Abstract
-
The purpose of this study was to prove that an intermediate resin layer (IRL) can increase the bond strength to dentin by reducing the permeability of single-step adhesives.
Flat dentin surfaces were created on buccal and lingual side of freshly extracted third molar using a low-speed diamond saw under copious water flow. Approximately 2.0 mm thick axially sectioned dentin slice was abraded with wet #600 SiC paper. Three single-step self-etch adhesives; Adper Prompt L-Pop (3M ESPE, St Paul, MN, USA), One-Up Bond F (Tokuyama Corp, Tokyo, Japan) and Xeno III (Dentsply, Konstanz, Germany) were used in this study. Each adhesive groups were again subdivided into ten groups by; whether IRL was used or not; whether adhesives were cured with light before application of IRL or not; the mode of composite application.
The results of this study were as follows;
1. Bond strength of single-step adhesives increased by an additional coating of intermediate resin layer, and this increasement was statistically signigicant when self-cured composite was used (p < 0.001).
2. When using IRL, there were no difference on bond strengths regardless the curing procedure of single-step adhesives.
3. There were no significant difference on bond strengths between usage of AB2 or SM as an IRL.
4. The thickness of hybrid layer was correlated with the acidity of adhesive used, and the nanoleakage represented by silver deposits and grains was examined within hybrid and adhesive layer in most of single-step adhesives.
5. Neither thickness of hybrid layer nor nanoleakage were related to bond strength.
-
Keywords: Intermediate resin layer; Bond strength; Permeability; Single-step adhesives; Composite; Acidity
I. INTRODUCTION
Currently used adhesives can be grouped into two categories according to their etching technique; total-etch or self-etch systems, although they are usually classified under the number of generation as 4
th to 7
th. Simplified adhesives, recently introduced, are preferred by widespread dentists for the easy bonding step and less time-consuming bonding. Also, the self-etching adhesives with simplified bonding steps do not require rinsing and can decrease the technical sensitivity due to the discrepancy between demineralization and resin infiltration associated with the use of total-etching system that needs wet-bonding technique. Depending on the number of steps involved in the adhesive application to the tooth substrate, the self-etch system can be further divided into two subgroups; two-step and single-step systems which combines two components before application, so called "all-in-one" systems
1-
4).
However, aside from the convenient use of single-step adhesives, they are reported incompatible with some composites with self-cure and dual-cure mode. In the first report
5) for incompatibility between self-cured composite and two-step total-etching adhesives, it was suggested that the chemical reaction might occur between the uncured, acidic resin monomers from the oxygen-inhibited layer of the adhesive and the initiator components such as basic amine in self-cured composite. This is named as the redox reaction
6). Three-step adhesive provides a layer of neutral bonding resin between the primer and the composite so that there will be no acid-base reaction in the intermixed zone and never have any compatibility issues with overlying composites. However, two-step total-etching adhesives and single-step adhesives do not provide this neutral layer because the bonding resin is mixed with the acidic monomers
7,
8).
The other mechanism for the issue of incompatibility between the adhesive and resin composite is explained as a result from the permeability of single-step adhesive. Single-step self-etching adhesives are even more acidic in nature by virtue of their self-etching capability
9,
10). Although single-step adhesives have a potential of incompatibility with self-cured composite due to its acidity, some of them contain tertiary initiator systems
11,
12). Osmotic blistering can occur if the cured adhesive layer acts as a semi-permeable membrane that was proven by delayed activation of light-cured composite
11,
13). There was a highly significant drop in tensile bond strength for all single-step adhesives when the light-cured composite was placed on the top of the cured adhesive and left in the dark for 20 minutes before activation, but no drop were shown in employed three-step total-etching adhesives. Thus, the drop in bond strength could not be explained by the adverse reaction between acidic resin monomers and aromatic tertiary amines used in self-cured composite. Oxygen-inhibited layer on the top of self-etching adhesive layer on the dentin surface contains many dissolved ionic species such as Ca
2+ and PO
43- in addition to acidic monomers, water and other solvent from the adhesive itself, as a result of the demineralization of the tooth during application of the acidic adhesive. The water remaining in the cured adhesive layer will serve as a water channel/water trees
14). The water and the ionic species in the oxygen-inhibited layer (high solute zone), sitting on top of the cured adhesive as a semipermeable membrane, over the hydrated dentin (low solute zone) will create an osmotic pressure gradient between the uncured layer and the dentin, providing an ideal condition for osmosis. The water that diffuses into the intermixed zone is trapped by the overlying hydrophobic composites, resulting in water blisters. This phenomenon is called osmotic blistering. Osmotic blistering of water droplets along the surface of the cured adhesive layer and emulsion polymerization of immersible resin components probably accounts for the compromised bond strength in single-step self-etching adhesives after delayed activation of light-cured composites
13). Cheong et al.
15) reported that transmission electron microscopy (TEM) revealed signs of frank composite uncoupling along the adhesive-composite interface, which may be attributed to the adverse chemical interaction between the acidic adhesive and the composite for single-step self-etching adhesives bonded to the self-cured composite. In addition, "water trees" that represent channels of increased permeability with the polymerized adhesive layer were also observed in the single-step self-etching adhesives. Both features were absent along the resin-dentin interfaces when self-cured composites were coupled to the two-step self-etching adhesives. Carvalho et al.
16) reported that the placement of an intermediate resin layer (IRL) was beneficial to increase bond strength for the coupling of adhesive resin cements that utilize self-etching primer to dentin.
Therefore the hypothesis of this study was that the permeability of single-step self-etching adhesives can be reduced if the IRL is applied between the adhesive and composite. The purpose of this study was to examine that an additional coat of intermediate hydrophobic resin can increase the bond strength to dentin by reducing the permeability of single-step adhesives and to determine the application mode of IRL. The micro-shear bond testing and Transmission Electron Microscope (TEM) examination for the adhesive interfaces were performed.
II. MATERIALS AND METHODS
Flat dentin surface were created on buccal and lingual side of freshly extracted third molar using a low-speed diamond saw (Isomet, Buehler Ltd, Lake Bluff, IL, USA) under copious water. Approximately 2.0mm thick axially sectioned dentin slice was abraded with wet #600 SiC paper.
