect of enamel demineralization. Caries Res 19:22-35. 1985.ArticlePubMed
- 9. Moreno EC, Zahradnik RT. Chemistry of enamel subsurface demineralization in vitro. J Dent Res 53:226-235. 1974.ArticlePubMedPDF
- 10. 이찬 영. 산 완충 용액을 이용한 인공 치아 우식 형성. 연세치대 논문집 7:34-41. 1992.
- 11. 박 정원, 허 복, 이 찬영. 유기산 완충 용액의 포화도가 법랑질 및 상아질의 재광화에 미치는 영향과 산화 인회석의 AFM 관찰. 대한치과보존학회지 25:459-473. 2000.
- 12. Aoba T, Okazaki M, Takahashi J, Moriwaki Y. X-ray diffraction study on remineralization using systhetic hydroxyapatite pellets. Caries Res 12:223-230. 1978.ArticlePubMed
- 13. Featherstone JDB, Mellerg JR. Relative rates of progress of artificial caries lesions in bovine, ovine and human enamel. Caries Res 15(1):109-114. 1981.ArticlePubMed
- 14. Featherstone JDB, Rodgers BE, Smith MW. Physicochemical requirements for rapid remineralization of early carious lesions. Caries Res 15:221-235. 1981.ArticlePubMed
