Abstract
-
The purpose of this study was to monitor the secretion of matrix metalloproteinase (MMP) and tissue inhibitor of metalloproteinase (TIMP) by human periodontal ligament (PDL) fibroblasts stimulated with Prevotella nigrescens lipopolysaccharide (LPS), and to examine the effect of calcium hydroxide treatment on P. nigrescens LPS.
LPS was extracted and purified from anaerobically cultured P. nigrescens. PDL fibroblasts were stimulated by the LPS (0, 0.1, 1, 10 ug/ml) or LPS (10 ug/ml) pretreated with 12.5 mg/ml of Ca(OH)2 for 3 days, for various periods of time (12, 24, 48 h). Immunoprecipitation were performed for protein level analysis of MMP-1, MMP-2 and TIMP-1. Total RNA was isolated and real-time quantitative polymerase chain reaction (PCR) was performed for quantification of MMP-1 mRNA.
According to this study, the results were as follows:
1. The production of MMP-1 by stimulation with P. nigrescens LPS increased in time-dependent manner, and showed maximum value at 48 h in both protein and mRNA level. But there was no dose-dependent increase.
2. MMP-2 production time-dependently increased when stimulated with 1 and 10 ug/ml LPS, but there was no dose-dependent increase.
3. TIMP-1 production increased to 24 h, but decreased at 48 h. It increased when stimulated with 0.1 and 1 ug/ml LPS, but suppressed at 10 ug/ml.
4. P. nigrescens LPS pretreated with Ca(OH)2 markedly downregulated MMP-1 gene expression.
-
Keywords: MMP-1; MMP-2; TIMP-1; P. nigrescens; LPS; Ca(OH)2; PDL fibroblast
I. INTRODUCTION
Bacteria in root canals can induce inflammatory reaction of the periapical tissue
1). The cells such as neutrophils, macrophages and lymphocytes are activated and release inflammatory mediators during this process. The activation of host cells by bacterial antigens is believed to trigger the release of inflammatory mediators that destroy periapical tissues. Tissue is made up of organized group of cells attached to an extracellular matrix and surrounded by a network of blood vessels. Tissue homeostasis is maintained by coordinating cell growth and proliferation with the production and turnover of the extracellular matrix
2). The proteolysis of extracellular matrix seems to be a key initiating event for progression of the inflammation process
3).
Matrix metalloproteinases (MMPs) are well known proteolytic enzymes responsible for the remodeling and degradation of extracellular matrix under both physiologic and pathologic conditions. They are a family of zinc endopeptidases and share a number of common structural and functional features but differ somewhat in terms of substrate specificity. There are more than twenty members of the family, including collagenases, gelatinases, stromelysins and membrane-type MMPs. MMP expression can be regulated transcriptionally by growth factors, cytokines, and others. MMP-1 and MMP-2 are typical MMPs and they play central roles in the destruction of connective tissue during inflammation.
MMP-1, fibroblast-type interstitial collagenase, degrades type I, II, III, VII, VIII, X collagen, gelatin and proteoglycan core protein. MMP-1 is the only enzyme that can degrade the native triple helix of type I collagen and render them sensitive to further digestion by other proteinases
4). MMP-1 is expressed after appropriate stimulation by fibroblasts, keratinocytes, endothelial cells, monocytes, macrophages, chondrocytes and osteoblasts.
MMP-2, or gelatinase A is perhaps the most widely distributed of all MMPs and has been identified in fibroblasts, keratinocytes, chondrocytes, endothelial cells, monocytes, osteoblasts and a number of transformed cells. Gelatinases have high affinity for gelatin. MMP-2 degrades gelatin, type IV, V, VII, X collagen, elastin and proteoglycan core protein. It involves in further extracellular degradation of denatured collagens and in degradation of basement membrane type IV collagen
5).
Naturally occurring inhibitors, tissue inhibitor of metalloproteinases (TIMPs) are important controlling factors in the actions of MMPs. The TIMP family is composed of at least four members (TIMP 1-4)
6). They are expressed by a wide range of cell types, both normal and transformed and widely distributed in different tissues. TIMPs form classic noncovalent bimolecular complexes with MMPs.
Tissue destruction in disease processes often correlates with an imbalance of MMPs over TIMPs
7-
9). Resident cells in connective tissue produce both MMPs and TIMPs in response to inflammatory mediators
10,
11). Investigation of the balance between the level of MMP and their inhibitor may reveal one of the host degradative pathways in the pathogenesis of bacterial-derived pulpal and periapical lesion.
It is well known that bacteria are one of the most important causative agents of pulpal and periapical inflammation. Recently, it was demonstrated that black-pigmented bacteria (BPB) play an important role in tissue disintegration in pulpal and periapical diseases
3,
12). The BPB have gained special prominence in the search for etiologic organisms associated with endodontic infections
13). BPB have been detected in human pulpal and periapical lesions
14,
15). The cells of these lesion respond to bacterial lipopolysaccharides (LPS) of these organisms and produce various inflammatory mediators. LPS is known to have a broad spectrum of biochemical and immunochemical activities that can lead to the destruction of host tissue
16). LPS are components of the outer membrane of Gram-negative bacteria and essential for bacterial viability. LPS are set free from bacteria during multiplication or lysis, and induce potent pathophysiological effects. LPS do not elicit their toxic effects by killing host cells nor by inhibiting cellular functions. LPS interact with several types of host cells and induce various bioactive endogenous lipid, peptide, and oxygen-derived mediators. Chemically, they are consisted of O-specific chain, a core oligosaccharides, and lipid A which determines the endotoxic activities
17). Lipid A consists of several ester bonds linking fatty acids to carbohydrate moieties. Each of these sites has the potential to undergo hydrolysis in the presence of alkali. The slightest variation of the fatty acid chains of the molecule's lipid A portion renders the entire molecule biologically inactive
18).
Calcium hydroxide has been exclusively used in endodontic treatments, for the treatment of traumatized teeth with necrotic pulps and teeth with open apex, for the management of root resorption, and for nonsurgical correction of root perforation. As a long term intracanal dressing, calcium hydroxide was shown to promote bony regeneration in periapical lesions. Hydroxyl ion dissociation of calcium hydroxide raise the pH sufficiently to kill bacteria
19). Use of calcium hydroxide as a routine intracanal dressing has been advocated in recent years to prevent the proliferation of remaining microorganisms in the root canals between visits. Even though bacteria are removed from the root canal, LPS are capable of maintaining apical periodontitis
20).