1. Experimental Design
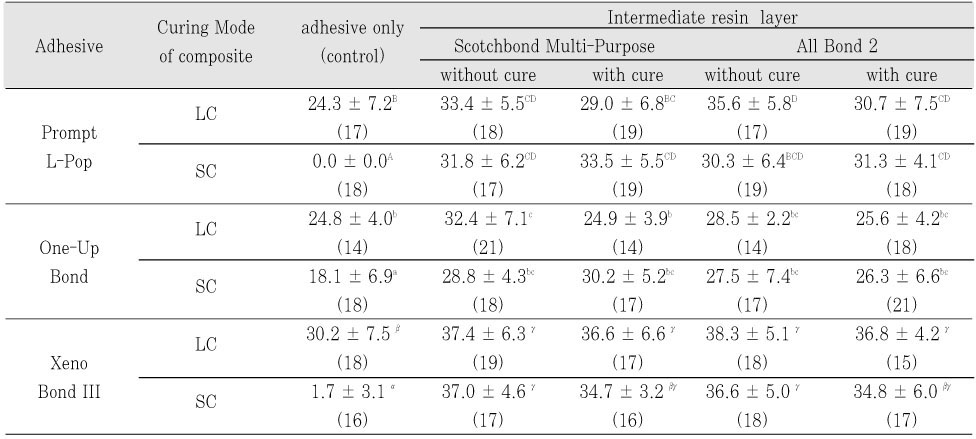
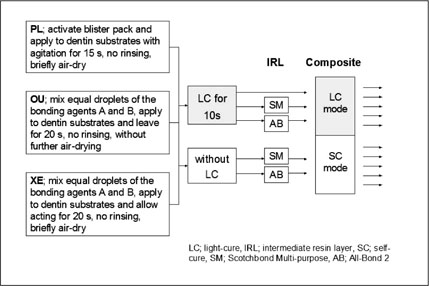
Three single-step self-etch adhesives (Adper Prompt L-Pop; PL, One-Up Bond F; OU, and Xeno III; XE) were used in this study (
Table 1). Each adhesive was divided into ten groups according to the use of IRL, the mode of composite applied, and the light-cure of adhesive before IRL application. Each adhesive was used according to the manufacturer's instructions as the control group. For experimental groups, two kinds of bonding resins, All-Bond 2 (AB2) and Scotchbond Multi-Purpose (SM) were used as IRL over single-step adhesives applied. Two experimental groups were further divided into four subgroups regarding whether an adhesive is light-cured before the application of IRL or not (
Figure 1).
A dual cure hybrid composite, Bis-Core was used. Coupling of composite to adhesive or/and IRL applied was performed under two different modes of activation. For self-cure mode, same amount of both composite from the base and the catalyst syringe of Bis-Core was hand mixed for 20 seconds under ambient light. For light-cured mode, only the base syringe, that is, a light-cured composite was used.
Composite was filled into an iris of Tygon tube (TYG-030, Small Parts Inc., Miami Lakes, FL, USA) with an internal diameter and a height of approximately 0.75 and 1.0mm, respectively. Tygon tube filled with composite was bonded to the dentin surface. Light-cured composite was cured for 20 seconds and self-cured composite was leaved for 30 minutes to be cure without disturbance. Four to six resin cylinders were attached on each dentin surface and 15 to 21 specimens were made for each experimental groups. Specimens were stored in water at 37℃ for 1 day. Tygon tube was removed with scalpel blade prior to testing. If resin cylinder had apparent interfacial gap formation, bubble inclusion, and any other defects, it was excluded from this study.
After storage of the specimens, micro-shear bond testing was performed. The dentin slice with resin cylinder was adhered to the testing device (jig) placed in a Universal testing machine (EZ-test; Shimadzu, Kyoto, Japan) with a cyanoacrylate adhesive. The tooth-resin interface for the test, the axis of rod, and the center of load cell were aligned as rectilinear as possible to ensure the desired orientation in the shear test force. A ligature wire (0.2mm in diameter) was looped around the resin cylinder to make contact through half its circumference, and gently held wire to contact the bonded surface. A shear force was applied to each specimen at a cross-head speed of 1mm/min until failure occurred. Fourteen to twenty one specimens were tested for each test group. The results of bond strength were statistically analyzed using one-way ANOVA and multiple comparisons were made using Tukey's test the 95% confidence level.
3. Nanoleakage Evaluation using TEM
To avoid damage to the diamond knife by glass fillers from hybrid composites, those composites were replaced with dual cure, microfilled experimental composite (Bisco Schaumburg, IL, USA). These composites were mixed and overlaid on the bonded dentin surface as before.
After each storage period, specimens were vertically, serially sectioned in the bucco-lingual direction into 0.9mm thick slabs using a diamond impregnated saw (Isomet, Buehler Ltd., Lake Bluff, IL, USA) under water lubrication. Four slabs were obtained from each tooth, forming a total of 8 specimens per group. Bonded slabs were coated with two layers of nail varnish applied up to within 1mm of the bonded interfaces. In order to rehydrate specimens, they were immersed in distilled water for ten minutes prior to immersion in the tracer solution for 24 hrs. Ammoniacal silver nitrate is prepared according to the protocol previously described by Tay et al.
17). Tooth slabs were placed in the ammoniacal silver nitrate in total darkness for 24 hrs, then rinsed thoroughly in distilled water, and immersed in photo-developing solution for 8 hrs under a fluorescent light to reduce silver ions into metallic silver grains within potential voids along the bonded interface.
Undemineralized, epoxy-resin embedded, ultrathin sections were prepared for TEM. One strip approximately 6 mm wide was sectioned from each slab perpendicular to the flat dentin surface using a diamond disk under copious water supply. Specimens were fixed in Karnovsky's solution, post-fixed in osmium tetroxide, dehydrated in ascending ethanol series (30 to 100%) and embedded in epoxy resin. Care was taken to ensure proper orientation of the resin-dentin interface. After initial screening of all semithin sections from each group, representative 90mm thick ultrathin sections were prepared with an ultramicrotome (MT-2C, RMC, FL, USA) using a diamond knife (Diatome, Biel/ Bienne, Switzerland) and collected on 100-mesh formvar-coated copper grids. Without additional staining, they were observed in a transmission electron microscope (Zeiss EM 900, Zeiss, Munich, Germany).