- 15. Margolis HC, Moreno EC, Murphy BJ. Effect of low levels of fluoride in solution on enamel demineralization. J Dent Res 65:23-29. 1986.ArticlePubMedPDF
- 16. Theuns HM, van Dijk JWE, Driessens FCM, Groeneveld A. Effect of the pH of buffer solution on artificial carious lesion formation in human tooth enamel. Caries Res 18:7-11. 1984.ArticlePubMed
- 17. Nikiforuk G. Formation, structure and metabolism of dental plaque. Understanding dental caries Vol. 1 Etiology and Mechanism. p. 119-157. Karger, Basel and New York; 1985.
- 18. Nikiforuk G. The use of topical fluoride. Understanding dental caries Vol. 11. Prevention. p. 63-86. Karger, Basel and New York; 1985.
- 19. Lammers PC. Borggreven JMPMm, Driessens FCM. Influence of fluorid on in vitro remineralization of artificial subsurface lesions determined with a sandwich technique. Caries Res 24:81. 1990.ArticlePubMed
- 20. 박 성호, 이 찬영, 이 정석. 유산 완충액을 이용한 인공 치아 우식 의 형성에 미치는 산의 농도와 pH 에 관한 연구. 대한치과보존 학회지 18:277-290. 1993.
- 21. 김 민경, 금 기연, 이 찬영. 법랑질 인공우식의 재광화에 미치는 pH의 영향에 관한 연구. 대한치과보존학회지 22:193-208. 1997.
- 22. 권 중원, 이 찬영. 완충 용액의 유산 농도와 pH가 법랑질의 재광 화에 미치는 영향. 연세대학교 대학원 치의학과 박사학위논문 2006.
- 23. Nancollas GH, Purdie N. The kinetics of crystal growth. Quart Rev 18:1-20. 1964.Article
- 24. ten Cate JM, Arends J. Remineralization of artificial enamel lesions in vitro. Caries Res 11:277. 1977.ArticlePubMed
- 25. Varughese , Moreno EC. Crystal growth of calcium apatites in dilute solutions containing fluorides. Calcified tissue Internationals 33(4):431-439. 1981.
- 26. Amjad Z, Nancollas GH. Effect of fluoride on the growth of hydroxyapatite and human dental enamel. Caries Res 13:250-258. 1979.ArticlePubMed
- 27. 한 원섭, 금 기연, 이 찬영. 인공 치아 우식의 재광화에 미치는 불 소의 영향. 대한치과보존학회지 21:161-173. 1996.
- 28. Silverstone LM. Remineralization phenomena. Caries Res 11:59-84. 1977.ArticlePubMed
