Articles
- Page Path
- HOME > Restor Dent Endod > Volume 29(1); 2004 > Article
- Original Article Tissue reactions to implanted intracanal medicaments in rats
- Miri Kim, Byunghyun Kim, Wonkyung Kim
-
2004;29(1):-29.
DOI: https://doi.org/10.5395/JKACD.2004.29.1.023
Published online: January 31, 2004
Department of Dentistry, Asan Medical Center, Seoul, Korea.
- Corresponding author: Miri Kim. Department of Dentistry, Asan Medical center, 388-1 Pungnap-2dong, Songpa-gu, Seoul 138-736, Korea. Tel: 02)3010-3824, Fax: 02)3010-6967, kmr333@unitel.co.kr
Copyright © 2004 Korean Academy of Conservative Dentistry
- 1,268 Views
- 2 Download
I. Introduction
The endodontic lesions are mainly caused by microorganisms in the root canal. The scientifically documented procedure for the best results in canal disinfection of teeth with apical periodontitis is based on complete debridement and irrigation of the root canal during the first appointment. Many investigators have shown that low intracanal bacterial counts can be achieved by mechanical debridement and flushing alone.1) But, intracanal medicaments had been used for root canal sterilization as part of controlled asepsis in infected root canals and their role is secondary to cleaning and shaping of the root canal.2)
Formaldehyde has been extensively used in endodontic therapy and still enjoys great popularity despite its high toxicity and mutagenic potential. Formocresol(19% formaldehyde, 35% cresol dissolved in 46% glycerine and water) was used routinely intracanal medicament in the past. Formaldehyde is volatile and, therefore, releases antimicrobial vapors if applied on a cotton pellet for pulp chamber disinfection. All formaldehyde preparations are potent toxins with an antimicrobial effectiveness much lower than their toxicity.3) The formaldehyde in contact with tissue in the pulp and periapical tissues is transportated to all parts of the body.4) Paraformaldehyde is a polymeric hydrate of formaldehyde. Paraformaldehyde, or trioxymethylene, is a powder, with a formaldehyde component of 91% to 99%. Recently Depulpin® (Voco Co., Cuxhaven, Germany) gains popularity as a devitalizing agent during root canal therapy in spite of high concentration of 49% paraformaldehyde because it facilitate devitalization of pulp and makes root canal therapy easier.
The use of calcium hydroxide in endodontics was introduced by Hermann in 1920.5) Calcium hydroxide cannot be categorized as a conventional antiseptic, but it does kill the bacteria in the root canal space. The value of calcium hydroxide in endodontic treatment of necrotic infected teeth is now well documented. Calcium hydroxide is a slowly working antiseptic. In clinical study, one week of intracanal dressing has been shown to safely disinfect a root canal system.6) In addition to killing the bacteria, calcium hydroxide has an extraordinary quality in its ability to hydrolyze the lipid moiety of bacterial lipopolysaccharide (LPS).7) This is a very desirable effect because dead cell wall material remains after killing of the bacteria that causes the root canal infection. Calcium hydroxide not only kills the bacteria, but it also reduces the effect of the remaining cell wall material LPS. Some of the effects of calcium hydroxide treatment may include reparative dentin formation. Recent data was shown that hydroxyl ions from the calcium hydroxide paste used as intracanal dressing in vitro diffused through the dentinal tubules of the apical region at pH 9.0 at a rate sufficient to inhibit most microorganisms.8) Calcium hydroxide may alter biological properties of bacterial LPS and the use of calcium hydroxide may result in detoxification of residual LPS in the root canals.9) And calcium hydroxide, being chemically a base, can react with gases and absorb them, thus it can react with carbon dioxide, producing calcium carbonate and water.10) Tempcanal® (Pulpdent Co., Watertown, MA, U.S.A) and Vitapex® (Neo Dental Chemical Products Co., Tokyo, Japan) are the most commonly used materials in Republic of Korea.
At present, there have been not enough studies about the reaction of pulp and periapical tissue caused by Depulpin®. The present work studied the tissue toxicity caused by three brands of intracanal medicaments in the connective tissue of rats.
