Abstract
-
Objectives
This study evaluated the effect of adjacent gingival blood flow on detection of pulpal blood flow (PBF) using ultrasound Doppler flowmetry (UDF) through animal study.
-
Materials and Methods
The study included 36 right and left maxillary the third incisors and canines in 9 experimental dogs. The study included 2 main steps: In the first step, the pulse sound level (PSL) was recorded on the cervical part of each tooth without flap elevation (Group 1), with flap elevation (Group 2), and after it was repositioned in place (Group 3). In the second step, the PSL was recorded on the cervical part of each tooth (Group 4), after pulpotomy (Group 5), after partial pulp extirpation (Group 6), after complete extirpation (Group 7), and after canal filling (Group 8). In Groups 5–8, the study was performed with and without flap elevation in the left and right teeth, respectively. The PSL was graded as follows: 0, inaudible; 1, heard faintly; and 2, heard well. The difference between each group was analyzed using Friedman’s test with Wilcoxon signed-rank tests (α = 0.05).
-
Results
In step 1, the PSL results were Group 1 > 2 and 3. In step 2, there was no significant difference between the groups when the flap was not elevated, while PSL results were Group 4 > 5 ≥ 6 and 7 ≥ 8 when the flap was elevated.
-
Conclusions
PBF is affected by gingival blood flow when measured with UDF. UDF measurements require isolation of gingiva from the tooth.
-
Keywords: Ultrasound doppler flowmetry; Pulpal blood flow; Gingival blood flow; Animal study
INTRODUCTION
Clinical diagnosis of pulpal status is challenging and occasionally confusing. Pulpal nerve sensibility is commonly evaluated using thermal (cold or hot) and electric pulp tests (EPT). However, these tests do not consider pulp vitality or blood flow. In addition, these tests rely on patients’ subjective responses and can be painful sometimes.
Several methods have been used to directly assess pulpal blood flow (PBF). These include invasive methods, such as radioisotope clearance tests, H
2 gas desaturation tests, and other non-invasive methods, such as laser Doppler flowmetry (LDF), pulse oximetry, and dual-wavelength spectrophotometry [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13]. Among these, LDF has been widely researched and is considered reliable.
Ultrasound Doppler flowmetry (UDF) as a non-invasive and radiation-free technique, is used in the medical field to assess blood flow in microvascular systems. Based on the detection of the microvascularity of the lesion, UDF has been successfully used in the differential diagnosis of periapical granulomas and cysts and follow-up evaluations of the healing of periapical lesions after root canal treatment [
14,
15]. Yoon
et al. [
16] first reported the possibility of using UDF as a diagnostic tool for evaluating dental pulp vitality. Their study measured and distinguished the PBF of root-filled teeth and contralateral vital teeth using UDF. PBF changes before and after infiltration anesthesia can also be measured using UDF [
17]. Cho and Park [
18] reported cases in which discolored teeth that did not respond to any sensibility test after traumatic injury showed vital signs with UDF and were treated successfully without endodontic treatment. In addition, the pulpal blood flow velocity (PBFV) of clinically normal maxillary anterior teeth in the resting position was measured successfully using UDF, with values of approximately 0.5–0.6 cm/s, regardless of the tooth type: central incisor, lateral incisor, or canine [
19]. In a retrospective study to examine the effects of age, sex, and blood pressure on the PBFV in human maxillary anterior teeth. Kim and Park [
20] reported that while PBFV increased with an increase in systolic blood pressure, age, sex, and tooth type had no significant effect on the PBFV. UDF can be successfully used to evaluate the pulpal status of traumatized teeth [
7,
21]. Retrospective studies that aimed to evaluate and compare the efficacy of UDF with that of EPT in assessing pulp vitality in traumatized teeth reported UDF to be more sensitive [
21]. In their retrospective study, long-term (> 3 years) follow-up results of teeth that exhibited contradictory results (UDF+, EPT-, in all cases) between the pulp sensibility test (thermal or EPT) and UDF until 1 year after trauma were used to determine the prognosis of the pulp. In that study with a follow-up of more than 3 years, pulp sensibility was recovered in 8 of the 13 teeth. Two teeth failed to recover pulp sensibility and became symptomatic; necessitating root canal treatment. The remaining three teeth were from patients with nerve damage; therefore, the pulp sensibility test was not feasible. They reported that UDF reduced unnecessary root canal treatment because it directly evaluated the pulp vascularity and reduced false negative responses; thus, the shortcomings of sensibility tests (EPT, thermal test) in patients with trauma could be overcome by using UDF [
7].
However, limitations of UDF are also indicated. First, false-positive responses occurred in 2/69 cases [
7]. The 2 cases showed a continuous positive response at 1.5 and 2 years after the trauma, but eventually received root canal treatment based on symptoms, discoloration, and radiographic evaluation. The teeth in the above 2 cases might have been affected by blood flow from the surrounding soft tissues. However, their effects have not been scientifically studied.