Collagen is a major structural protein of the extracellular matrix of all human tissues
21). The extracellular matrix of periodontal ligament (PDL) tissue is composed mainly of type I collagen
4). The degradation of these collagens is therefore thought to play a major role in tissue destruction during inflammation. The fibroblast is a prominent cellular component of the pulp and periapical tissue. Fibroblasts have the ability to both synthesize and degrade organic matrices including collagen
22), and are believed to play an important role in collagen metabolism in health and disease. PDL fibroblast is the first cell in periapical tissue to encounter spreading bacteria and their byproducts from infected root canal.
Prevotella nigrescens is the most frequently isolated BPB from the infected root canals
13,
23,
24), and thought to play significant roles in pulpal and periapical pathosis. Moreover, previously identified
P. intermedia were now distinguished and actually isolated as
P. nigrescens25). However, there was little information concerning the effects of
P. nigrescens LPS on the production of MMPs from PDL fibroblasts. There are differences in the structure and function of LPS among bacterial species
26). The effects of bacterial LPS on tissue destruction and the responsiveness of host tissue are also different
9). Because of the observed interspecies variation, the ability and efficiency of calcium hydroxide to inactivate LPS from different bacterial species should be investigated, particularly since endodontic infections are characteristically polymicrobial
19).
Thus, the purpose of this study is to monitor the secretion of MMP and TIMP by human PDL fibroblasts stimulated with P. nigrescens LPS, and to examine the effect of calcium hydroxide treatment on P. nigrescens LPS.
II. MATERIALS AND METHODS
1. Culture of Bacteria and Purification of LPS
P. nigrescens (ATCC 33563, Rockville, MD, USA) was cultured in brain heart infusion broth supplemented with hemin (10 µg/ml), vitamin K1 (1 µg/ml) and yeast. Bacterial cells were grown anaerobically at 37℃. After confluency, the bacterial cell pellet was obtained by centrifugation at 10,000 xg for 10 min. This pellet was washed with distilled water and then lyophilized. LPS was extracted and purified by the method as described by Eidhin and Mouton
27). The lyophilized bacteria was mixed with 1 ml of distilled water and then boiled for 15 min. Following centrifugation at 12,000 xg for 5 min, cellular debris was removed, and the supernatant was collected. Then proteinase K (1 mg / 50 µl water) was added and incubated for 1 h at 60℃. After the tube was boiled for 5 min to precipitate any residual proteinase K, the supernatant was dialyzed against distilled water. After lyophilization of the supernatant, the final powder is crude LPS. Preparation of higher purity could be obtained by an additional proteinase K digestion. The purified LPS was redissolved to a concentration of 1 mg/ml of distilled water and confirmed in 12 % sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and silver staining (
Figure 1).
Commercial preparations of Escherichia coli (E. coli) LPS (Sigma chemical company, St Louis, MO, USA) were obtained as lyophilized powder and used as positive control.
2. Culture of PDL fibroblast and stimulation by LPS
PDL cells were obtained from PDL explants dissected from the mid-root of premolars extracted for orthodontic reasons, according to the method described by Oates et al
28). PDL tissue was scraped from the middle third of the root surface with sharp blade under sterile conditions. The tissue explants were placed in 60 mm tissue culture plates containing Dulbecco's modified eagle's medium (DMEM) supplemented with high glucose (4500 mg/L), 5% L-glutamine, and 10% heat inactivated fetal bovine serum (FBS). The cells were incubated in humidified air at 37℃ with 5% CO
2 for 2 to 4 weeks. The medium was replaced every 2 or 3 days until sufficient cell proliferation was evident. Brief incubation with 0.25% trypsinethylenediamine tetraacetic acid (EDTA) was employed to remove the cells, which were then transferred into 100 mm dish in α-MEM supplemented with 10% FBS, 1 mM sodium pyruvate, 1 mM non-essential amino acid and antibiotics for continued growth. The cells were grown in 35 mm dish or on glass coverslips for immunohistochemical staining with 5B5 kit (Dako) for the confirmation of cytoplasmic marker (β-subunit of proly-4-hydroxlyase and disulfide-isomerase) of fibroblasts (
Figure 2). After confluency, the cells were passed on 6 well plate in culture media containing DMEM, 0.1% FBS, 5% L-glutamine and antibiotics. They were stimulated by LPS (0, 0.1, 1, 10 µg/ml) of
P. nigrescens and
E. coli (positive control) for various periods of time (12, 24, 48 h). In addition, they were stimulated with LPS (10 µg/ml) treated with 12.5 mg/ml Ca(OH)
2 for 3 days.
Stimulated PDL cells were sonicated with 200 µl of lysis buffer (20 mM Tris-Cl pH 7.4, 150 mM NaCl, 1 M EDTA, 1 mM ethylene glycerol bis (b-aminoethylether) tetraacetic acid, 1 % Triton X-100, 2.5 mM sodium pyro phosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 µg/µl leupeptin, 1 mM phenylmethylsulfonyl fluoride) for 20 sec and centrifuged at 13000 rpm for 30 min at 4℃. Protein was quantified by BCA method. 100 µg of protein was incubated for 1 h at 4℃ with 5 µg of anti-MMP1, anti-MMP2, and anti-TIMP1 goat polyclonal antibodies (Santacruz Biotech Inc., USA), respectively. Immunoprecipitation was performed according to standard techniques using Protein-A Sepharose (Sigma-Aldrich Co., USA). Protein-A sepharose incubated with protein and antibody mixture for 2 h at 4℃. They were washed five times with phosphate buffered saline including 280 mM NaCl and 0.2% Tween-20. SDS buffer was added to the precipitate and incubated for 5 min at 90℃. Supernatants were electrophoresed in 12 % SDS-PAGE gel and transferred to nitrocellulose membrane (Amersham pharmacia biotech). The membranes were incubated with anti-MMP1, anti-MMP2, or anti-TIMP1 antibodies diluted 1/200 (v/v) in blocking solution, for overnight at 4℃. Membranes were washed four times with phosphate buffered saline with Tween (PBST) and incubated with horseradish peroxidase-linked anti-mouse IgG antibody diluted 1/10000 (v/v). They were then washed extensively with PBST and developed with DAB kit (Vector Co., USA). Protein quantification was performed using Image Analyzer (BIO-PROFIL BIO-1D, Window application V99.04, Vilber Lourmat, France).