II. Materials & Methods
Twenty male Sprague-Dawley rats were housed in metal cage at a constant temperature of 24℃ with commercial rat chow available ad libitum. Anesthesia of the rat was obtained using a combination of ketamin(5mg/kg, yoohan chemical co. Korea), xylazine hydrochloride(0.15mg/kg, Bayel korea co. Korea), and 2% Xylocaine (Including 1/100000 epi. Astra, MA, USA).
For implantation, the teflon-coated poly-tubes about 3mm length & diameter cylinder type were used. Each rat received three implants in ventral subcutaneous regions; Tempcanal®, Vitapex® and Depulpin®. Rats were sacrificed by IV injection of an overdose of pentobarbital sodium and perfused with 10% buffered formalin after 7, 30days respectively. After perfusion procedures, implant areas were carefully excised and further fixed in formaldehyde.11-13)
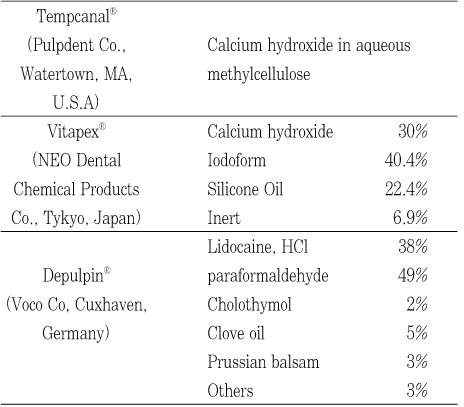
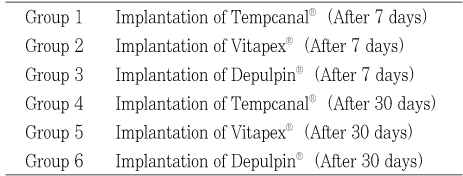
The intracanal medicaments of this experiment were shown in Table 1 and the classification of experimental groups was shown in Table 2.
Block biopsies around implant were sectioned 5mm width with sharp knives. And then the block sections were dehydrated in alcohol, embedded in paraffin, serially sectioned at 4µm and stained with hematoxylin and eosin.
The type of tissue adjacent to the implanted materials, the presence of inflammation, predominant cell type, and thickness of fibrous connective tissue next to each implant were recorded by three examiners who were unaware of the type of implanted materials in the tissues. The three evaluators were blinded to the treated groups and evaluated the histological sections according to the following category;
Data were statistically analyzed with the Kruskall-Wallis test. The level of significance for the overall differences was set at p<0.05.
III. Results
At group 1, most of histologic sections showed mild inflammation. The heavily inflammed tissues were composed of many infiltrated inflammatory cells such as lymphocytes, macrophages and plasma cells. And histologic slides at group 2 indicated that lesions were composed acute inflammatory cells such as macrophages and giant cells containing particles from test materials. But, at group 3, most of histologic sections had zones of severe inflammation. They had large numbers of inflammatory cells including granulocytes, macrophages, lymphocytes, plasma cells and showed large areas of tissue necrosis.
Histologic evaluation of group 4 indicated that lesions healed, but most of histologic slides were remained granulation tissue and inflammatory area. Remnants of test implant were surrounded by fibrous tissue. Some material particles exist in macrophages and vessles. At group 5, chronic inflammation was remained around the material particles. Some slides showed empty area of necrotic change.
In case of group 6, there was chronic inflammatory tissue in most histologic sections. Necrotic area indicated abscess formation.
The statistical analysis of inflammatory score was done with the Kruskall-Wallis test. The level of significance for the overall differences was set at p<0.05. It did not show significant differences among group 1, group 2, and group 3. However, it showed significant differences between group 3, 4 and group 6.