Another challenge is the absence of a clear standard in UDF parameters to differentiate between normal and pathologic pulpal status [
21]. Some cases showed unclear and unstable UDF signals and sounds, leading to difficulty in determining normal pulpal status [
21]. However, this may only be confirmed through an animal study.
If the cause of the false positive reaction in the UDF can be identified and the standard for normal blood flow signal and sound can be secured, the accuracy of the UDF can be further increased and it will be able to contribute to more accurately measuring the vitality of the pulp.
The present study aimed to evaluate the effect of adjacent gingival blood flow on PBF detection using UDF in an animal model. This study also aimed to investigate the UDF pulse spectrum (PS) of vital pulp.
MATERIALS AND METHODS
The manuscript of this animal study has been written according to Preferred Reporting Items for Animal studies in Endodontology (PRIASE) 2021 guidelines [
22].
The study included 36 right and left maxillary the third incisors and canines in 9 healthy male mongrel dogs, aged approximately 10 months and weighing approximately 25–30 kg. The sample size was determined with reference to a previous human study [
17]. The power was 0.9 and the standardized effect size was 1.2.
The animals were housed and monitored daily for the study duration at the Department of Laboratory Animal Resources, Yonsei Biomedical Research Institute, Seoul, Republic of Korea. They were allowed to acclimatize for 1 week before the experiment. They were kept in individual cages at 22°C with a relative humidity of 50% and a 12-hour light/dark cycle. Approximately 500 g of solid food (Purina; Nestle SA, Vevey, Switzerland) was provided to each animal daily, and as much water as desired was available during the study period. The study complied with the Animal Research: Reporting of In Vivo Experiments guidelines and was performed in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals. This study was approved by the Institutional Animal Care and Use Committee (IACUC), Seoul, Republic of Korea (IACUC approval No. 2020-0325).
The study consisted of 2 main steps: The first step was conducted to determine how the PSL generated through the UDF was affected by the presence or absence of flaps. The second step was conducted to investigate the effect of PSL at each stage of root canal treatment, according to the presence or absence of flaps.
An MM-D-K (Minimax, Moscow, Russia) ultrasound Doppler imaging instrument with 20 MHz probe was used to measure pulp blood flow velocity. An ultrasound gel (Pro-gel II; Dayo Medical, Seoul, Korea) was applied as a coupling agent.
General anesthesia was induced by alfaxalone (Alfaxan; Jurox Pty Ltd., Rutherford, NSW, Australia), medetomidine hydrochloride (Tomidine; Provet Ltd., Istanbul, Turkey) intravenously and was maintained by 2% isoflurane (Ifran Liq; Hana Pharm Co Ltd., Seoul, Korea) in conjunction with pure oxygen by inhalation.
In the first step, after drying the tooth surface, the ultrasound gel was applied to the cervical area of the tooth, and UDF probe tip with a custom-made cap was placed at about 60° angle to the labial surface on the cervical part of each tooth, and the resulting PSL and PS were recorded (Group 1). Then, using a periosteal elevator, the gingiva was separated from the alveolar bone to elevate a flap, isolating it from the tooth contact. Hemostasis around the tooth was achieved using compression. Subsequently, the UDF was placed in the cervical region of each tooth as aforementioned, and the PSL and PS were measured and recorded (Group 2). The flap was then reapproximated using 3-0 black silk, and PSL and PS were measured and recorded using UDF as aforementioned (Group 3). Three observers agreed to the PSL while listening to the sound together and recorded the score (0: inaudible; 1: audible when putting the ear close to the device; 2: audible even at a distance of 2–3 m from the device). The steps in Groups 1–3 were performed continuously, and in the case of flap elevation, the experiments were conducted together with the third incisor and canine on the same side.
The second step was performed on the same test dog, 1 month after the first step. For the maxillary right the third incisor and canine of each experimental dog, the UDF probe tip was placed with a custom-made cap on the cervical part of each tooth, and the PSL and PS were recorded (Group 4). After pulpotomy (Group 5), partial pulp extirpation (up to 5 mm below the cementoenamel junction of each tooth) (Group 6), complete extirpation (Group 7), and canal filling with calcium paste (Well-Paste; Vericom, Anyang, Korea)(Group 8), PSL and PS were measured and recorded.
After the surgical procedures, the animals were administered ketorolac thromethamine (Keromin inj; Hana Pharm Co Ltd.), Cefazolin sodium (30 mg/kg; Chong Kun Dang, Seoul, Korea), Meloxicam (0.2 mg/kg; Boehringer Ingelheim, Kallithea, Greece) for a week.
PSL and PS were measured and recorded for each experimental dog’s left the third incisor and canine teeth similar to the right teeth in Group 4. In Groups 5–8, the gingiva was detached entirely from the alveolar bone using a periosteal elevator to elevate a flap, and 3-0 black silk was used for suturing to prevent it from coming in contact with the teeth. After hemostasis was achieved by compressing the alveolar bone area around the teeth for 5–10 minutes, PSL and PS were measured and recorded using the UDF similar to the right tooth group. Steps in Groups 4–8 were performed continuously, and in the case of flap elevation, steps were carried out with the third incisors and canines on the same side.