III. RESULTS
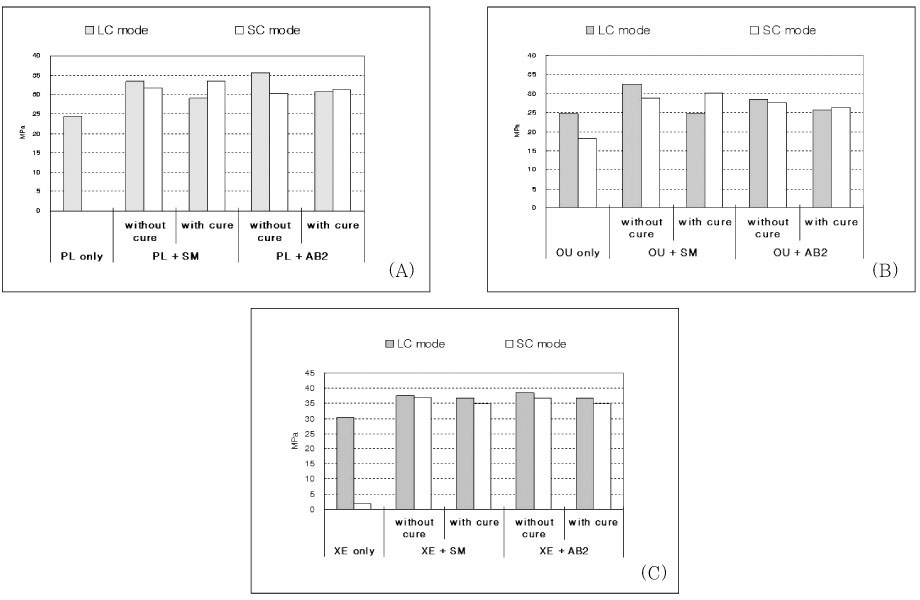
The bond strength of the six control groups and twenty four experimental groups are represented in
Table 2 and
Figure 2. Two-way ANOVA revealed that the bond strength results were significantly influenced by the adhesive type (p < 0.001), composite curing mode (p < 0.001), and the use of IRL (p < 0.001).
Tukey's multiple comparison tests showed that for the groups using PL, there was a statistically significant difference between manufacturer's recommended bonding protocol (PL only) and the additional use of a coating of IRL prior to placement of both light-cured and self-cured composites (p < 0.05, except for light-cured composite with SM after adhesive cured). The bond strength of only PL group with self-cured composite was zero, which means they were never bonded to each other. There was no significant difference among all of groups using IRL if the adhesive was previously cured or not.
The bond strength of OU coupled with self-cured composite was higher than other different adhesives used as recommended bonding protocol (adhesive only). For groups using OU, the bond strength with self-cured composite was significantly increased by applying IRL. The cure of OU before applying SM as IRL made bond strength significantly decrease when light-cured composite was bonded (p < 0.01).
For the groups using XE, the bond strength was significantly increased by the additional application of IRL before placement of both light-cured and self-cured composites (p < 0.05). There was no significant difference between the results of all groups using IRL if the adhesive was cured or not and even bonded with light-cured or self-cured composite.
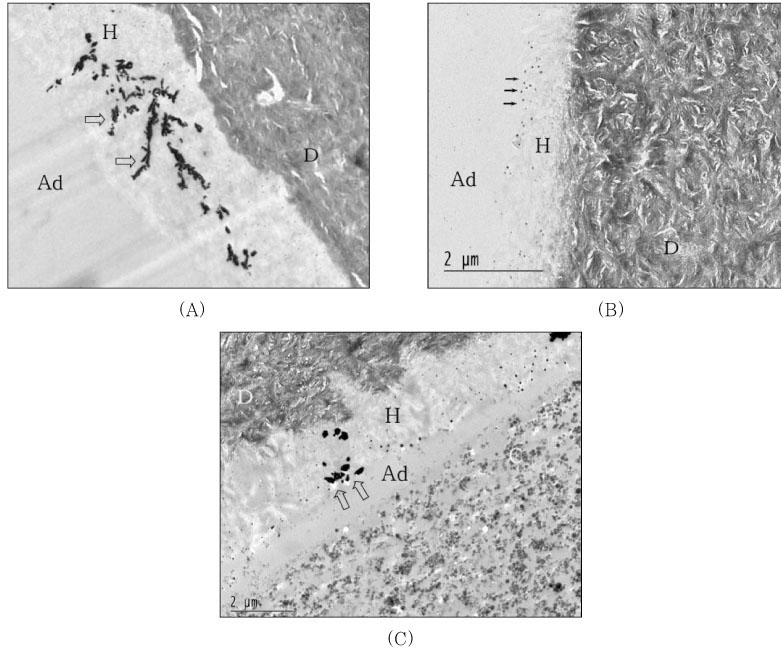
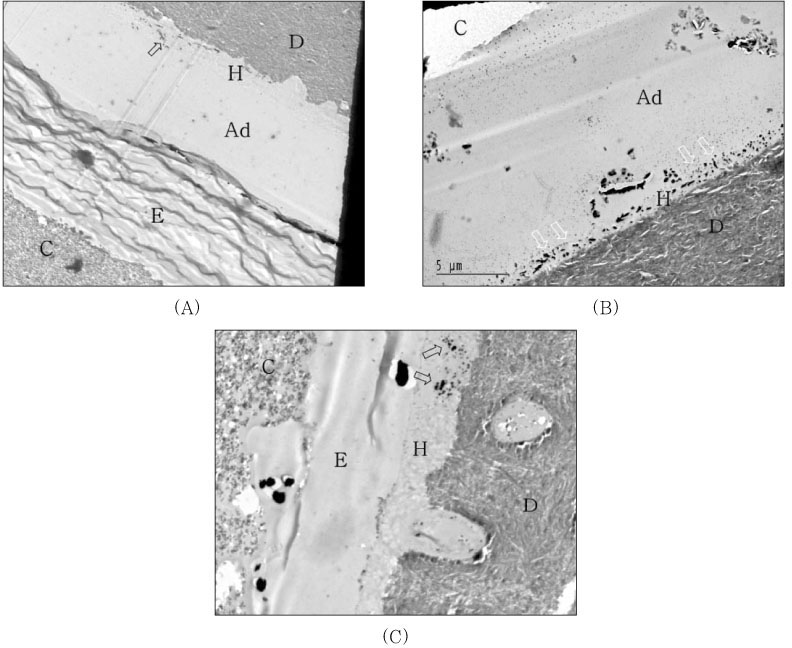
For undemineralized, unstained TEM micrographs, most of the resin-dentin interfaces of the single-step adhesives revealed the presence of silver deposits and grains varied from moderate to severe within the hybrid layer and adhesive layer. When light-cured composite was coupled with each adhesive only, hybrid layer thickness was approximately 5µm and silver deposits were identified within all hybrid layer thickness and water-tree appearance were showed for PL (
Figure 3A). For OU group, hybrid layer was very thin approximately 1.0 to 1.5µm and the isolated tiny silver grains were scattered at the top and within hybrid layer (
Figure 3B). Not much of silver deposits and grains were observed at the top of hybrid layer and adhesive layer of XE was very thinner than other adhesive groups (
Figure 3C). When self-cured composite was bonded, epoxy resin infiltrated into detached interface between the adhesive and composite layer before TEM preparation for PL (
Figure 4A). For OU group, the amount of silver deposits and grains was increased within either all hybrid layer or adhesive layer (
Figure 4B). Epoxy resin was infiltrated into debonded interface between hybrid layer and composite layer of which interface was hollowed with some void and there were some silver grains within the hybrid layer (
Figure 4C).