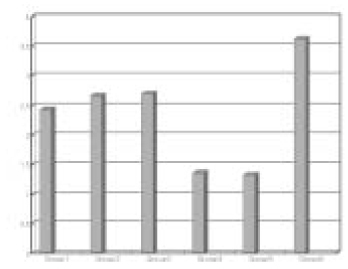
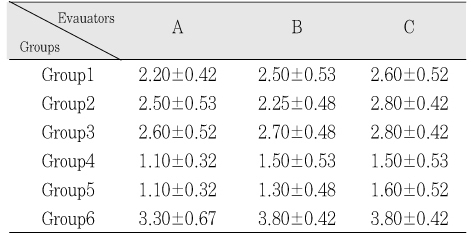
The obtained mean and standard deviation results were as follows (Table 3). The inflammatory scores at group 1, 2, 3, were higher than group 4, 5. But, it did not show statistical differences between groups or materials. But, at group 6, it showed significant differences in comparison with other groups. The degree of inflammation of six groups is summarized in Fig. 1. (Figure 2, 3, 4, 5, 6, 7)
IV. DISCUSSION
Several medicaments are used empirically in endodontics: formaldehyde, phenolic compounds, halogens, calcium hydroxide etc. The primary purpose of an intracanal medication is to reduce the root canal microflora following cleaning of the root canal while producing a minimal effect on normal host tissue. A secondary reason for use is to reduce postinstrumentation pain. Factors that influence the effect of intracanal medication were length of treatment, form of the drug, periodontal status, volume, and size of the apical foramen.14)
Among these materials, formaldehyde formulas have been routinely used by many dentists during the last 40 years. Formocresol may produce a humoral response in animals immunized with formocresol treated pulps then rechallenged with formocresol placed into root canals. In addition, formocresol appears to be mutagenic and carcinogenic.15)
In this experiment, Depulpin® had showed severe inflammation at 7 and 30 days. In early stage, it showed acute inflammatory reactions, but after 30 days, it showed chronic inflammation and pus formation. Martin and others16) reported extensive inflammation of the surrounding periapical tissue, including damage to attachment apparatus, as a result of toxicity penetrating power of formocresol. Because of the alkylating effect of formaldehyde, formaldehyde is extremely cytotoxic, causing widespread necrosis of vital tissue.2)
Moon and others17) reported the apical tissue of Depulpin®-treated tooth had showed large lesions at 4weeks. They concluded Depulpin® could cause more adverse reaction to periapical tissue than formocresol. In comparison our study, it had showed similar result. The more potent medications are also the more irritating to host tissues. So, It seems likely that Depulpin® has a great amount of cytotoxicity.
In contrast, calcium hydroxide has a great value in endodontics. It is a strong alkaline substance, which has a pH of approximately 12.5. In an aqueous solution, calcium hydroxide dissociates into calcium and hydroxyl ions. Various biological properties have been attributed to this substance, such as antimicrobial activity, tissue-dissolving ability, the inhibition of tooth resorption and induction of repair by hard tissue formation. Because of such effects, calcium hydroxide has been recommended for use in several clinical situations.10)
Most of the endopathogens are unable to survive in the highly alkaline environment provided by calcium hydroxide. Antimcobial activity of calcium hydroxide is related to the release of hydroxyl ions in an aqueous environment. Hydroxyl ions are highly oxidant free radicals that show extreme reactivity, reacting with several biomolecules. Hydroxyl ions induce lipid peroxidation, resulting in the destruction of phopholipids, structural components of the cellular membrane.18-20). But, the exact mechanism of action of calcium hydroxide is not clearly understood.
Several brands of calcium hydroxide preparation had been sold in dental market. Among these preparations, Tempcanal®, and Vitapex® were widely have been used in endodontic area in Republic of Korea. Tempcanal® was composed of true calcium hydroxide, but Vitapex® was composed of calcium hydroxide and iodoform(Table 1). Iodoform has several disadvantages, that is very potent disinfection matrial and possibility of discoloring tooth. But, in our experiment, Vitapex® showed desirable properties.
After seven days, histologic sections of calcium hydroxide indicated that lesions with acute inflammatory change. But after 30 days, histologic evaluation of this sites indicated that lesion healed, whereas remnants of test implant surrounded by fibrous tissue. Some material particles exist in macrophages and vessels. The main component of the tissue response to the Vitapex® was the foreign body reaction caused by the dispersion of material particles mediated by macrophages and giant cells.