4. Analysis of RNA
Primer Design
For real-time polymerase chain reaction (PCR) amplification, optimal primers were designed using Primer express™ software (PE). As a housekeeping gene, glyceraldehydes-3-phosphate dehydrogenase (GAPDH) was used to compare assay reproducibility. They are as follows;
MMP-1:
GAPDH:
Isolation of RNA
After stimulation of cells with LPS and Ca(OH)2 treated LPS, total RNA were isolated using TRIzol Reagent (Gibco) as described below. The cells were lysed with 0.5 ml of TRIzol reagent and then 100 ml of chloroform was added and inverted mixed. Following centrifugation at 15000 rpm for 30 min at 4℃, aqueous phase was collected into a fresh tube and mixed with additional 0.25 ml of isopropyl alcohol. After incubation for 10 min on ice, the samples were centrifuged at 15000 rpm for 10 min at 4℃ to precipitate RNA. The RNA pellet was washed with 1 ml of 75% ethanol and centrifuged at 15000 rpm for 10 min at 4℃. The isolated RNA was dissolved in diethyl pyrocarbon - ate (DEPC) - treated water and quantified using spectrophotometric method.
Real-time PCR assay
Reverse transcription (RT) reaction mixture containing dNTP, oligo(dT)20 primer, 1µg of total RNA, and Reverse Transcriptase (Invitrogen) was heated at 65℃ for 2 min. Thermal cycling parameters for RT reactions are as follows; RT for 60 min at 37℃, RT inactivation for 10 min at 72℃.
Real-time quantitative PCR was performed by a GeneAmp 5700 sequence detection system with SYBR Green Master Mix (Applied Biosystems, USA). SYBR Green is highly specific to double strand (ds)-DNA: upon binding to ds-DNA, it exhibits a large increase in fluorescence. Direct detection of PCR product is monitored by measuring the increase in fluorescence. The reaction mixture (25 µL) contains 0.5 µL of the cDNA sample with 0.25 µM of the PCR primer. Two tube method for target genes and control gene was used in 96 well plate. The cycle profile was as follows; 1 cycle at 50℃ for 2 min, 1 cycle at 95 ℃ for I min, 40 cycles at 95℃ for 15 sec, and 60 ℃ for 1 min. Experiments were performed in triplicate and two times.
III. RESULTS
After exposure to various concentrations of
P. nigrescens LPS, the protein level of MMP-1, MMP-2, and TIMP-1 in PDL fibroblast was evaluated by immunoprecipitation.
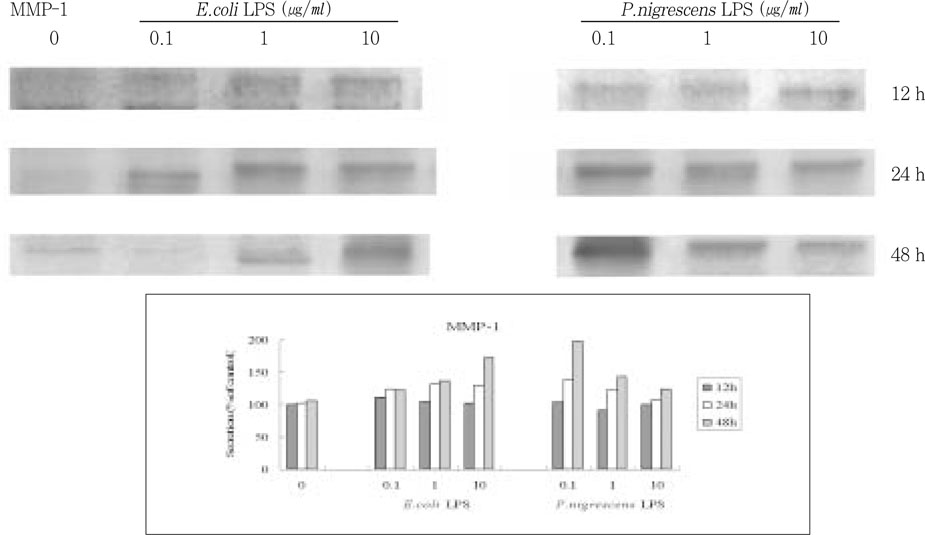
P. nigrescens LPS and control
E.coli LPS increased the secretion of MMP-1 in time-dependent manner (
Figure 3). The secretion of MMP-1 was highest in cells that were stimulated with 0.1 µg/ml of
P. nigrescens LPS for 48 h. But there was no dose-dependent increase, and the most effective LPS concentration was different between
E.coli and
P. nigrescens.
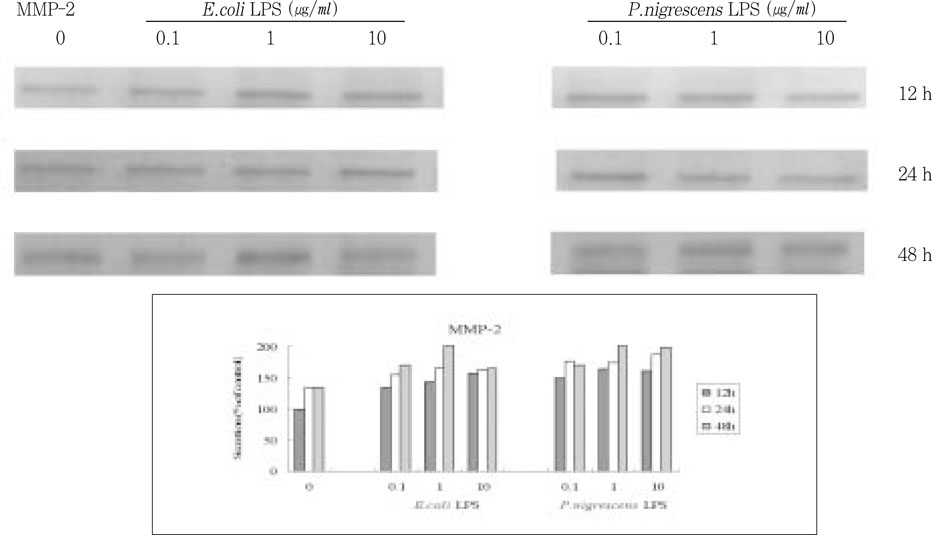
MMP-2 secretion time-dependently increased when stimulated with 1 and 10 µg/ml LPS, but there was no dose-dependent increase (
Figure 4).