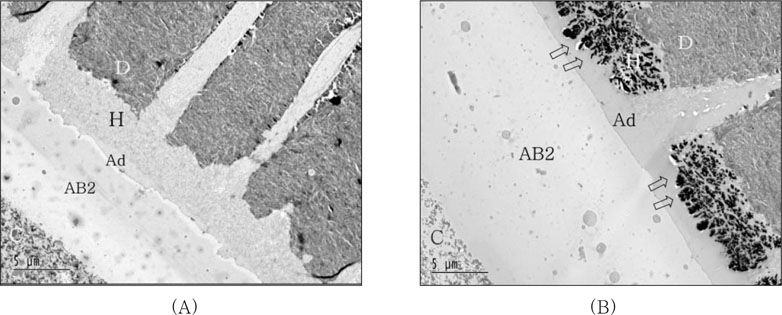
Figure 5A showed the resin-dentin interfaces of PL coupled with self-cured composite after IRL application. No silver deposit within hybrid layer was observed and the interface of adhesive layer with IRL was well demarcated when adhesive layer was not cured before IRL application. For IRL application after cure of adhesive layer, massive silver deposition was observed within hybrid layer and protruded into adhesive layer. Relatively thicker adhesive and IRL layer was observed (
Figure 5B).
For self-cured composite coupled with IRL (SM) application after curing of OU (
Figure 6), typical water-tree appearances were represented by silver impregnation into adhesive layer approximately 1.5µm from the hybrid layer. Agglomerated fluoroalumino-silicate glass particles were shown in adhesive layer and some of silver deposits were observed at dentin layer under hybrid layer in magnified image (
Figure 6B).
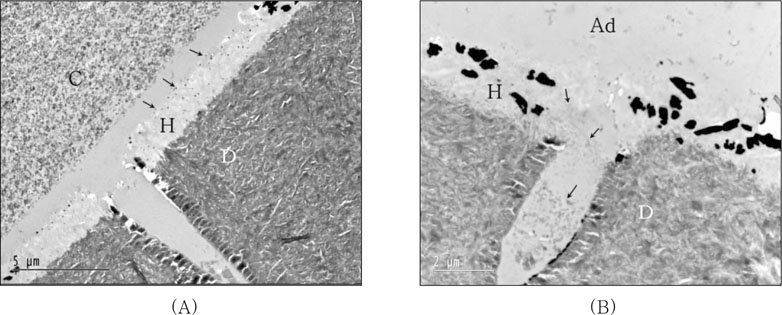
For the resin-dentin interfaces of XE bonded with light-cured composite after IRL (AB2) application without curing of adhesive layer (
Figure 7A), both well developed hybrid layer and resin tag into dentinal tubule were observed. Silver grains were observed at top of hybrid layer, and no demarcation was detected between IRL and adhesive layer due to absence of curing of adhesive layer. When IRL (SM) was applied after XE cured, silver deposition was observed within hybrid layer and protruding into adhesive layer. Notably microfiller particles were dispersed in resin tag and hybrid layer limitedly (
Figure 7B).
IV. DISCUSSION
Potential water-binding domains within hybrid layers and adhesive layers in resin-dentin interfaces are traced by ammoniacal silver nitrate
18). Differences in hydrophilicity and water content have an important role in nanoleakage patterns presented by adhesive systems. All bonding agents tested in this study contain some amount of water and hydrophilic monomers in their composition (
Table 1). The presence of water within dentin adhesives composition plays an important role in both total- and self-etching techniques. Water is an essential component in self-etching systems, in order to enable ionization of acidic monomers and demineralization of underlying enamel and/or dentin
9). Besides the presence of water in their composition, the ionizable moieties of acidic monomers are hydrophilic. The application of comparatively hydrophobic intermediate resin layer could increase the bond strength.
All adhesives studied showed a certain degree of nanoleakage. Nanoleakage was originally defined as leakage within hybrid layer that contained regions of incomplete resin infiltration which demonstrated variable degree of a reticular pattern in self-etching adhesives
19,
20). The presence of residual water within the adhesive may lead to domains of incomplete polymerization of the adhesive in the particular regions. This may account for the presence of vertical streaks of silver deposits that were seen directly above the hybrid layer.
When light-cured composite was coupled with each adhesive only, the bond strength did not show any difference among each single-step adhesives employed in this study. Hybrid layer thickness for PL was approximately 5µm and silver deposits were identified within all hybrid layer thickness and water-tree appearances were shown. For OU group, hybrid layer was very thin, approximately 1.0 to 1.5µm, and the isolated tiny silver grains were scattered at the top and within hybrid layer. For XE used, not much of silver deposits and grains were observed at the top of hybrid layer and adhesive layer was very thinner than other adhesive groups. All of the self-etching adhesives contain specially collaborated acidic functional monomer varying with acidity (pH). Acidity of PL, OU, and XE adhesives in this study is 1.3, 2.3, and 1.5 respectively
21). The thickness of hybrid layer was correlated with the acidity of these adhesives as same as previous report
9).