Fuang and others21) reported that the calcium hydroxide sealer the least toxic in primary human PDL cultures in vitro. The helpful properties reported for calcium hydroxide included creation being indicated for several clinical conditions. In our work, calcium hydroxide containing paste had showed desirable tissue biocompatibility. However, we think that it is not a panacea. Calcium hydroxide has a limited antibacterial spectrum that does not affect all members of the endodontic microbiota. In addition, physicochemical properties of this substance may limit its effectiveness in disinfecting the entire root canal system after a short-term use6).
In endodontics, the use of intracanal medicament has decreased with time. But the endodontists had encountered many difficult cases in endodontic treatment; large perforations, endoperio involvement lesions, bucco-palatal connecting large lesions etc. We suggest that we should make an effort to overcome the difficulty in endodontic problem by intracanal medicaments. The new materials emerged in dental research; Bone Morphogenetic Proteins, Growth factors etc.22-25)
Maybe the promising drugs will be possible to raise the success rate of endodontic treatment, so we should to concentrate our efforts in developing new intracanal medications.
V. CONCLUSION
Intracanal medicaments had been used for root canal disinfection as a part of controlled asepsis in infected root canals and their role is secondary to cleaning and shaping of the root canal. The present work studied the reactions caused in the connective tissue of rats by implanting three brands of intracanal medicaments. Twenty male Sprague-Dawley rats were used. Each rat received three implants in ventral subcutaneous regions; Tempcanal®, Vitapex® and Depulpin®. Animals were sacrificed 7, 30 days after implantation. After processing of H-E staining, three evaluators examined and graded none (1), mild (2), moderate (3), and severe (4) inflammation. Data were statistically analyzed with the Kruskall-Wallis test (P<0.05).
At day 7, all three materials showed a mederate to severe inflammatory reactions and it was not statistically different from each other. After 30 days of implantation, Tempcanal® and Vitapex® showed none to mild inflammation but Depulpin® showed large numbers of inflammatory cells including granulocytes, macrophages, lymphocytes, plasma cells and large areas of tissue necrosis.
These findings suggest that Tempcanal® and Vitapex® showed compatible biological behavior, but Depulpin® showed severe cytotoxicity.
- 1. Lewis BB, Chestner SB. Formaldehyde in dentistry: a review of mutagenic and carcinogenic potential. J Am Dent Assoc. 1981;103: 429-434.ArticlePubMed
- 2. Foreman PC, Barnes F. A review of calcium hydroxide. Int Endod J. 1990;23: 283-297.ArticlePubMed
- 3. Spangberg L, Rutherg , Rydinge . Biological effect of endodontic antimicrobial agents. J Endod. 1979;5: 166-175.PubMed
- 4. Araki K, Isaka H, Ishii T, Suda H. Excretion of 14C-formaldehyde distributed systemically through root canal following pulpectomy. Endod Dent Traumatol. 1993;9: 196-199.Article
- 5. Hermann BW. Calciumhydrxyd als Mittel zum Behandel und Fullen von Zahnwur zel kanalen. 1920 09;V 29: Wurzburg: Med Diss.