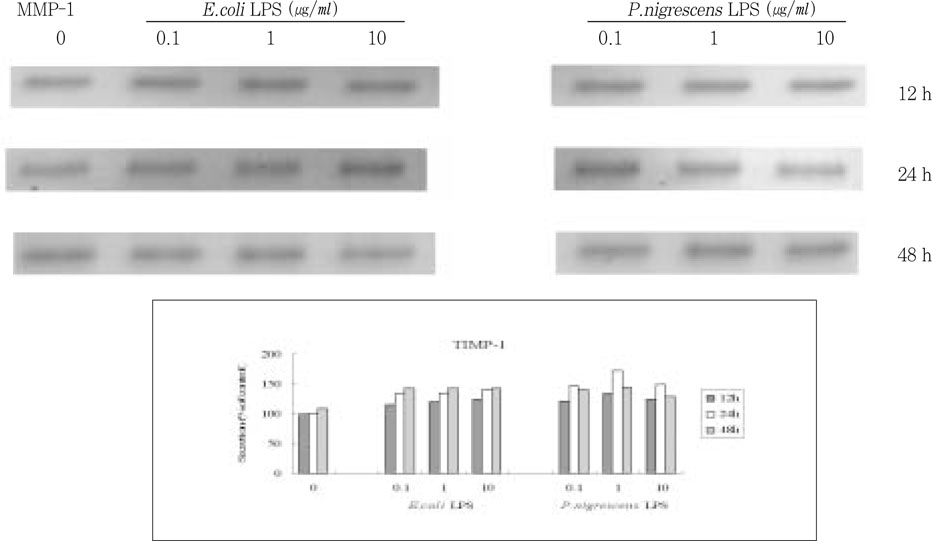
TIMP-1 secretion increased up to 24 h, but decreased at 48 h. It increased when stimulated with 0.1 and 1 µg/ml LPS, but suppressed at 10 µg/ml (
Figure 5).
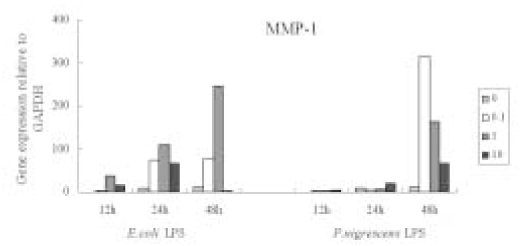
MMP-1 gene expression increased time-dependently by stimulation with
P. nigrescens LPS, but there was no dose-dependent increase. At 48 h, MMP-1 gene expression was highly increased at all concentration of
P. nigrescens LPS. MMP-1 gene expression increased until 1 µg/ml of
E. coli LPS, and then decreased at 10 µg/ml (
Figure 6).
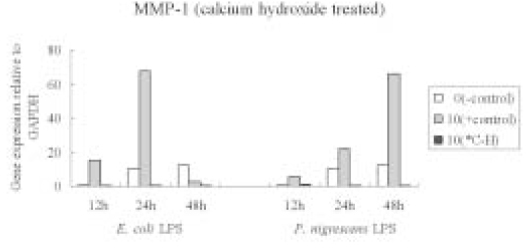
Calcium hydroxide pretreatment to both
P. nigrescens and
E. coli LPS markedly downregulated MMP-1 gene expression (
Figure 7).
IV. DISCUSSION
The production of MMP-1, MMP-2 and TIMP-1 in PDL fibroblasts was monitored employing immunoprecipitation, and MMP-1 by real-time PCR.
The advantages of real-time (during PCR) acquisition of data over end point are; fewer processing steps reduce the opportunity for errors, the time and efforts are substantially reduced. Northern blotting works well for quantification, but it requires a large amount of high quality total RNA and is time consuming. PCR can be used to amplify relatively degraded RNA, with small amount
29). It can be used to quantitate large numbers of samples in a relatively short time
30).
In situations without inflammatory cells, tissue destruction is initiated by specific attack on matrix macromolecules by members of the family of MMPs, synthesized by stimulated connective tissue cells. These process may be augmented by the infiltration of inflammatory cells, mechanical damage, and bacterial infection
9). The proteolysis of extracellular matrix by MMPs is believed to play an essential role in the development and progression of pulpal and periapical inflammation
3). Shin et al.
31) demonstrated that the tissue level of MMP-1, -2, and -3 in pulpal and periapical lesion were higher than those of normal tissues. Recent studies have shown that elevated levels of MMPs may be involved in tissue destruction in inflamed dental pulps
3,
10,
11,
32-
34). Moreover, anaerobic bacterial extracts was reported to accelerate the production of MMP-1 and MMP-2 by human dental pulp(HDP) cells
35).
Many studies have confirmed MMPs and TIMPs production in human gingival fibroblasts and HDP cells by various cytokines, growth factors, or other stimuli. However, the activity of MMP and their inhibitor in human PDL fibroblast has not been well studied. The PDL fibroblast is believed to play an important role in collagen metabolism in pulpal and periapical disease. The turnover of collagen in the periodontal ligament is controlled by the balance between collagen synthesis and degradation. The family of matrix metalloproteinases and their inhibitors is one of the mechanisms which regulate this balance
36).