- 29. Haikel Y, Frank RM, Voegel JC. Scanning electron microscopy of the human enamel surface layer of incipient caries lesions. Caries Res 17(1):1-13. 1983.ArticlePubMed
- 30. Featherstone JDB. Comparison of artificial caries like lesions by quantitative microradiography and microhardness profiles. Caries Res 17:385-391. 1983.ArticlePubMed
- 31. Groeneveld A, Jongebloed W, Arends J. The mineral content of decalcified surface enamel. A combined microprobe-quantitative microradiography study. Caries Res 8(3):267-274. 1974.ArticlePubMed
- 32. Darling AI. Studies of the early lesion of enamel caries with transmitted light, polarized light and radiography. Brit Dent J 6:289-341. 1956.
- 33. Darling AI. Studies of the early lesion of enamel caries its nature, mode of spread and points of entry. Brit Dent J 8:119-135. 1958.
- 34. Silverstone LM, Wefel JS, Zimmerman BF, Clarkson BH, Featherstone MJ. Re-mineralization of natural and artificial lesions in human dental enamel in vitro effect of calcium concentration of the calcific fluid. Caries Res 15:138-157. 1981.ArticlePubMed
- 35. White DJ, Chen WC, Nacollas GH. Kinetic and physical aspects of enamel remineralization-A constant composition study. Caries Res 22:11-19. 1988.ArticlePubMed
REFERENCES
Tables & Figures
REFERENCES
Citations
Citations to this article as recorded by 

- Effect of fluoride concentration in pH 4.3 and pH 7.0 supersaturated solutions on the crystal growth of hydroxyapatite
Haneol Shin, Sung-Ho Park, Jeong-Won Park, Chan-Young Lee
Restorative Dentistry & Endodontics.2012; 37(1): 16. CrossRef
THE DYNAMIC CHANGE OF ARTIFICIALLY DEMINERALIZED ENAMEL BY DEGREE OF SATURATION OF REMINERALIZATION SOLUTION AT pH 4.3
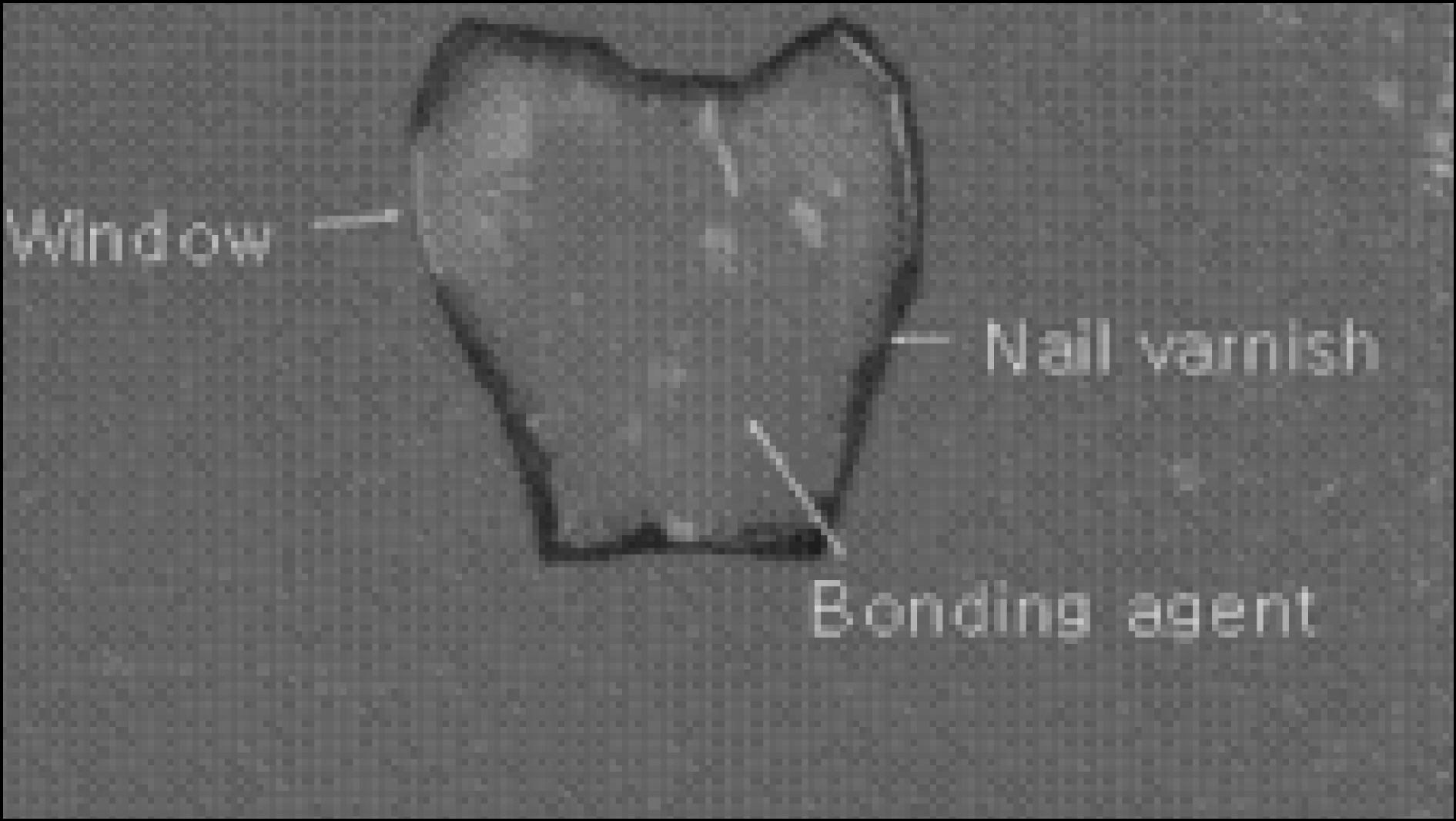

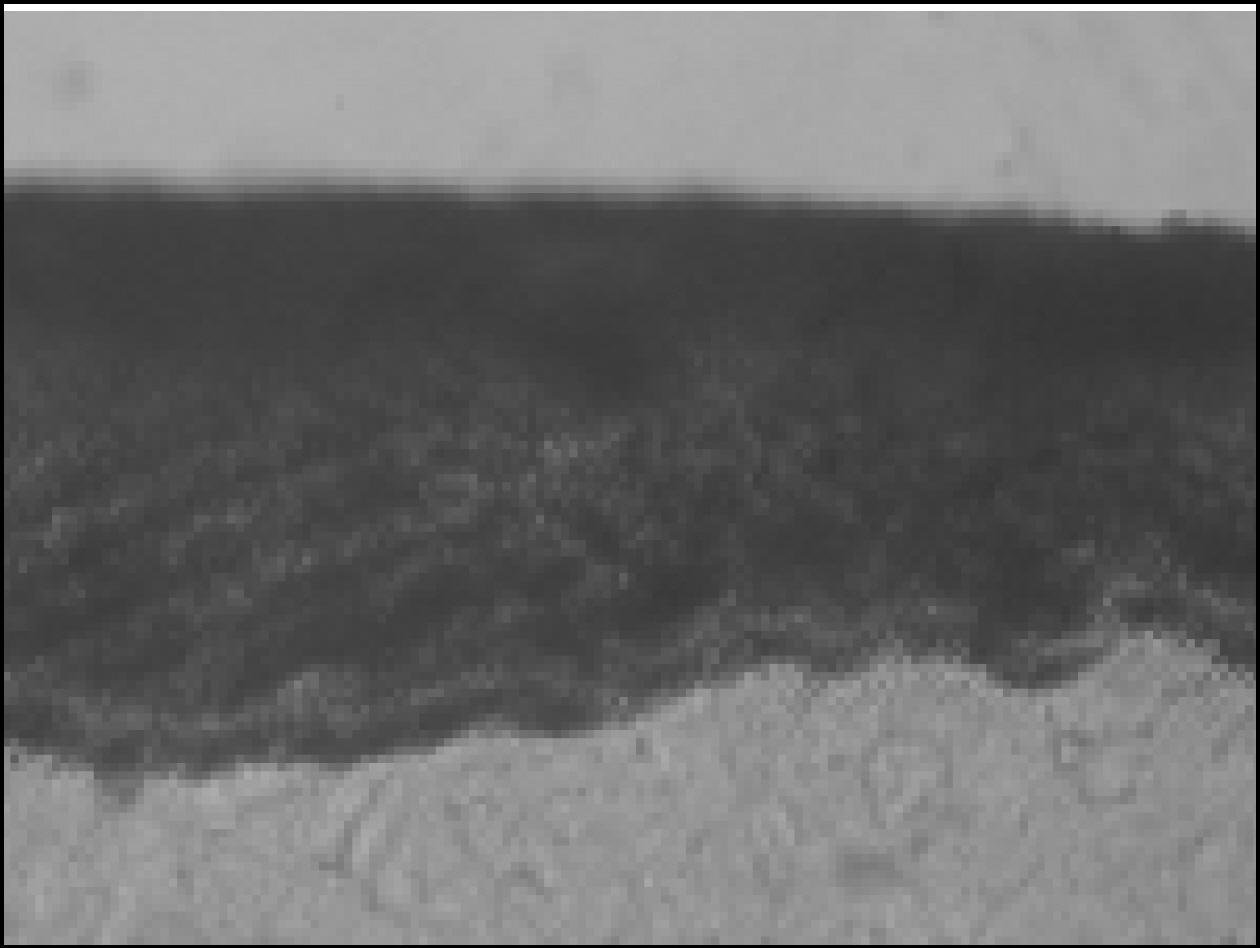
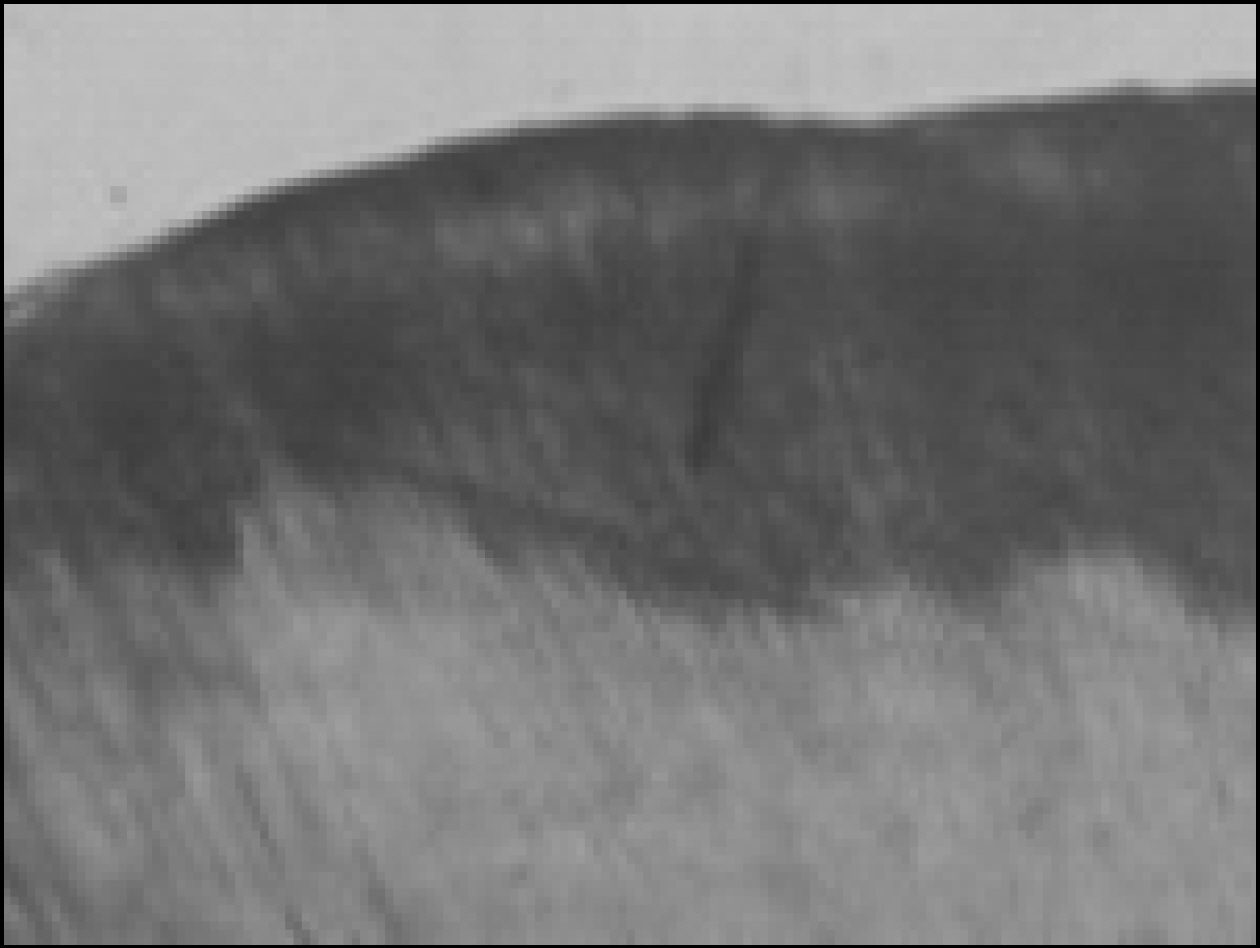
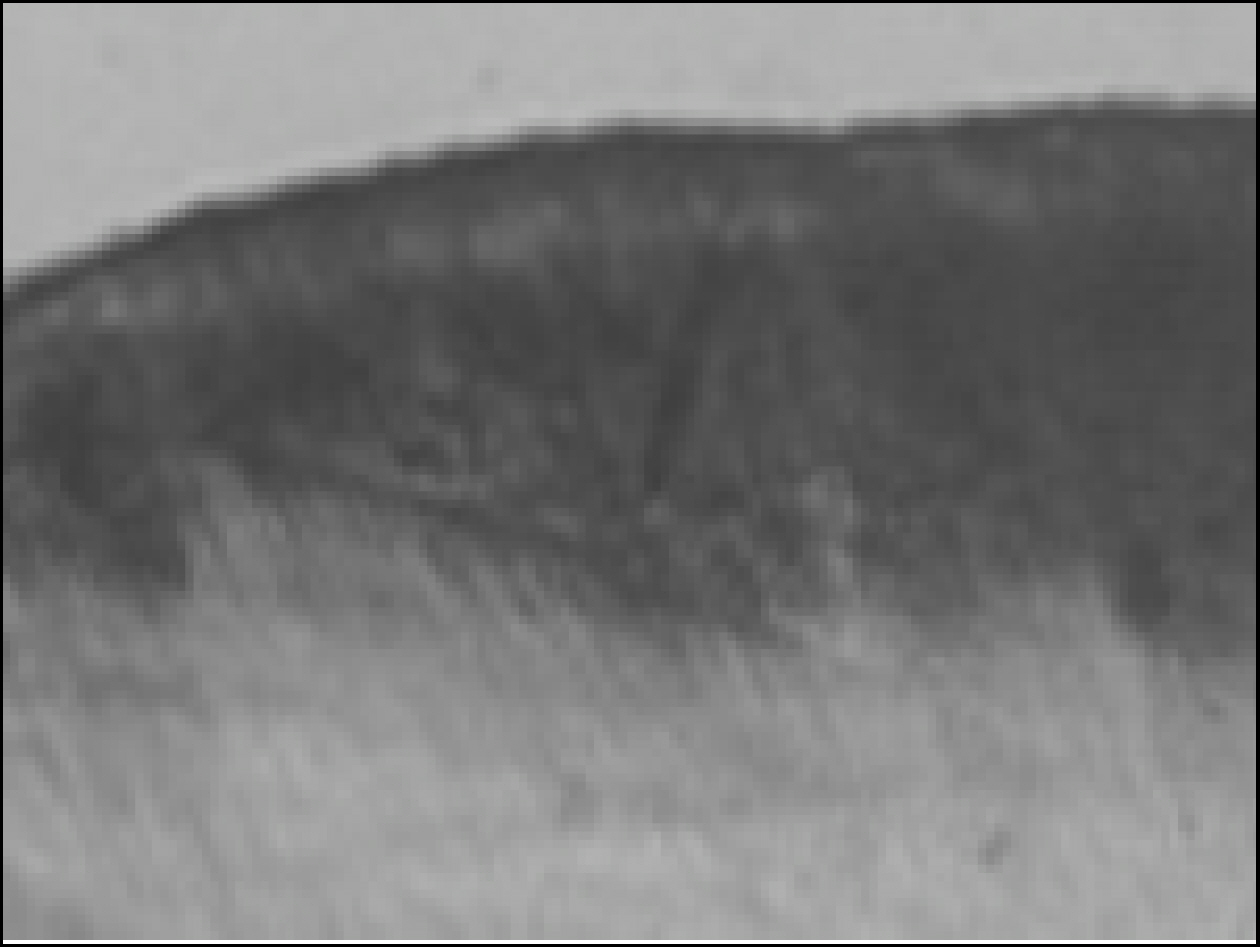