- 6. Sjogren U, Figdor D, Spanberg L, Sundqvist G. The antimicrobial effect of calcium hydroxide as a short-term intracanal dressing. Int Endod J. 1991;24: 119-125.ArticlePubMed
- 7. Safavi KE, Nichols FC. Alteration of biological properties of bacterial lipopolysacchride by calcium hydroxide treatment. J Endod. 1994;20: 127-129.PubMed
- 8. Safavi KE, Spanberg L, Langerland K. Root canal dentinal tubule disinfection. J Endod. 1990;16: 207.ArticlePubMed
- 9. Kontakiotis E, Nakou M, Georgopoulou M. In vitro study of the indirect action of calcium hydroxide on the anaerobic flora of the root canal. Int Endod J. 1995;28: 285-289.ArticlePubMed
- 10. Siqueira JF, Lopes HP. Mechanisms of antimicrobial activity of calcium hydroxide: a critical review. Int Endod J. 1999;32: 361-369.ArticlePubMed
- 11. Pettis GY, Kaban LB, Glowacki J. Tissue response to composite ceramic hydroxyapatite/Demineralized bone implants. J Oral Maxillofac Surg. 1990;48: 1068-1074.ArticlePubMed
- 12. Tavares T, Soares IJ, Silveira NL. Reaction of rat subcutaneous tissue to implants of gutta-percha for endodontic use. Endod Dent Traumatol. 1994;10: 174-178.ArticlePubMed
- 13. Torabinejad M, Pitt Ford TR, Abedi HR, Kariyawasam SP. Tissue reaction to implanted root-end filling materials in the tibia and mandible of guinea pigs. J Endod. 1998;24(7):468-471.ArticlePubMed
- 14. Madison S, Anderson RW. Medications and temporaries in endodontic treatment. Dent Clin North Am. 1992;36: 343-356.ArticlePubMed
- 15. Block RM, Lewis RD, Sheats JB, Burke SG, Richmond VA. Antibody formation to dog pulp tissue altered by formocresol within the root canal. Oral Surg Oral Med Oral Pathol. 1978;45: 282-292.ArticlePubMed
- 16. Martin H, et al. Permeability of the apical third of the root to drugs used in endodontic therapy: an in vitro study. J Oral Ther Pharmacol. 1968;4: 451-455.PubMed
- 17. Moon HI, et al. Pulpal and Periapical Reaction to Formocresol and Depulpin® in the Rat Tissue. J Korean Acad Conserv Dent. 2002;27: 355-362.Article
- 18. Yang SF, Rivera EM, Baumgardner KR, Walton RE, Stanford C. Anaerobic Tissue-dissolving abilities of calcium hydroxide and sodium hypochlorite. J Endod. 1995;21: 613-616.ArticlePubMed
- 19. Foster KH, Kulild JC, Weller RN. Effect of smear layer removal on the diffusion of calcium hydroxide through radicular dentin. J Endod. 1993;19: 136-140.ArticlePubMed
- 20. Katebzadeh N, Hupp J, Trope M. Histological periapical repair after obturation of infected root canals in dogs. J Endod. 1999;25: 364-368.ArticlePubMed
- 21. Huang FM, Tai KM, Chou MY, Chang YC. Cytotoxicity of resin-, zinc oxide-, and calcium hydroxide-based root canal sealers on human periodontal ligaments cells and permanent V79 cells. Int Endod J. 2002;35(2):153-158.PubMed
- 22. Masatsugu I, Yasuharu Y, Masahiro M, Teruo A. Bone regeneration produced in rat femur defects by polymer capsules containing recombinant human bone morphogenetic protein-2. J Oral Maxillofac Surg. 1999;57: 695-698.ArticlePubMed
- 23. Rodgers JB, Vasconez MD, Wells MD, Deruca PP, Faugere MC, Fink BF, Hamilton D. Two lyophilized polymer matrix recombinant human bone morphogenetic protein-2 carriers in rabbit calvarial defects. J Craniofac Surg. 1998;9: 147-153.ArticlePubMed
- 24. King GN, King N, Hughes FJ. Effect of tow delivery systems for recombinant human bone morphogenetic protein-2 on periodontal regeneration in vivo. J Periodont Res. 1998;33: 226-236.
- 25. Uludag H, Darren D, Janet G, Jian Li, Wozney JM. Implantation of recombinant human bone morphogenetic proteins with biomaterial carriers: A correlation between protein pharmacokinetics and osteoinduction in the rat ectopic model. J Biomed Mater Res. 2000;50: 227-238.ArticlePubMed
REFERENCES
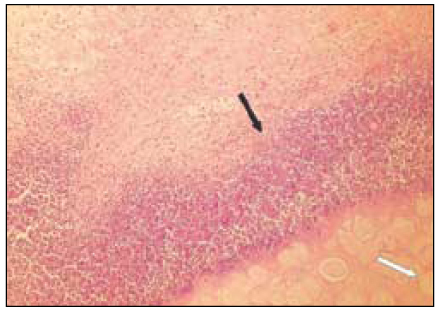
Fig. 2
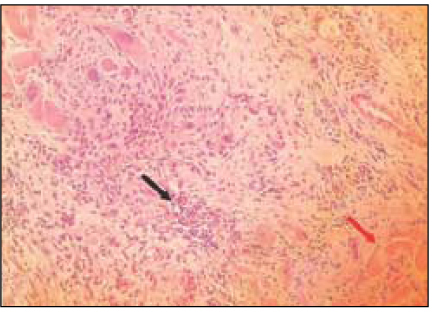
Group 1 (Tempcanal® 7Ds)(H&E stain, ×200)
Black Arrow: Inflammatory infiltrate.