In this study, we quantified the MMP-1 mRNAs extracted from PDL fibroblasts after LPS stimulation, and normalized with GAPDH mRNA levels
37). As it was confirmed by immunoprecipitation analysis, mRNA level of MMP-1 showed time-dependent increase and significantly increased at 48 h after the exposure to the bacterial LPS. A low amount of MMP-1 expression in the negative control group could be explained as follows; Low amount of constitutive MMP-1 serves as a remodeling element of connective tissue under physiological condition
22). However, Tamura et al reported that unstimulated human dental pulp fibroblasts did not express MMP-1 mRNA
11), which is somewhat different from our result.
There are many reports to investigate MMP-2 activity in HDP cells. Ueda et al
35) reported that TNF-α stimulated MMP-2 activity in HDP cells. Chang et al proposed that cytokines can regulate MMP-2 production by HDP cells, and MMP-2 may play a role in pulpal inflammation
32). They also reported that the supernatants from
P. endodontalis and
P. gingivalis elevated MMP-2 production in time and dose-dependent manner both in HDP and PDL cell cultures
3).
MMP-2 secretion in this study showed time and dose-dependent increase, but the extent of increase was lower than that of MMP-1. This result is consistent with the fact that gelatinases are expressed constitutively by most cells in culture but only moderately responsive to stimulation
5).
TIMP-1 serves as a regulator throughout the collagenolytic process. TIMP-1 can form 1:1 complex with and inactivate MMP-1
6). Because the same cells that produce MMP-1 are also capable of synthesizing TIMP-1, the overall molar ratio between MMP-1 and TIMP-1 plays a significant role in connective tissue remodeling
38). The role of TIMPs may be to tightly control metalloproteinase activity both at the level of activation and subsequent substrate degradation. TIMPs inhibit the activity of the fully competent MMPs and also appear to block or retard MMP precursor activation. The induction of TIMP-1 by cytokines has been reported in several cell types with different results, which may represent tissue-specific regulatory mechanisms
22). Yang et al demonstrated that in human gingival fibroblasts, cytokines differentially and specifically regulate expression of TIMP-1 mRNA
2).
In this study, TIMP-1 secretion was increased up to 24 h after the exposure to the bacterial LPS, then the level start to drop. The downregulation of TIMP-1 at 48 h could cause MMP/TIMP imbalance and culminate in tissue destruction by the bacterial LPS. This result is consistent with the findings of Nakata et al
35) that sonicated bacterial extracts (SBEs) of BPBs stimulated HDP cells to produce MMP-1 and MMP-2, with lesser extent, TIMP-1 production. They proposed that SBEs may cause dental pulp tissue to be destroyed by changing the balance of the production between MMPs and TIMPs by HDP cells. Alvares et al reported that IL-1β exerted profound effect in MMP-1 synthesis in PDL fibroblasts, but no effects in TIMP-1 at the mRNA level. They suggested that cytokines differentially and specifically regulate expression of collagenase mRNA in human PDL
36).
LPS is known to exert profound effect on the protein expression by host cells. High concentration of
E.coli LPS inhibited the production of DNA and protein from HDP cells
39). High level of LPS was obviously toxic to connective tissue and would result in tissue necrosis
40). A significant correlation has been demonstrated between intracanal LPS contents and specific clinical symptomatology of pulp and periapical tissues
41-
43). There are increasing evidences that calcium hydroxide intracanal dressings may alter biological properties of LPS
18-
20,
44). Calcium hydroxide has alkaline pH of 10 - 12, which can neutralize LPS by hydrolysis of ester bonds in the lipid A chains, resulting in detoxification of LPS. The neutralizing capacity of calcium hydroxide is concentration-dependent, with increased concentrations of LPS being able to exhaust inhibitory effects of this medicament. Clinically, it is observed that some cases require multiple changes of calcium hydroxide to effect healing
20). The fact that the expression of MMP was downregulated when stimulated with either
P. nigrescens or control
E. coli LPS pretreated with calcium hydroxide indicate that calcium hydroxide is effective in preventing extracellular matrix degradation when bacterial infection exists by reducing MMP production from PDL fibroblast.
P. nigrescens LPS was used in this study because infection of the root canals is frequently associated with BPB and among them, this microorganism is most commonly isolated from infections of endodontic origin. LPS from different organisms vary in their effects on host cells. This may be related to differences in the chemical structure of the LPS
26). LPS from
P. gingivalis has been reported to display significantly less biological potency than
E. coli LPS
45). But Koga et al reported that biological effects of purified LPS from
P. gingivalis were comparable to those of
E. coli LPS
16).
The regulation of MMP synthesis and secretion from the PDL fibroblast by
P. nigrescens has not been reported previously. The immunoprecipitation and RT-PCR analysis revealed that the levels of MMP-1, MMP-2 and TIMP-1 were elevated after addition of
P. nigrescens LPS. These results suggest that MMP pathway may be one of the mechanism of periapical breakdown by
P. nigrescens LPS. In addition,
P. nigrescens has been implicated in a number of virulence factors in the pathogenesis of pulpal and periapical infections. Among these virulence factors, proteolytic enzymes may destroy connective tissues or molecules of the host defense system
46).
The effects of cytokines and LPS on MMP and TIMP production are variable depending on cell types and species
10). The difference of proteinases and inhibitor levels in different sources of cells may reflect the phenotypic and biochemical differences between cell populations and may represent tissue-specific regulatory mechanisms
2). Many of the MMP activities are specifically regulated at the level of gene expression, but post-translational extracellular processing to the active form is an another important level of regulation. Interaction between multiple factors is important when considering cells in tissue
in vivo. However, this study has not examined the effects of LPS on post-translational modifications, and the activation of the latent form of enzymes into its active form. In the present study, we report that
P. nigrescens LPS directly triggers PDL fibroblasts to express degradative enzymes including MMP-1 and MMP-2.
P. nigrescens may be involved in tissue destruction at the site of periapical infections. Further study of the detailed mechanism of actions of
P. nigrescens LPS and MMP may be helpful to understand the pathogenesis of periapical lesions.
V. CONCLUSION
The production of MMP and TIMP by human PDL fibroblasts stimulated with P. nigrescens LPS and the effect of calcium hydroxide treatment were monitored.
According to this study, the results were as follows:
P. nigrescens LPS increased the production of MMP-1 in time-dependent manner. MMP-1 at 48 h in both protein and mRNA level showed maximum value. But there was no dose-dependent increase.