Conversely, for self-cured composite, the bond strength was varied from zero to 18.1 MPa. It was the highest in OU, but the lowest in PL which was not bonded to each other. Thus, epoxy resin infiltrated totally into the detached interface between adhesive and composite layer during TEM preparation for PL. Similarly, some of partially debonded interface was observed in XE specimen of which interface was hollowed with some void and there were some silver grains within the hybrid layer (
Figure 4C). As previously mentioned, some of single-step adhesives contain ternary initiator system such as aryl sulphinate salts, barbituric acid, and tertiary butyl peroxymaleic acid in order to overcome the possible adverse effect of acidic component on the amine used with camphoroquinone
11,
12). Similar ternary catalyst (that is) contained in One-up Bond is aryl borate. This is why the bond strength of OU without IRL showed higher than any other adhesives, though the amount of silver deposits and grains were increased within either all hybrid layer or adhesive layer.
The bond strength results were significantly influenced by the adhesive type, composite curing mode and the use of IRL, which was revealed by two-way ANOVA. Tukey's multiple comparison tests further showed that there was a statistically significant difference between manufacturer's recommended bonding protocol (PL only) and the additional use of a coating of IRL prior to placement of both light-cured and self-cured composites for the groups using PL (except for light-cured composite with SM after adhesive cured). These results were supported by TEM examination. Coupling PL with self-cured composite after IRL application, no silver deposits within hybrid layer were observed and the interface of adhesive layer with IRL was well demarcated when adhesive layer was not cured before IRL was applied. Although there was no significant difference among all of groups using IRL if adhesive was cured previously or not, massive silver deposition was observed within hybrid layer and protruded into adhesive layer and relatively thicker adhesive and IRL layer were shown for IRL application after curing of adhesive (
Figure 5B).
For groups using OU, the bond strength with self-cured composite was significantly increased by applying IRL. The cure of OU before applying IRL (SM) made bond strength significantly decrease when light-cured composite was bonded. From TEM examination, typical water-channel was observed by silver impregnation into adhesive layer approximately 1.5µm from the hybrid layer. These vertical streaks of silver deposit in One-up Bond were as same as what Tay et al.
18) observed. Also, agglomerated glass particles were shown in adhesive layer and some of silver deposits were observed at dentin layer under hybrid layer in magnified image (
Figure 6B).
The bond strength of XE was significantly increased by the additional use of IRL before placement of both light-cured and self-cured composites. There were no significant difference between the results of all groups using IRL regardless whether the adhesive was cured or not and even coupled with light-cured or self-cured composite. Bonded with light-cured composite after IRL (AB2) applied without cure of XE (layer), both well developed hybrid layer and resin tag into dentinal tubule were observed. Silver grains were observed at top of hybrid layer, and no demarcation was detected between IRL and adhesive layer due to without cure of adhesive layer (
Figure 7A). When IRL (SM) was applied after XE cured, silver deposition was observed within hybrid layer and protruding into adhesive layer. Microfiller particles of adhesive were dispersed in resin tag and hybrid layer limitedly (
Figure 7B). This result is somewhat different from a previous reported by Tay et al.
17) that the nanofillers could not infiltrated into interfibrillar spaces of hybrid layer since aggregation of filler and retention of ground substance.
From the results of this study, the permeability of single-step adhesive layer was reduced with use of IRL, consequently its bonding procedure resulted in the same as two-step self-etching systems. Thicker adhesive layer resulted from additional application may also have contributed to the relief of contraction stresses in non-compliant adhesive joints as well as increase of bond strength
22). Recently, Reis et al.
23) reported that two-step total-etch and single-step systems were more prone to nanoleakage than two-step self-etching systems that presented the lowest degree of nanoleakage, which was almost same with the result of other previous study.
Ideally, an adhesive system should provide adequate bond strength and sealing of dentin surfaces and be long-lasting. Water sorption by hydrophilic resin monomers within both the hybrid layer and the adhesive layer has been thought to contribute to the degradation of resin-dentin bond strength over time
24). This phenomenon is aggravated by the incorporation of increased concentrations of hydrophilic resin components into contemporary self-etch adhesives
25), since hydrophilicity and hydrolytic stability of resin monomers are generally antagonistic properties. Long-term water storage of hydrophilic resin blends such as those employed in dentin adhesive, resulted in a marked reduction in their mechanical strength that may compromise the durability of resin-dentin bonds
26). Water-rich domains, as represented by the reticular mode of nanoleakage expression and manifested as self-propagating water trees along the adhesive-dentin junction, may result in a rapid deterioration of mechanical properties of adhesive along this region, resulting in adhesive failure along the surface of the hybrid layer. All of two-step self-etching adhesive showed a bonding durability in short-term evaluation in our previous study
28). A recent
in vivo study showed that deterioration of resin-dentin bonds occurs predominantly via the leaching of resins instead of degradation of collagen fibrils within the hybrid layer
29). To date, the routine use of simplified adhesive systems in combination with resin composites to restored cavities with exposed dentin margins is a questionable recommendation30).
Next thing to take interest in should be the bonding durability of single-step self-etching adhesives though the bond strength was increased by the application of an IRL. The result of initial bond strength may not be a predictable criteria for successful adhesion. Although we used two commercial bonding resins that contain hydrophilic monomer (HEMA) in this study, the hydrocity of IRL layer may affect both the adhesive strength and the durability.
V. CONCLUSION
The bond strength of single-step adhesive to resin composite was improved by applying an intermediate resin layer to reduce the permeability of adhesive layer. This means that single-step adhesive can not be used in multi-purpose clinically, and consequently similar to the procedure of two-step self-etching adhesive. Therefore, the choice of simplified adhesive seduced by time-saving procedures and easy application should be meditated.
REFERENCES
- 1. Kanca J. Improving bond strength through acid etching of dentin and bonding to wet dentin surfaces. J Am Dent Assoc. 1992;123: 35-42.ArticlePubMed
- 2. Bertolotti R. total-etch the rational dentin bonding protocol. J Esthet Dent. 1991;3: 1-6.ArticlePubMed
- 3. van Meerbeek B, Perdigao J, Lambrechts P, Vanherle G. The clinical performance of adhesives. J Dent. 1998;26: 1-20.ArticlePubMed
- 4. Frey O. Creating a reliable bond. An all-in-one system. Am J Dent. 2000;85D-87D.PubMed
- 5. Swift EJ Jr, Perdigao J, Combe EC, Simpson CH 3rd, Nunes MF. Effects of restorative and adhesive curing methods on dentin bond strengths. Am J Dent. 2001;14: 137-140.PubMed
- 6. Sanares AM, Itthagarun A, King NM, Tay FR, Pashley DH. Adverse surface interactions between one-bottle light-cured adhesives and chemical-cured composites. Dent Mater. 2001;17: 542-556.ArticlePubMed
- 7. Schiltz MY, Suh BI. Effect of pH of single-bottle adhesives on shear bond strength. J Dent Res. 2001;80(special issue):50.