Red Arrow: Material particles.

Fig. 3
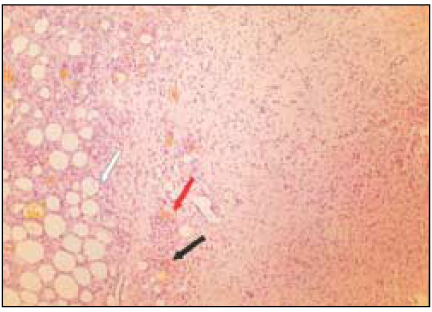
Group 2 (Vitapex® 7Ds) (H&E stain, ×200)
Black Arrow: Macrophages and giant cells containing particles from test materials.
Red Arrow: Material particles.
White Arrow: Necrotic area.

Fig. 4
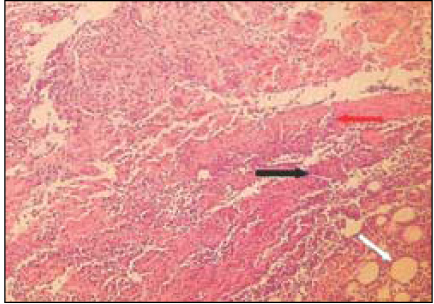
Group 3 (Depulpin® 7Ds) (H&E stain, ×200)
Black Arrow: Foci of reaction to material particles.
Red Arrow: Material particles.
White Arrow: Necrotic area.

Fig. 5
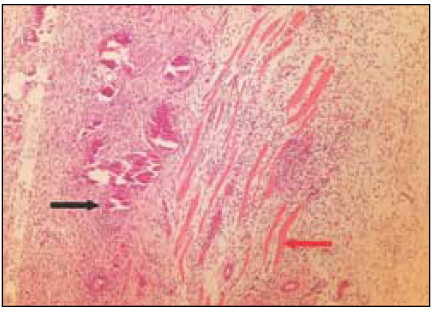
Group 4 (Tempcanal® 30Ds) (H&E stain, ×200)
Black Arrow: Material particles in macrophages and vessles. Remnants of implant material surrounded by fibrous capsule.
Red Arrow: Material particles.

Fig. 6
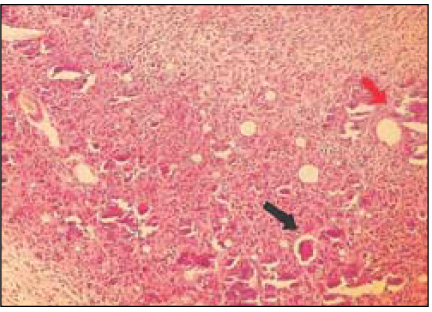
Group 5 (Vitapex® 30Ds) (H&E stain, ×200)
Black Arrow: Material particles in macrophages.
Red Arrow: Necrotic area.