1 and 10 µg/ml of P. nigrescens LPS increased MMP-2 production time-dependently, but there was no dose-dependent increase.
0.1 and 1 µg/ml of P. nigrescens LPS increased, but 10 µg/ml LPS suppressed TIMP-1 production. It increased to 24 h, but decreased at 48 h.
P. nigrescens LPS pretreated with Ca(OH)2 markedly downregulated MMP-1 gene expression.
REFERENCES
- 1. Trowbridge HO, Stevens BH. Microbiologic and pathologic aspects of pulpal and periapical disease. Curr Opin Dent. 1992;2: 85-92.
- 2. Yang YY, Tsai HF, Lu SC, Huang YF, Chang YC. Regulation of tissue inhibitors of metalloproteinase-1 gene expression by cytokines in human gingival fiboblasts. J Endod. 2002;28(12):803-805.PubMed
- 3. Chang YCH, Lai CC, Yang SF, Chan Y, Hsieh YS. Stimulation of matrix metalloproteinases by black-pigmented bacteroides in human pulp and periodontal ligament cell cultures. J Endod. 2002;28(2):90-93.ArticlePubMed
- 4. Lin SK, Wang CC, Huang S, Lee JJ, Chiang CP, Lan WH, Hong CY. Induction of dental pulp fibroblast matrix metalloproteinase-1 and tissue inhibitor of metalloproteinase-1 gene expression by interleukin-1α and tumor necrosis factor-α through a prostaglandin-dependent pathway. J Endod. 2001;27(3):185-189.ArticlePubMed
- 5. Birkedal-Hansen H, Moore WBI, Bodden MK, Winsor LJ, Brikedal-Hansen B, DeCarlo A, Engler JA. Matrix metalloproteinases: A review. Crit Rev Oral Biol Med. 1993;4(2):197-250.PubMed
- 6. Douglas DA, Shi YE, Sang QA. Computational sequence analysis of the tissue inhibitor of metalloproteinase family. J Protein Chem. 1997;16: 237-255.ArticlePubMedPDF
- 7. Seguier S, Gogly B, Bodineau A, Godeau G, Brousse N. Is collagen breakdown during periodontitis linked to inflammatory cells and expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in human gingival tissue? J Periodontol. 2001;72(10):1398-1406.ArticlePubMed
- 8. Reynolds JJ. Collagenases and tissue inhibitors of metalloproteinases: a functional balance in tissue degradation. Oral Dis. 1996;2: 70-76.ArticlePubMed
- 9. Reynolds JJ, Hembry RM, Meikle MC. Connective tissue degradation in health and periodontal disease and the roles of matrix metalloproteinases and their natural inhibitors. Adv Dent Res. 1994;8(2):312-319.ArticlePubMedPDF
- 10. Panagakos FA, O'Boskey FJ Jr, Rodriguez E. Regulation of pulp cell matrix metalloproteinase production by cytokines and lipopolysaccharides. J Endod. 1996;22(7):358-361.ArticlePubMed
- 11. Tamura M, Nagaoka S, Kawagoe M. Interleukin-1α stimulates interstitial collagenase gene expression in human dental pulp fibroblast. J Endod. 1996;22(5):240-243.ArticlePubMed
- 12. Hosoya S, Matsushima K. Stimulation of interleukin-1β production of human dental pulp cells by Porphyromonas endodontalis lipopolysaccharides. J Endod. 1997;23(1):39-42.PubMed
- 13. Sundqvist G, Jahansson E, Sjogren U. Prevalence of black-pigmented bacteroides species in root canal infections. J Endod. 1989;15(1):13-19.ArticlePubMed
- 14. Baumgartner JC, Watkins BJ, Bae KS, Xia T. Association of black-pigmented bacteria with endodontic infections. J Endod. 1999;25(6):413-415.ArticlePubMed
- 15. Siqueira JF, Rocas I, Oliveira JCM, Santos KRN. Molecular detection of black-pigmented bacteria in infections of endodontic origin. J Endod. 2001;27(9):563-566.ArticlePubMed
- 16. Koga T, Nishihara T, Fujiwara T, Nisizawa T, Okahashi N, Noguchi T, Hamada S. Biochemical and immunological properties of lipopolysaccharide (LPS) from Bacteroides gingivalis and comparison with LPS from Escherichia coli. Infect Immun. 1985;47(3):638-647.ArticlePubMedPMCPDF
- 17. Rietschel ET, Kirikae T, Schade FU, Mamat U, Schmidt G, Loppnow H, Ulmer AJ, Zahringer U, Seydel U, Padova F, Schreier M, Brade H. Bacteial endotoxin: molecular relationships of structure to activity and function. FASEB J. 1994;8: 217-225.ArticlePubMedPDF
- 18. Buck RA, Cai J, Eleazer PD, Staat RH, Hurst HE. Detoxification of endotoxin by endodontic irrigants and calcium hydroxide. J Endod. 2001;27(5):325-327.ArticlePubMed
- 19. Safavi KE, Nichols FC. Effects of calcium hydroxide on bacterial lipopolysaccharide. J Endod. 1993;19(2):76-78.PubMed
- 20. Barthel CR, Levin LG, Reisner HM, Trope M. TNF-α release in monocytes after exposure to calcium hydroxide treated Escherichia coli LPS. Int Endod J. 1997;30: 155-159.ArticlePubMed
- 21. Cury JD, Campbell EJ, Lazarus CJ, Albin RJ, Welgus HG. Selective up-regulation of human alveolar macrophage collagenase production by lipopolysaccharide and comparison to collagenase production by fibroblasts. J Immunol. 1988;141(12):4306-4312.ArticlePubMedPDF
- 22. Otsuka K, Sodek J, Limeback H. Synthesis of collagenase and collagenase inhibitors by osteoblast-like cells in culture. Eur J Biochem. 1984;145(1):123-129.ArticlePubMed