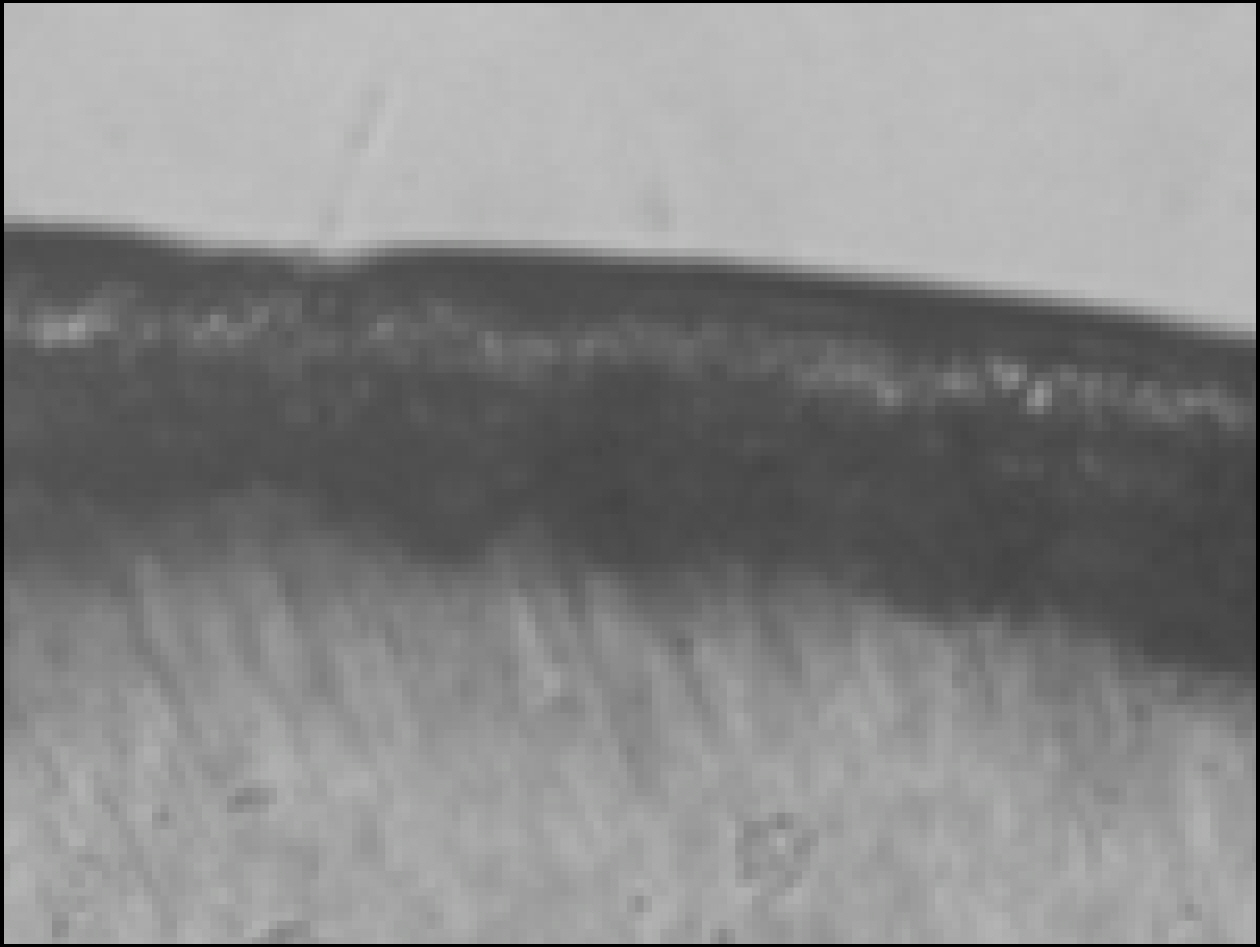
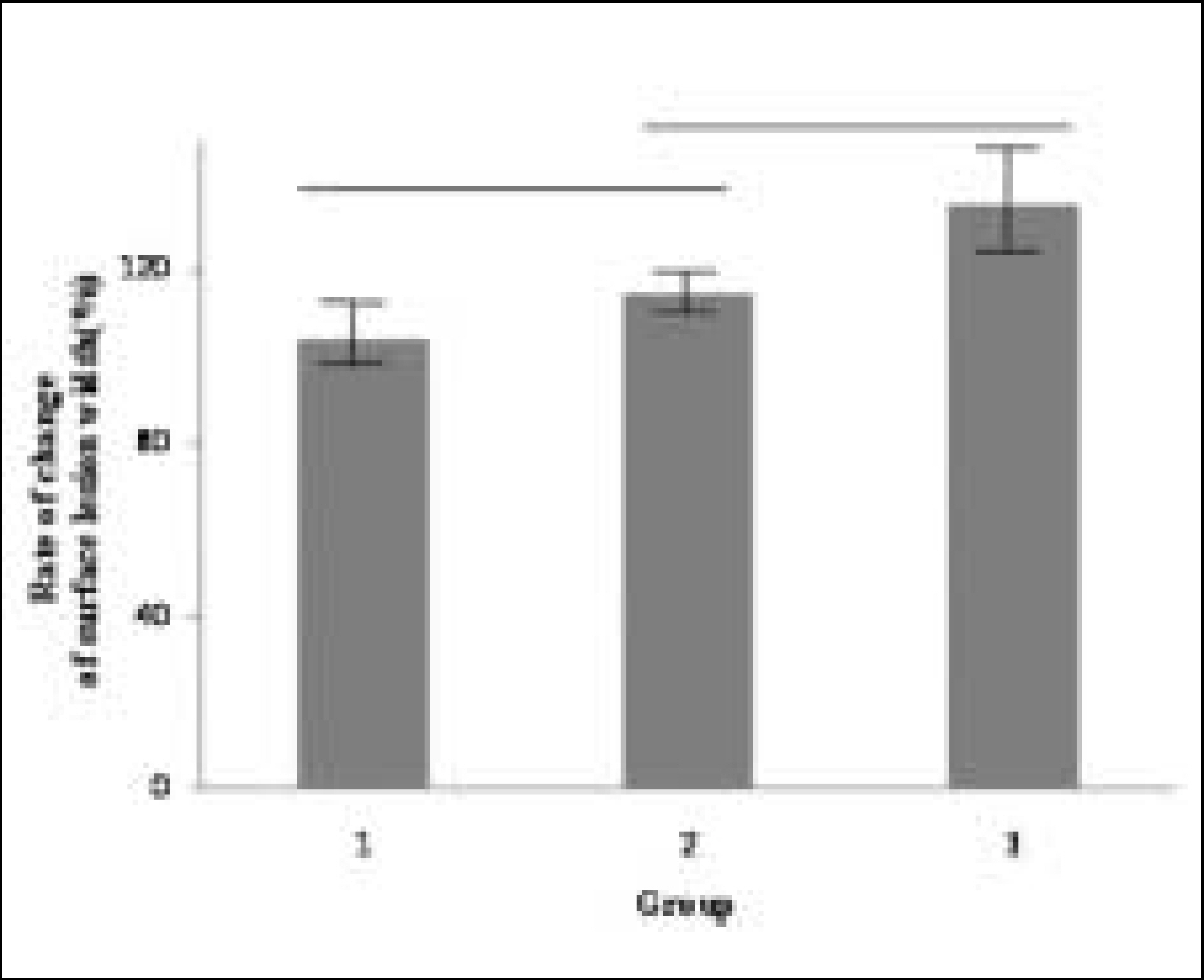
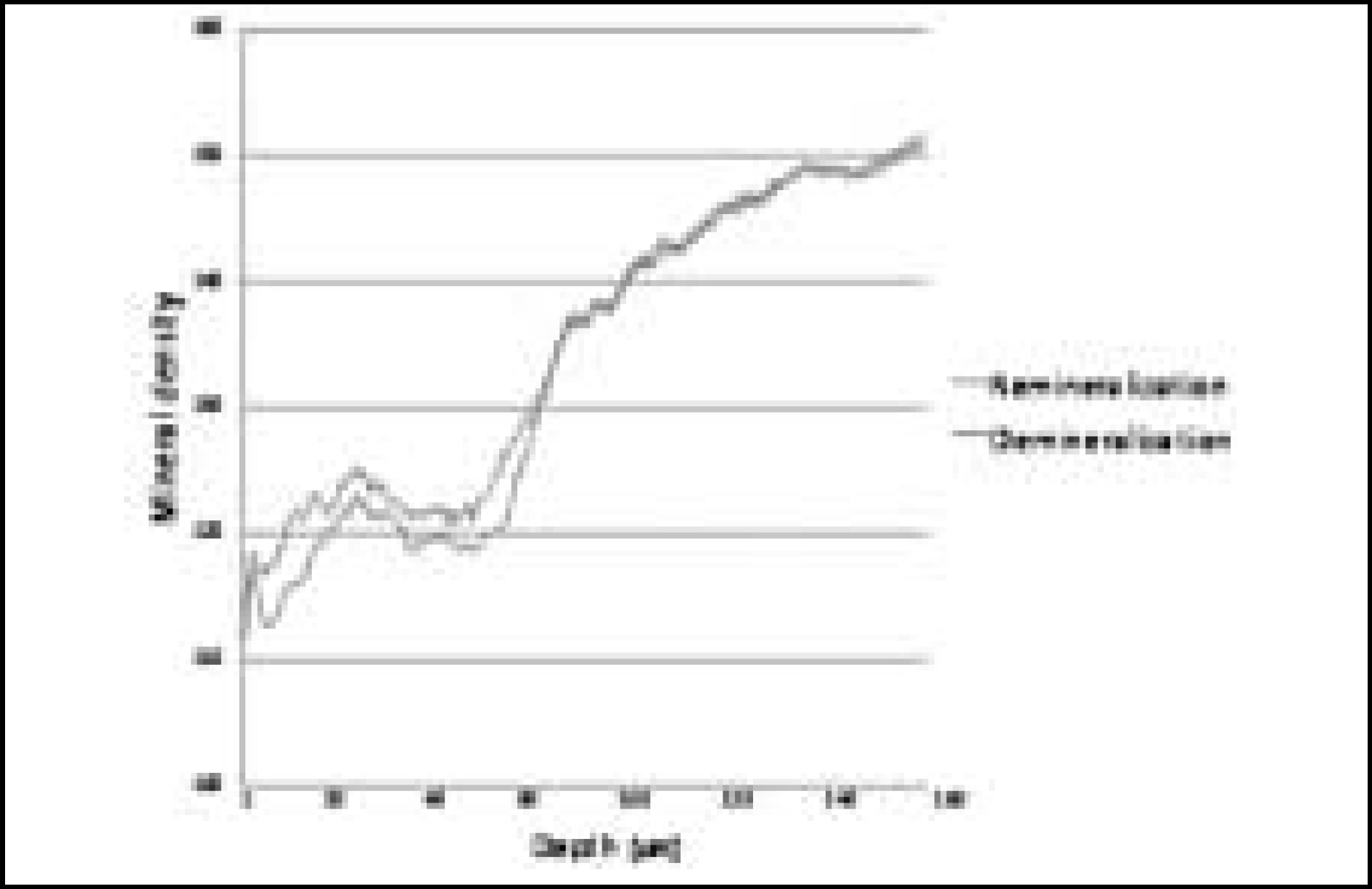
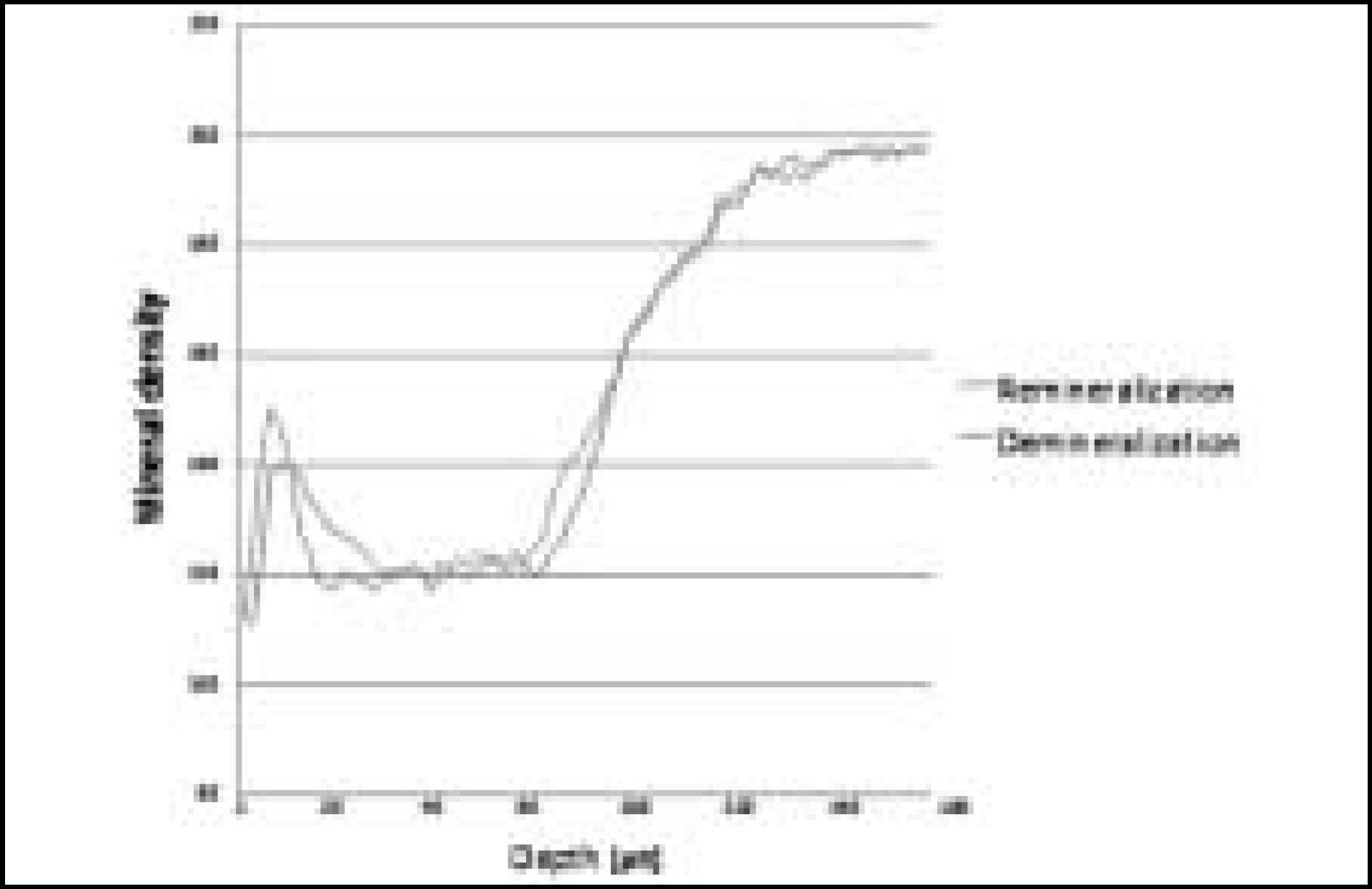
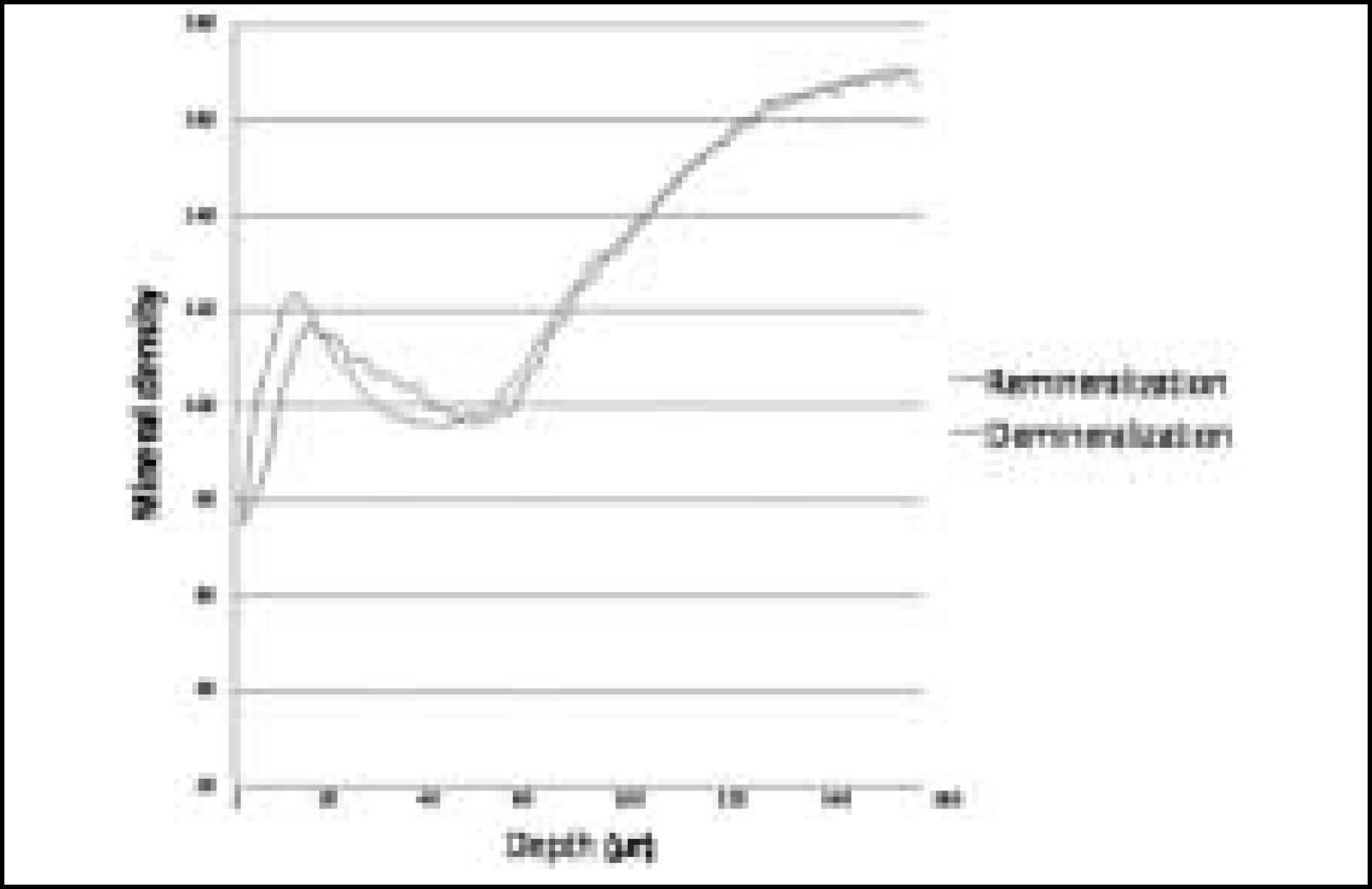
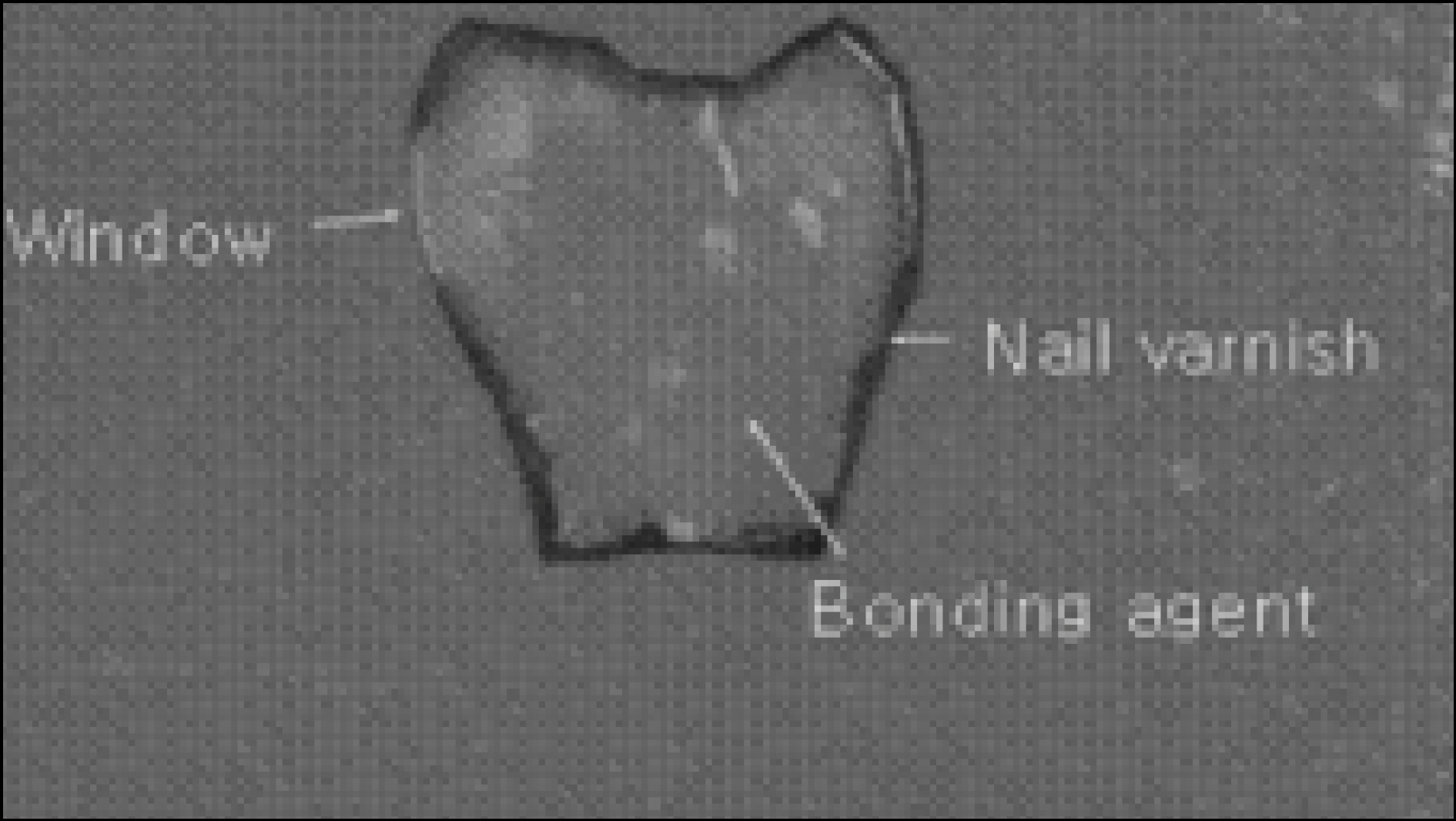
Figure 1. Enamel specimen used in the experiment
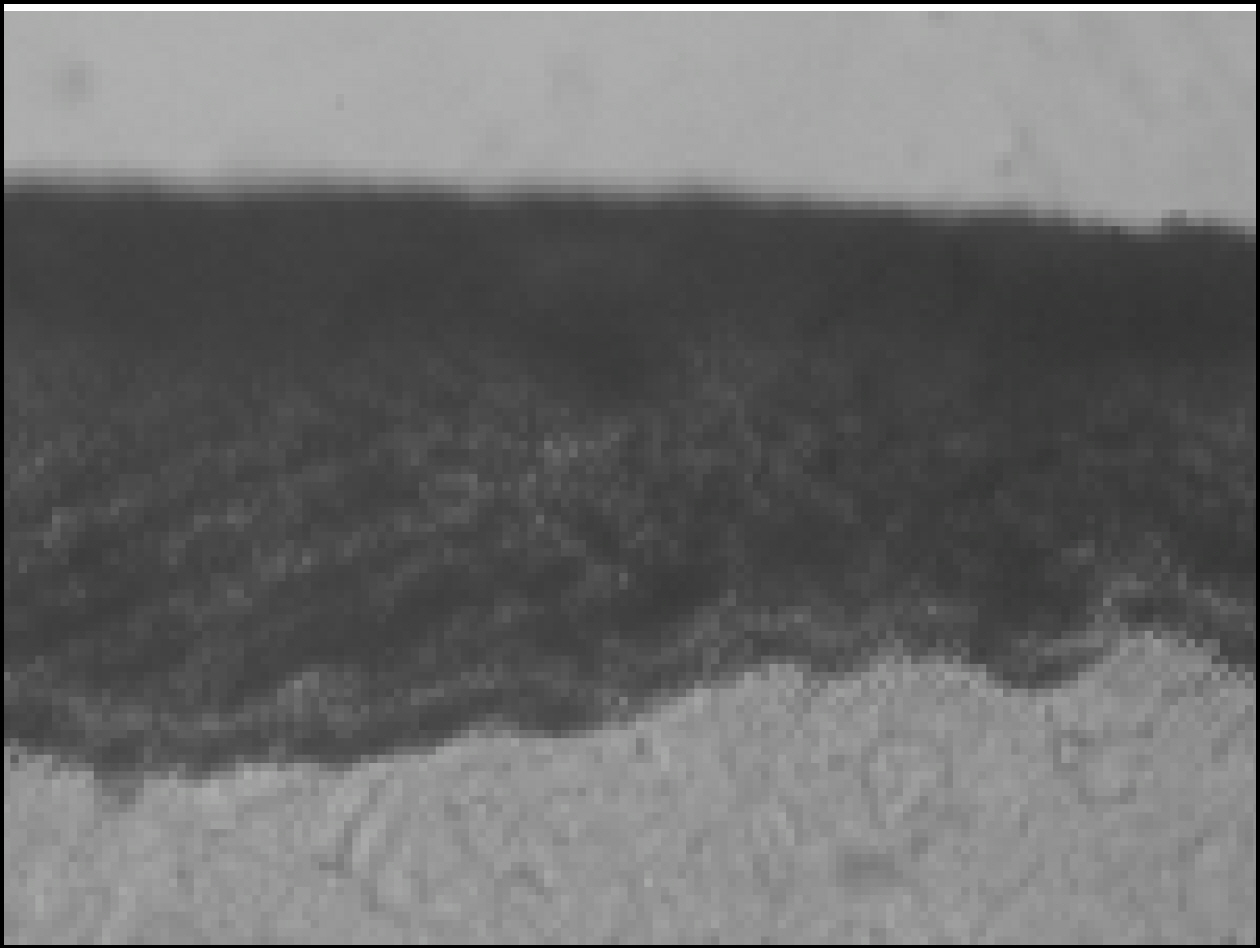
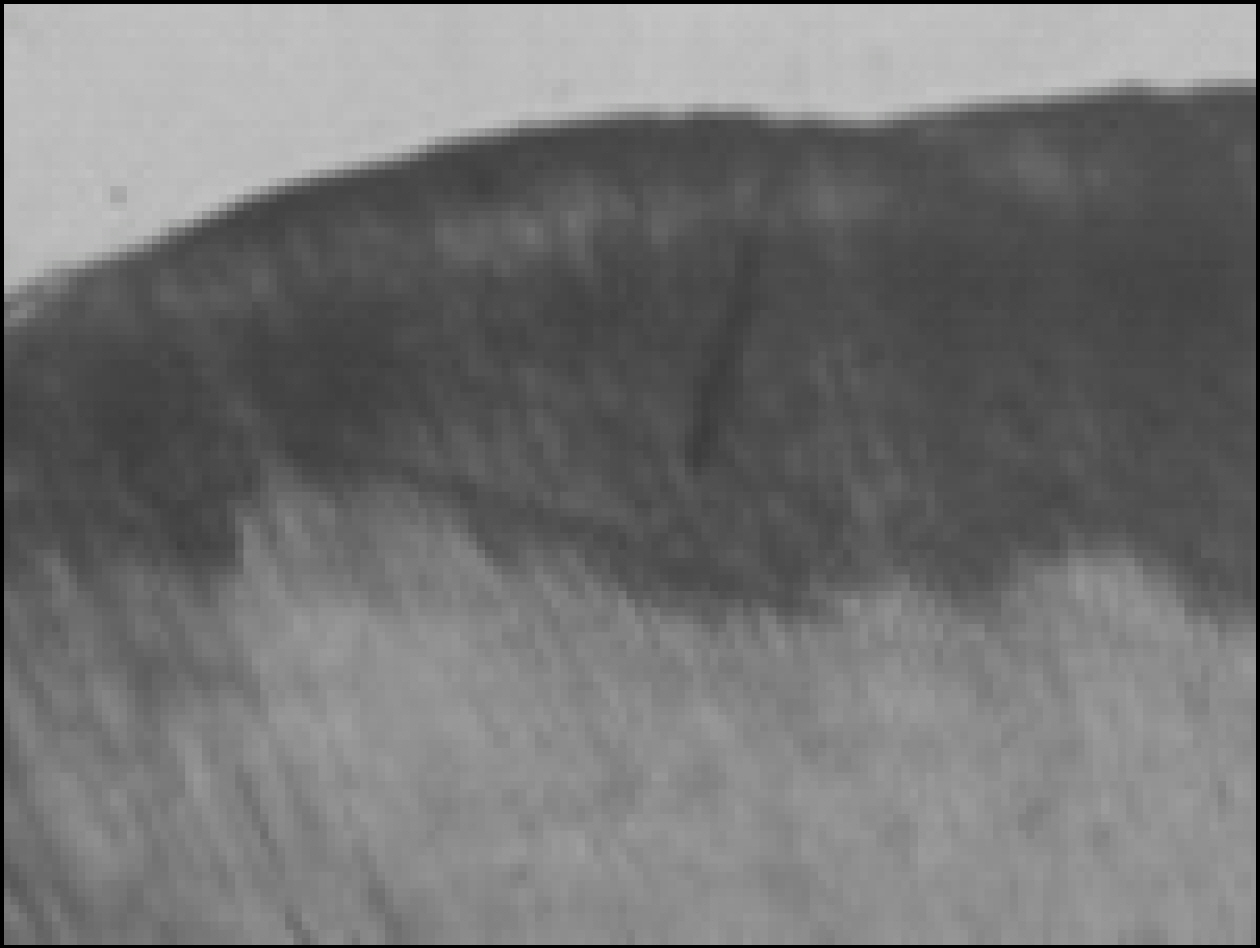
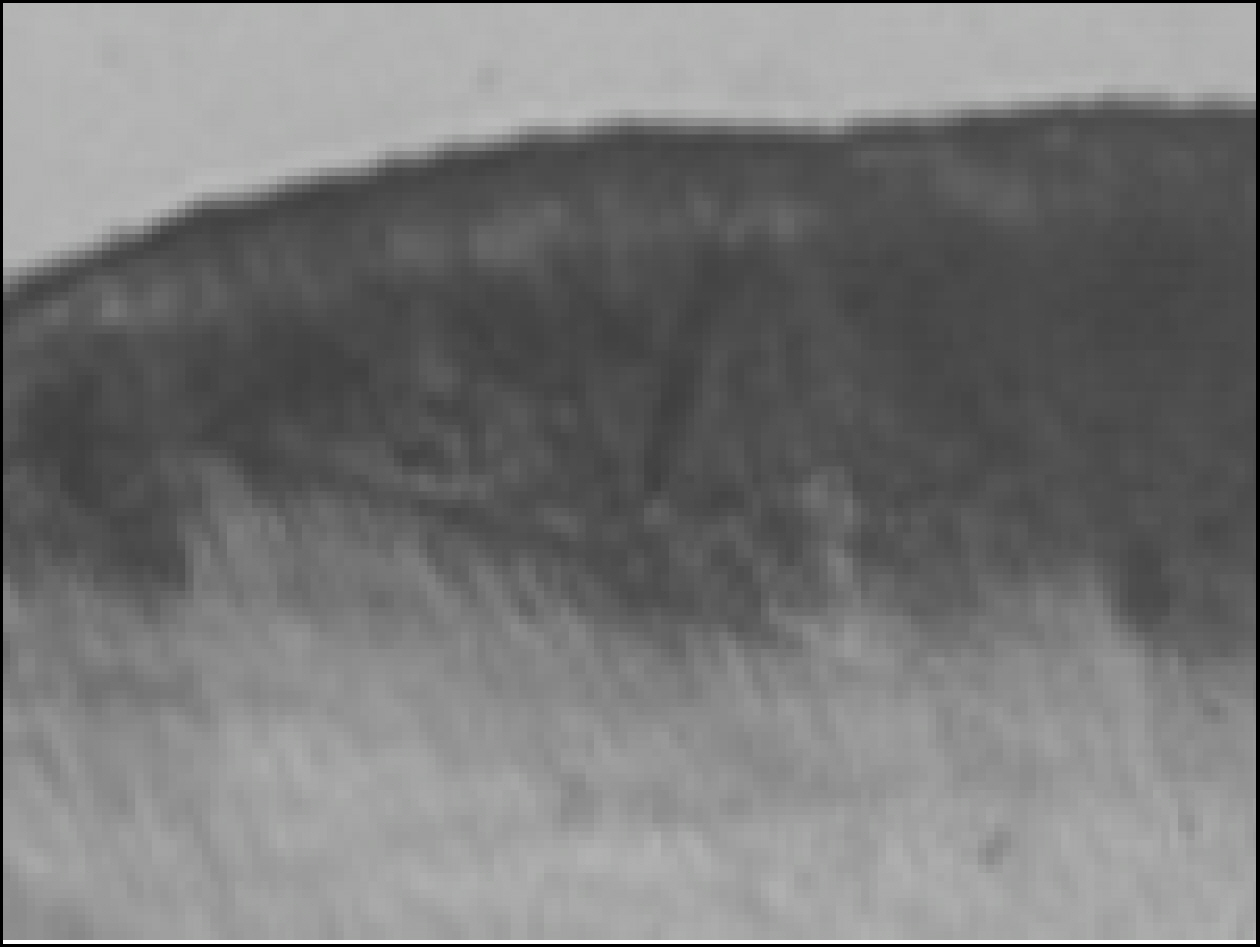
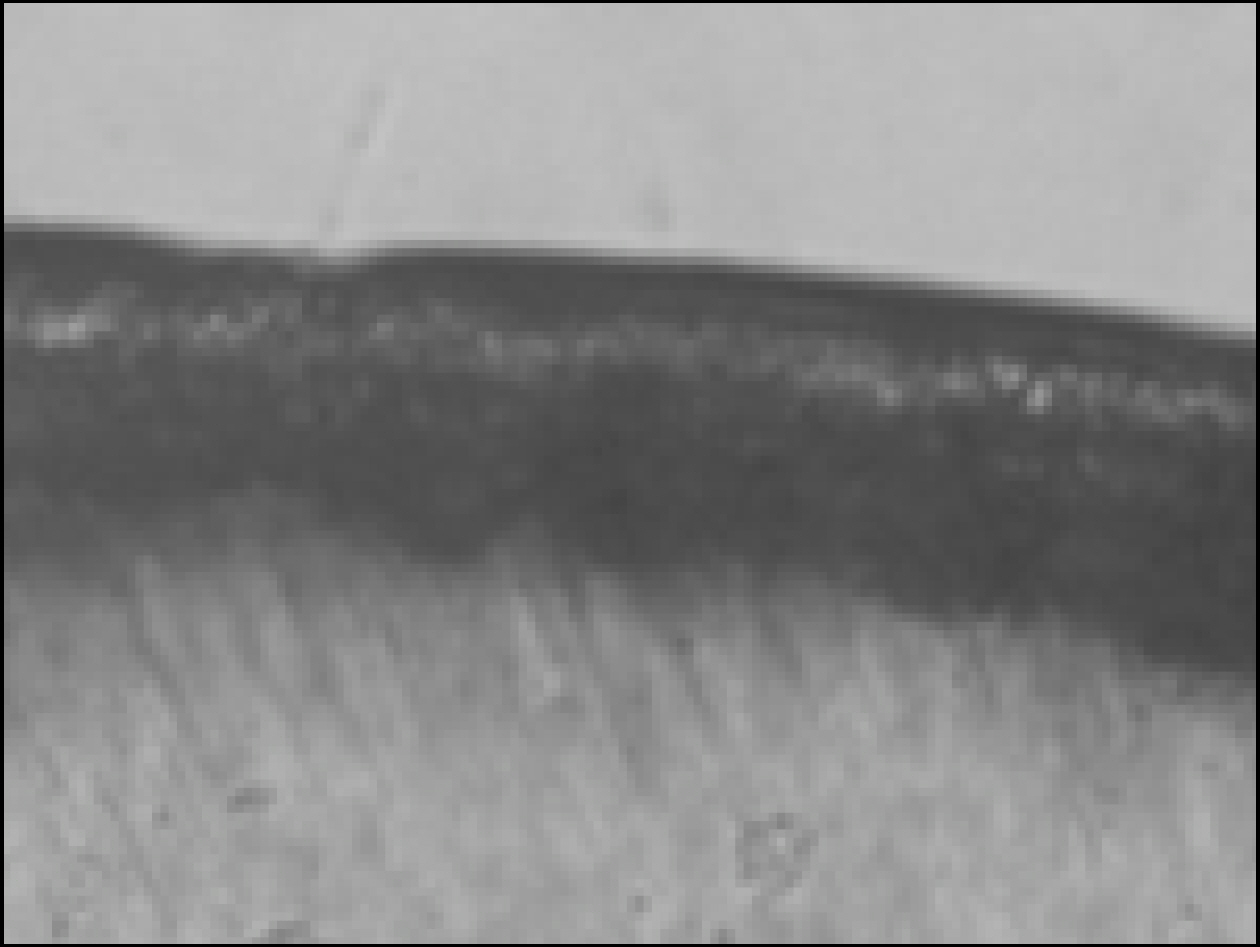
Figure 2. Polarizing microscopic observation of demineralized enamel (Group1, × 100)
Figure 3. Polarizing microscopic observation of remineralized enamel (Group1, × 100)
Figure 4. Polarizing microscopic observation of demineralized enamel (Group 2, × 100)
Figure 5. Polarizing microscopic observation of remineralized enamel (Group 2, × 100)
Figure 6. Polarizing microscopic observation of demineralized enamel (Group 3, × 100)
Figure 7. Polarizing microscopic observation of remineralized enamel (Group 3, × 100)