- 8. Tay FR, Pashley DH, Suh BI, Carvalho RM, Itthagarun A. Single-step adhesives are permeable membranes. J Dent. 2002;30: 371-382.ArticlePubMed
- 9. Tay FR, Pashley DH. Aggressiveness of contemporary self-etching systems. I: Depth of penetration beyond dentin smear layers. Dent Mater. 2001;17: 296-308.PubMed
- 10. Pashley DH, Tay FR. Aggressiveness of contemporary self-etching adhesives. Part II: etching effects on unground enamel. Dent Mater. 2001;17: 430-444.PubMed
- 11. Tay FR, Pashley DH, Yiu CK, Sanares AM, Wei SH. Factors contributing to the incompatibility between simplified-step adhesives and chemically-cured or dual-cured composites. Part I. Single-step self-etching adhesive. J Adhes Dent. 2003;5: 27-40.PubMed
- 12. Tay FR, Suh BI, Pashley DH, Prati C, Chuang SF, Li F. Factors contributing to the incompatibility between simplified-step adhesives and chemically-cured or dual-cured composites. Part II. Single-bottle, total-etch adhesive. J Adhes Dent. 2003;5: 91-105.PubMed
- 13. Tay FR, King NM, Suh BI, Pashley DH. Effect of delayed activation of light-cured resin composites on bonding of all-in-one adhesives. J Adhes Dent. 2001;3: 207-225.PubMed
- 14. Tay FR, Pashley DH. water-treeing- A potential mechanism for degradation of dentin adhesives. Am J Dent. 2003;16: 6-12.PubMed
- 15. Cheong C, King NM, Pashley DH, Ferrari M, Toledano M, Tay FR. Incompatibility of self-etch adhesives with chemical/dual-cured composites: two-step vs one-step systems. Oper Dent. 2003;28: 747-755.PubMed
- 16. Carvalho RM, Pegoraro TA, Tay FR, Pegoraro LF, Silva NR, Pashley DH. Adhesive permeability affects coupling of resin cements that utilise self-etching primers to dentine. J Dent. 2004;32: 55-65.ArticlePubMed
- 17. Tay FR, Moulding KM, Pashley DH. Distribution of nanofillers from a simplified-step adhesive in acid-conditioned dentin. J Adhes Dent. 1999;1: 103-117.PubMed
- 18. Tay FR, Pashley DH, Yoshiyama M. Two modes of nanoleakage expression in single-step adhesives. J Dent Res. 2002;81: 472-476.ArticlePubMedPDF
- 19. Sano H, Takatsu T, Ciucchi B, Horner JA, Matthews WG, Pashley DH. Nanoleakage: leakage within the hybrid layer. Oper Dent. 1995;20: 18-25.PubMed
- 20. Sano H, Yoshiyama M, Ebisu S, Burrow MF, Takatsu T, Ciucchi B, Carvalho R, Pashley DH. Comparative SEM and TEM observations of nanoleakage within the hybrid layer. Oper Dent. 1995;20: 160-167.PubMed
- 21. Suh BI. Current issues in adhesion dentistry; Classification, curing, compatibility and sensitivity. 2005;Bisco Inc; 26. (in Press).
- 22. Choi KK, Kim SW, Choi HY. Effect of filler addition to bonding agents on the physical properties and the bond strength to bovine teeth. 2002;The First International Congress on Adhesive Dentistry IAD; 434. Program and Abstracts.
- 23. Reis AF, Arrais CAG, Novaes PD, Carvalho RM, De Goes MF, Giannini M. Ultramorphological analysis of resin-dentin interfaces produced with water-based single-step and two-step adhesives; Nanoleakge expression. Oper Dent. 2005;in Press.
- 24. Hashimoto M, Ohno H, Kaga M, Endo K, Sano H, Oguchi H. In vivo degradation of resin-dentin bonds in humans over 1 to 3 years. J Dent Res. 2000;79: 1385-1391.ArticlePubMedPDF
- 25. Tanaka J, Ishikawa K, Yatani H, Yamashita A, Suzuki K. Correlation of dentin bond durability with water absorption of bonding layer. Dent Mater J. 1999;18(1):11-18.ArticlePubMed
- 26. Yiu CK, King NM, Pashley DH, Suh BI, Carvalho RM, Carrilho MR, Tay FR. Effect of resin hydrophilicity and water storage on resin strength. Biomaterials. 2004;25: 5789-5796.ArticlePubMed
- 27. Chang YI, Choi KK, Park SJ. Compatibility between two-step self-etching adhesives and composites with different curing mode. J Korean Acad Conserv Dent. 2005;in Press.
- 28. Sano H, Yoshikawa T, Pereira PN, Kanemura N, Morigami M, Tagami J, Pashley DH. Long-term durability of dentin bonds made with a self-etching primer, in vivo. J Dent Res. 1999;78: 906-911.ArticlePubMedPDF
- 29. Göhring TN, Schönenberger KA, Lutz F. Potential of restorative systems with simplified adhesives: Quantitative analysis of wear and marginal adaptation in vitro. Am J Dent. 2003;16: 275-282.PubMed
Figure 1Bonding protocol for each experimental group.
Figure 2Bar charts showing the micro-shear bond strength for Prompt L-Pop (A), One-up Bond (B), and Xeno III (C) according to the bonding protocols.