Tables & Figures
REFERENCES
Citations
Citations to this article as recorded by 

Tissue reactions to implanted intracanal medicaments in rats
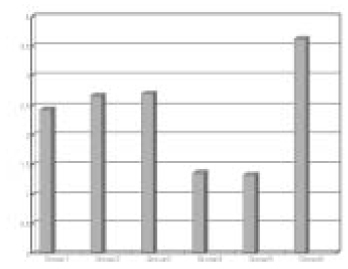
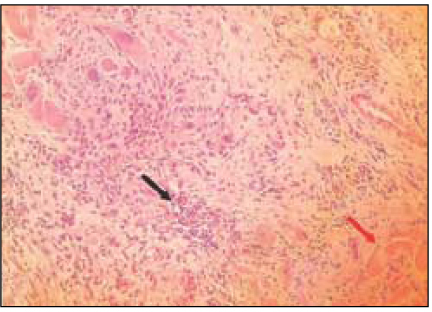
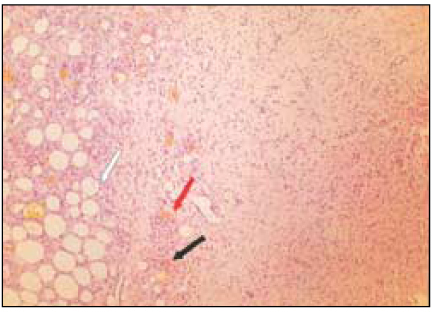
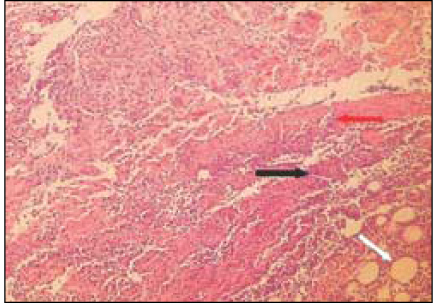
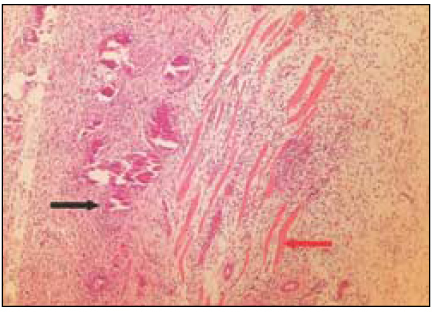
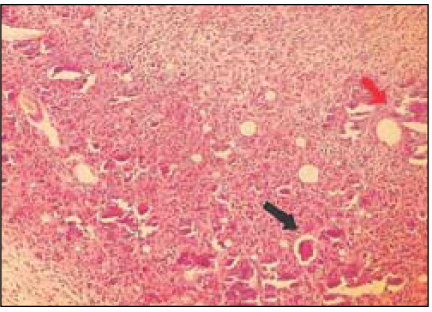
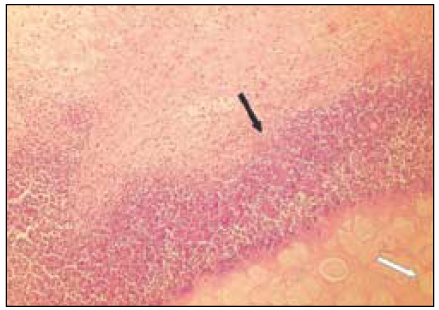
Fig. 1
Grade scores in each group.
Fig. 2
Group 1 (Tempcanal® 7Ds)(H&E stain, ×200)
Black Arrow: Inflammatory infiltrate.
Red Arrow: Material particles.
Fig. 3
Group 2 (Vitapex® 7Ds) (H&E stain, ×200)
Black Arrow: Macrophages and giant cells containing particles from test materials.
Red Arrow: Material particles.
White Arrow: Necrotic area.
Fig. 4
Group 3 (Depulpin® 7Ds) (H&E stain, ×200)
Black Arrow: Foci of reaction to material particles.
Red Arrow: Material particles.
White Arrow: Necrotic area.
Fig. 5
Group 4 (Tempcanal® 30Ds) (H&E stain, ×200)
Black Arrow: Material particles in macrophages and vessles. Remnants of implant material surrounded by fibrous capsule.
Red Arrow: Material particles.
Fig. 6
Group 5 (Vitapex® 30Ds) (H&E stain, ×200)
Black Arrow: Material particles in macrophages.
Red Arrow: Necrotic area.
Fig. 7
Group 6 (Depulpin® 30Ds) (H&E stain, ×200)
Black Arrow: Inflammatory infiltrate.
White Arrow: Necrotic area.
Fig. 1
Fig. 2
Fig. 3
Fig. 4
Fig. 5
Fig. 6
Fig. 7
Tissue reactions to implanted intracanal medicaments in rats
Composition of intracanal medicaments
The classification of experimental groups
Inflammatory scores of each group (Mean+SD).
Table 1
Composition of intracanal medicaments
Table 2
The classification of experimental groups
Table 3
Inflammatory scores of each group (Mean+SD).

 KACD
KACD
 ePub Link
ePub Link Cite
Cite