Figure 8. Rate of change of surface lesion width at Group 1, 2, 3 ((width of surface lesion after remineralization / width of surface lesion before remineralization) × 100(%)). Horizontal bars represent no statistically significant differences (P > .05).
Figure 9. Comparison of mineral density in enamel before and after remineralization (Group 1)
Figure 10. Comparison of mineral density in enamel before and after remineralization (Group 2)
Figure 11. Comparison of mineral density in enamel before and after remineralization (Group 3)
Figure 1.
Figure 2.
Figure 3.
Figure 4.
Figure 5.
Figure 6.
Figure 7.
Figure 8.
Figure 9.
Figure 10.
Figure 11.
THE DYNAMIC CHANGE OF ARTIFICIALLY DEMINERALIZED ENAMEL BY DEGREE OF SATURATION OF REMINERALIZATION SOLUTION AT pH 4.3
| Composition | Concentration |
|---|---|
| Lactic acid (mM) | 100 |
| Calcium (mM) | 15.5 |
| Phosphate (mM) | 8.5 |
| Sodium azide (mM) | 3.08 |
| pH | 4.3 |
| Composition | Group |
||
|---|---|---|---|
| 1 | 2 | 3 | |
| Lactic acid (mM) | 10.00 | 10.00 | 10.00 |
| Calcium (mM) | 19.98 | 27.56 | 33.17 |
| Phosphate (mM) | 7.94 | 10.25 | 12.52 |
| Sodium azide (mM) | 3.08 | 3.08 | 3.08 |
| Fluoride (ppm) | 2.00 | 2.00 | 2.00 |
| pH | 4.30 | 4.30 | 4.30 |
| Degree of saturation | 0.22 | 0.30 | 0.35 |
| Condition | Demineralized | Surface lesion | Mineral |
|---|---|---|---|
| Depth (%) |
Width (%) |
Change (%) | |
| Group | (Mean ±S.D.) | (Mean ±S.D.) ( | (Mean ±S.D.) |
| 1 | 115.1 ±11.4 % | 109.8 ±3.8 % | |
| 2 | 112.6 ±7.9 % | 101.9 ±2.2 % | |
| 3 | 111.4 ±6.5 % | 102.7 ±4.7 % |
Table 1. Initial composition of demineralization solution
Table 2. Initial composition of remineralization solution
Table 3. Rate of change of quantitative value during demineralization & remineralization at enamel

 KACD
KACD


 ePub Link
ePub Link Cite
Cite