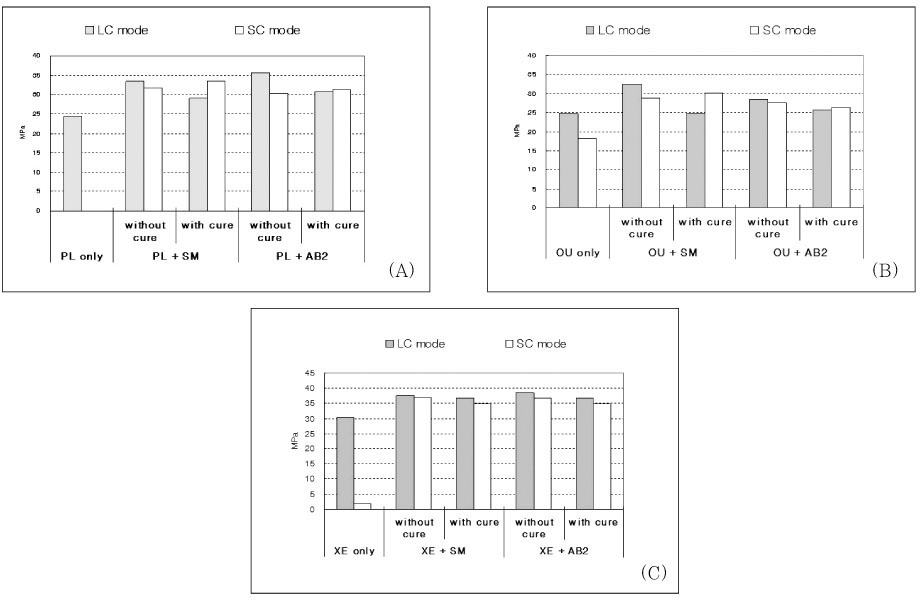
Figure 3
Undemineralized, unstained TEM micrographs of the resin-dentin interfaces of the single-step adhesives with light-cure mode composite.
(A) Prompt L-Pop; Hybrid layer (H) thickness was approximately 5µm and silver deposits were identified within all hybrid layer thickness and water-tree appearances (open arrow) were showed.
(B) One-Up Bond; Very thin hybrid layer (1.0-1.5µm) and isolated tiny silver grain scattered at the top and within hybrid layer.
(C) Xeno III; Not much of silver deposits and grains were observed at the top of hybrid layer and adhesive layer (Ad) was very thinner than other adhesive groups.
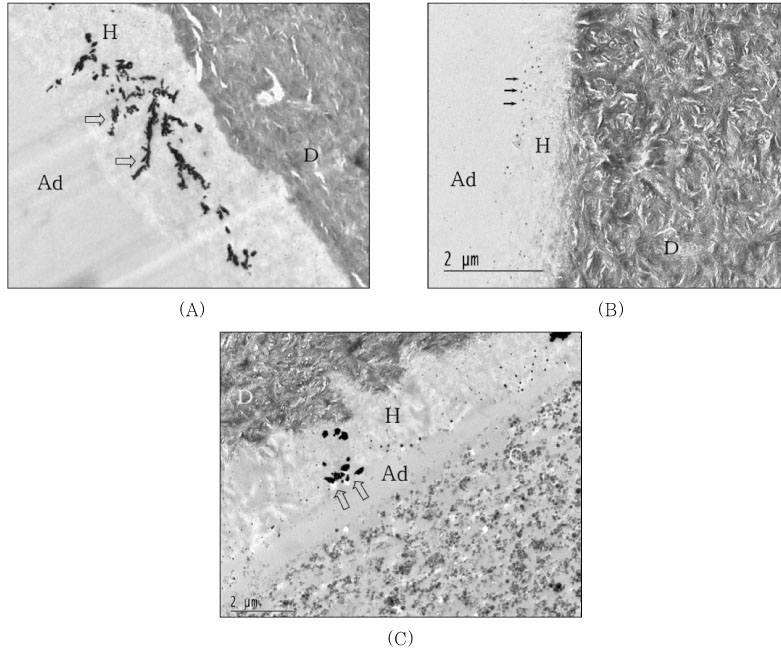
Figure 4
TEM micrographs of the resin-dentin interfaces of the single-step adhesives with self-cure mode composite.
(A) Prompt L-Pop; Epoxy resin (E) infiltrated into debonded interface between adhesive and composite layer before TEM preparation. Silver deposits were showed in hybrid layer (open arrow).
(B) One-Up Bond; The amount of silver deposits and grains increased within either all hybrid layer or adhesive layer (white arrow).
(C) Xeno III; Epoxy resin (E) infiltrated into debonded interface between hybrid layer and composite layer of which interface hollowed with some void. Note silver grains within the hybrid layer (open arrow).
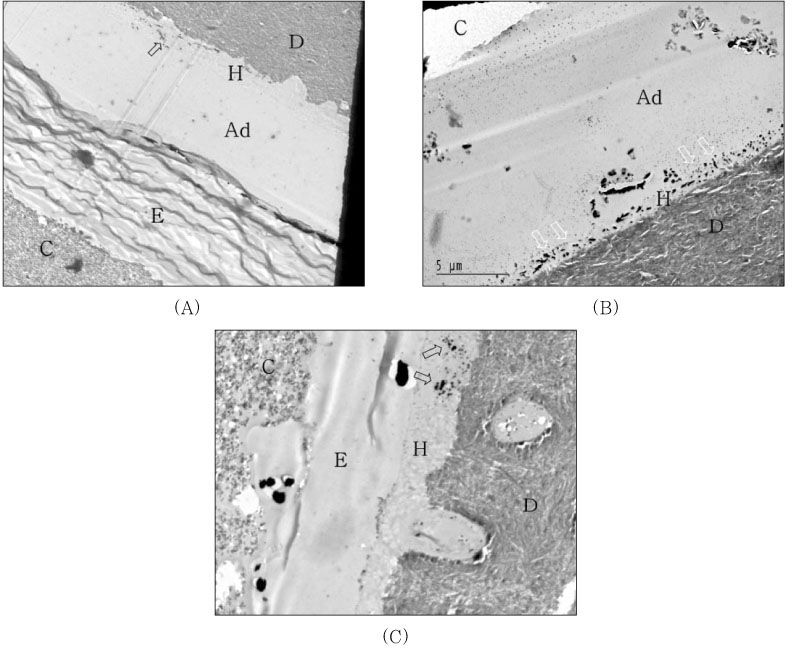
Figure 5
TEM micrographs of the resin-dentin interfaces of Prompt L-Pop bonded with self-cured composite after intermediate resin layer (IRL-AB2) applied.
(A) IRL applied without cure of adhesive layer; No silver deposit within hybrid layer was observed and the interface of adhesive layer with IRL was well demarcated.
(B) IRL applied after cure of adhesive layer; Massive silver deposition was observed within hybrid layer and protruded into adhesive layer. Relatively thicker adhesive and IRL layer than (A) was observed by means of cure previously.