- 23. Dougherty WJ, Bae KS, Watkins BJ, Baumgartner JC. Black-pigmented bacteria in coronal and apical segments of infected root canals. J Endod. 1998;24(5):356-358.ArticlePubMed
- 24. Bae KS, Baumgartner JC, Shearer TR, David LL. Occurrence of Prevotella nigrescens and Prevotella intermedia in infections of endodontic origin. J Endod. 1997;23: 620-623.ArticlePubMed
- 25. Gharbia S, Haapasalo M, Shah H, et al. Characterization of Prevotella intermedia and Prevotella nigrescens isolates from periodontic and endodontic origin. J Periodontol. 1994;65: 56-61.PubMed
- 26. Firoozkoohi J, Zandi H, Olsen I. Comparison of lipopolysaccharides from Bacteroides, Porphylomonas, Prevotella, Camphylobacter and Wolinella spp. by Tricine-SDS-PAGE. Endod Dent Traumatol. 1997;13: 13-18.PubMed
- 27. Eidhin DN, Mouton C. A rapid method for preparation of rough and smooth lipopolysaccharides from Bacteroides, Porphylomonas and Prevotella. FEMS Microbiol Lett. 1993;110: 133-138.PubMed
- 28. Oates TW, Rouse CA, Cochran DL. Mitogenic effects of growth factors on human periodontal ligament cells in vitro. J Periodontol. 1993;64: 142-148.ArticlePubMed
- 29. Freeman WM, Walker SJ, Vrana KE. Quantitative RT-PCR: Pitfalls and potential. Biotechniques. 1999;26(1):112-125.ArticlePubMed
- 30. Gibson UEM, Heid CA, Williams PM. A novel method for real time quantitative RT-PCR. Genome Res. 1996;6: 995-1001.ArticlePubMed
- 31. Shin SS, Lee JI, Baek SH, Lim SS. Tissue levels of matrix metalloproteinases in pulps and periapical lesions. J Endod. 2002;28(4):313-315.ArticlePubMed
- 32. Chang YC, Yang SF, Hsieh YS. Regulation of matrix metalloproteinase-2 production by cytokines and pharmacological agents in human pulp cell cultures. J Endod. 2001;27(11):679-682.ArticlePubMed
- 33. O'Boskey FJ Jr, Panagakos FS. Cytokines stimulate matrix metalloproteinase production by human pulp cells during long-term culture. J Endod. 1998;24(1):7-10.ArticlePubMed
- 34. Ueda I, Matsushima K. Stimulation of plasminogen activator activity and matrix metalloproteinases of human dental pulp-derived cells by tumor necrosis factor-α. J Endod. 2001;27(3):175-179.ArticlePubMed
- 35. Nakata K, Yamasaki M, Iwata T, Suzuki K, Nakane A, Nakamura H. Anaerobic bacterial extracts influence production of matrix metalloproteinases and their inhibitors by human dental pulp cells. J Endod. 2000;26(7):410-413.ArticlePubMed
- 36. Alvares O, Klebe R, Grant G, Cochran DL. Growth factor effects on the expression of collagenase and TIMP-1 in periodontal ligament cells. J Periodontol. 1995;66: 552-558.ArticlePubMedPDF
- 37. Boyle DL, Rosengren S, Bugbee W, Kavanauph A, Firestein GS. Quantitative biomarker analysis of synovial gene expression by real-time PCR. Arthritis Res Ther. 2003;5(6):R352-R360.ArticlePubMedPMCPDF
- 38. Dean DD, Martel-Pelletier J, Pelletier JP, Howell DS, Woessner JF Jr. Evidence for metalloproteinase and metalloproteinase inhibitor imbalance in human osteoarthritic cartilage. J Clin Invest. 1989;84: 678-685.ArticlePubMedPMC
- 39. Nakane A, Yoshida T, Nakata K, Horiba N, Nakamura H. Effects of lipopolysaccharides on human dental pulp cells. J Endod. 1995;21(3):128-130.ArticlePubMed
- 40. Pinero G, Kiatpongsah S, Hutchins MO, Hoover J. The effect of endotoxin on the synthesis of connective tissue matrix components by pulp fibroblasts in vitro. J Endod. 1983;9: 2-7.ArticlePubMed
- 41. Schein B, Schilder H. Endotoxin content in endodontically involved teeth. J Endod. 1975;1(1):19-21.ArticlePubMed
- 42. Horiba N, Maekawa Y, Abe Y, Ito M, Matsumoto T, Nakamura H. Correlations between endotoxin and clinical symptoms or radiolucent areas in infected root canals. Oral Surg Oral Med Oral Pathol. 1991;71: 492-495.ArticlePubMed
- 43. Yamasaki M, Nakane A, Kumazawa M, Hashioka K, Horiba N, Nakaura H. Endotoxin and gram-negative bacteria in the rat periapical lesions. J Endod. 1992;18: 501-504.ArticlePubMed
- 44. Safavi KE, Nichols FC. Alteration of biological properties of bacterial lipopolysaccharide by calcium hydroxide treatment. J Endod. 1994;20(3):127-129.ArticlePubMed
- 45. Nair BC, Mayberry WR, Dzaiak R, Chen PP, Levine MJ, Hausmann E. Biological effects of purified lipopolysaccharide from Bacteroides gingivalis. J Periodontal Res. 1983;18: 40-49.PubMed
- 46. Jun KC, Barua PK, Zambon JJ, Neiders ME. Proteolytic activity in black-pigmented bacteroides species. J Endod. 1989;15(10):463-467.ArticlePubMed
Figure 1
E. coli (left) and P. nigrescens (Right) LPS confirmed by SDS-PAGE and silver staining.

Figure 2Immunohistochemical staining of human PDL cells for the confirmation of cytoplasmic marker (β-subunit of proly-4-hydroxlyase and disulfideisomerase) of fibroblasts.
Figure 3
Effects of LPS on MMP-1 protein production in PDL fibroblasts.
upper. immunoprecipitation analysis, lower. quantification of band intensity relative to negative control (0 µg/ml, 12 h).