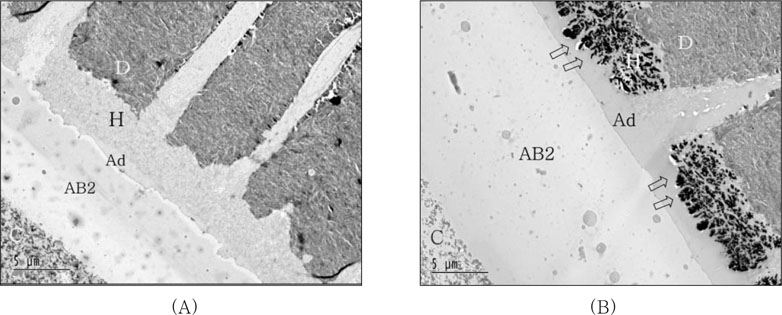
Figure 6
TEM micrographs of the resin-dentin interfaces of One-Up Bond bonded with self-cured composite after intermediate resin layer (IRL-SM) applied.
(A) and (B) IRL applied after cure of adhesive layer; Silver impregnation into adhesive layer approximately 1.5µm from the hybrid layer represented typical water-trees (arrow). Agglomerated fluoroalumino-silicate glass particles were shown in adhesive layer (open arrow). Black box area was magnified at 11,500. Some of silver deposits were observed at dentin layer under hybrid layer.
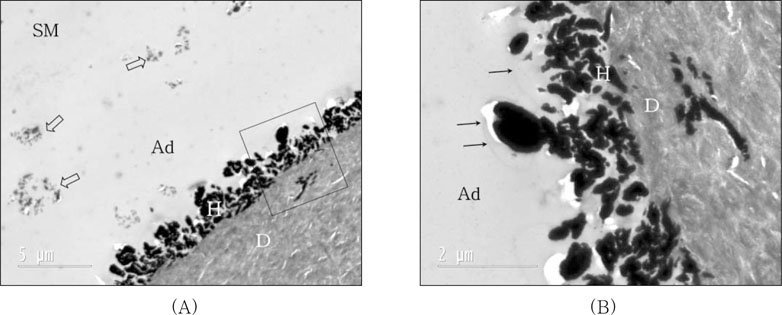
Figure 7
(A) TEM micrographs of the resin-dentin interfaces of Xeno III bonded with light-cured composite after intermediate resin layer (IRL-AB2) applied without cure of adhesive layer; Both of well developed hybrid layer and resin tag into dentinal tubule were observed. Silver grains were observed at top of hybrid layer (arrow). No demarcation was detected between IRL and adhesive layer due to without cure of adhesive layer.
(B) Xeno III bonded with light-cured composite after IRL (SM) applied after cure of adhesive layer -opklbn m,(× 7,100); Silver deposition was observed within hybrid layer and protruding into adhesive layer. Microfiller particles were dispersed in resin tag and hybrid layer limitedly (arrow).
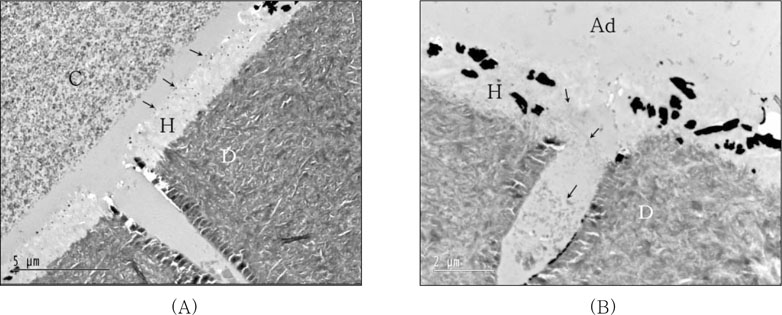
Table 1The materials used in this study
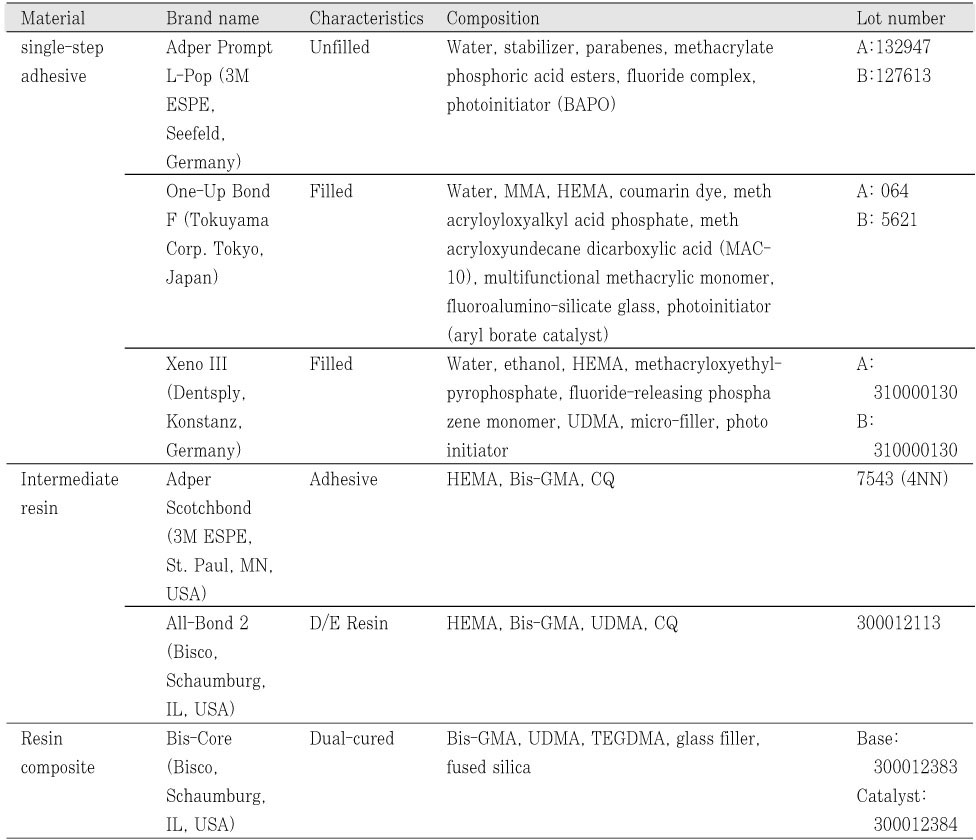
Table 2Micro-shear bond strength (MPa, mean ± SD) of single-step adhesives using intermediate resin layer











 KACD
KACD

 ePub Link
ePub Link Cite
Cite