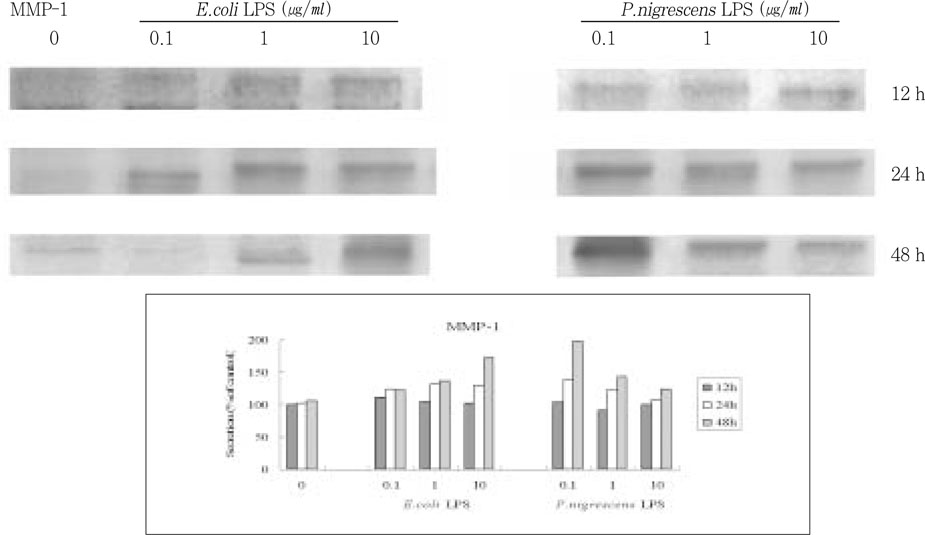
Figure 4
Effects of LPS on MMP-2 protein production in PDL fibroblasts.
upper. immunoprecipitation analysis, lower. quantification of band intensity relative to negative control (0 µg/ml, 12 h).
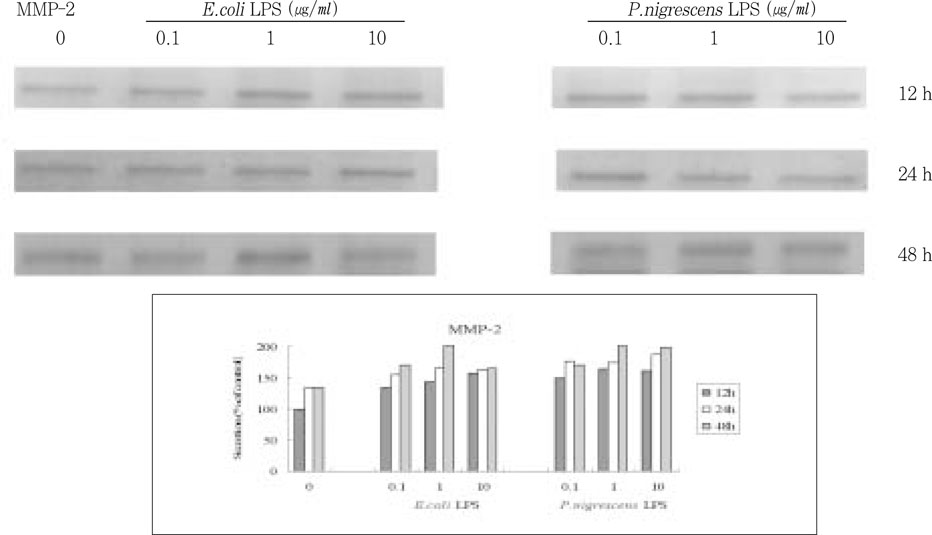
Figure 5
Effects of LPS on TIMP-1 protein production in PDL fibroblasts.
upper. immunoprecipitation analysis, lower. quantification of band intensity relative to negative control (0 µg/ml, 12 h).
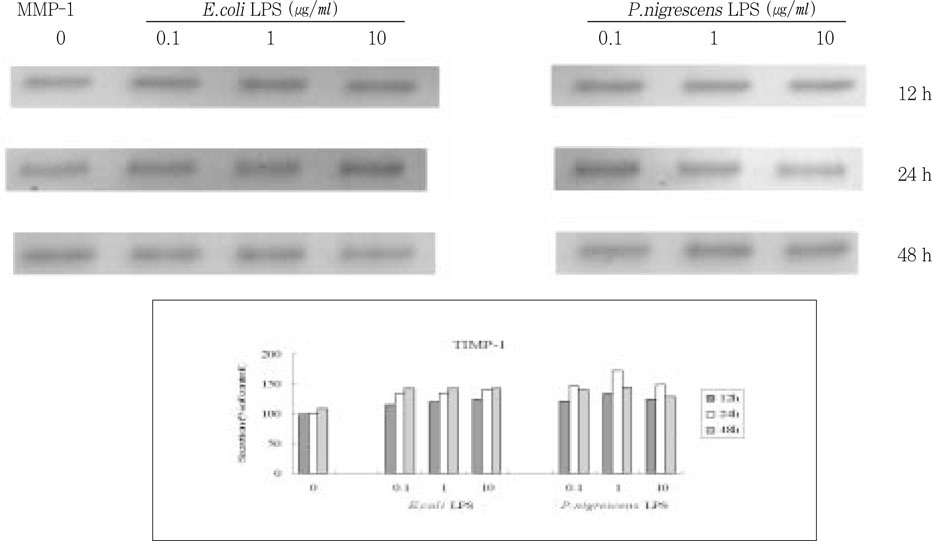
Figure 6Effects of LPS on MMP-1 mRNA expression in PDL fibroblasts analyzed by real-time PCR and normalized with GAPDH mRNA level, relative to negative control (0 µg/ml, 12 h).
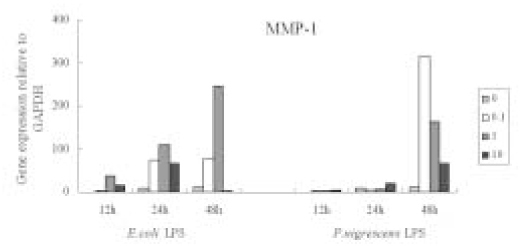
Figure 7
Effects of LPS pretreated with Ca(OH)2 on MMP-1 mRNA expression in PDL fibroblasts analyzed by real-time PCR and normalized with GAPDH mRNA level, relative to negative control (0 µg/ml, 12 h).
*C-H; calcium hydroxide treated LPS








 KACD
KACD



 ePub Link
ePub Link Cite
Cite