For the PSL data, the differences between each group on the maxillary left and right sides were analyzed using Friedman’s test (α = 0.05). When a post hoc test was required, Wilcoxon signed-rank tests were conducted with Bonferroni correction.
For the PS data, the spectrum corresponding to PSL levels 1–3 was first classified, and then a representative spectrum shape of each level was selected. To observe the spectrum under the same conditions, the brightness of the UDF device was set to 75, in contrast to 42, and the scale was set to 2 (left and right) and 4 (top and bottom) stages.
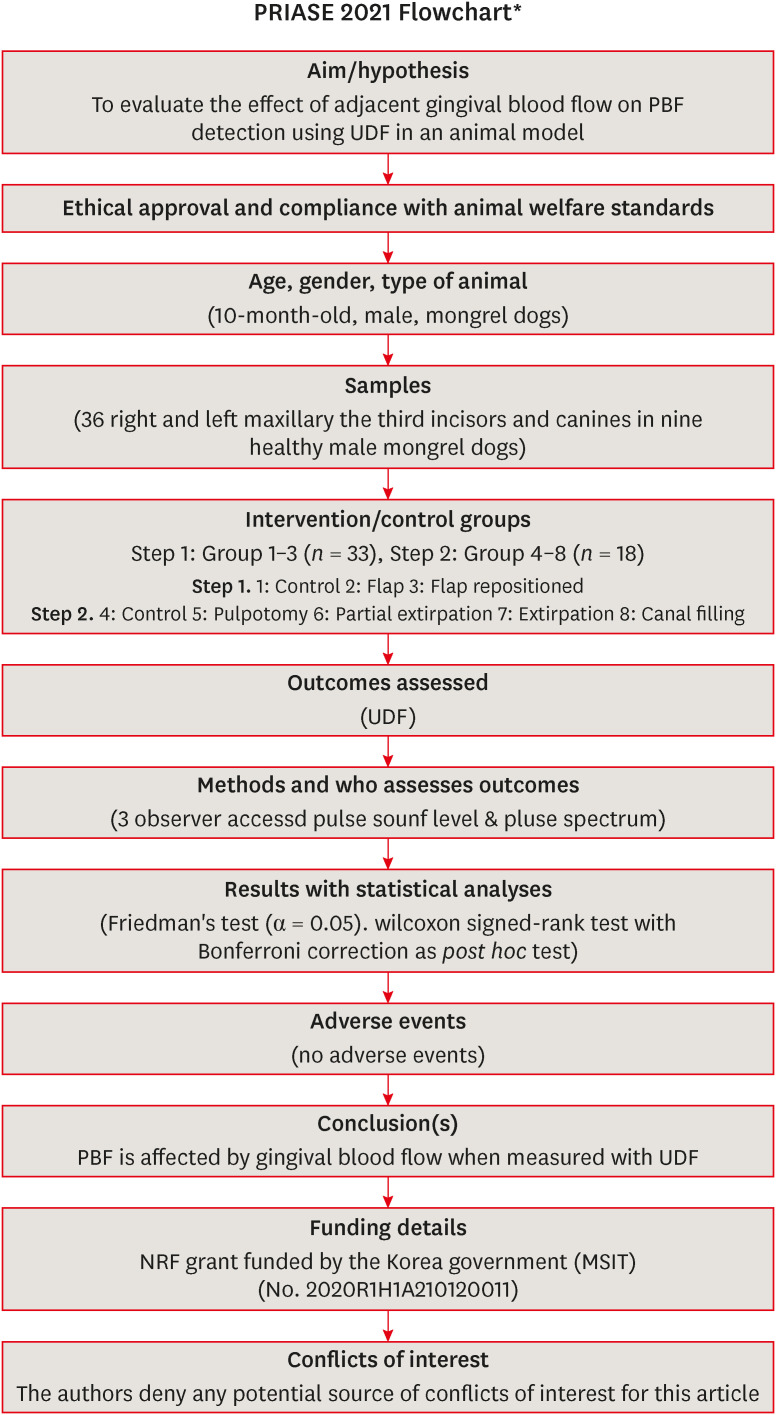
The flow of the present study is summarized in
Figure 1.
Figure 1
Preferred Reporting Items for Animal studies in Endodontology (PRIASE) 2021 Flowchart of present study.
UDF, ultrasound Doppler flowmetry; PBF, pulpal blood flow; NRF, National Research Foundation of Korea.
RESULTS
Step 1. Statistical analysis was performed on 33 data because three data were damaged during computer works. The mean, standard deviation, and median of the PSLs of the non-flap groups and those with flaps are summarized in
Table 1, and Friedman’s test showed that there was a difference between the groups (χ
2[
2] = 36.400;
p < 0.001).
Post hoc analysis using Wilcoxon signed-rank tests was conducted with Bonferroni correction, resulting in a significance level of 0.017. PSL was classified as Group 1 > 2 and 3 (
Table 2). The distribution of scores in each group is shown in
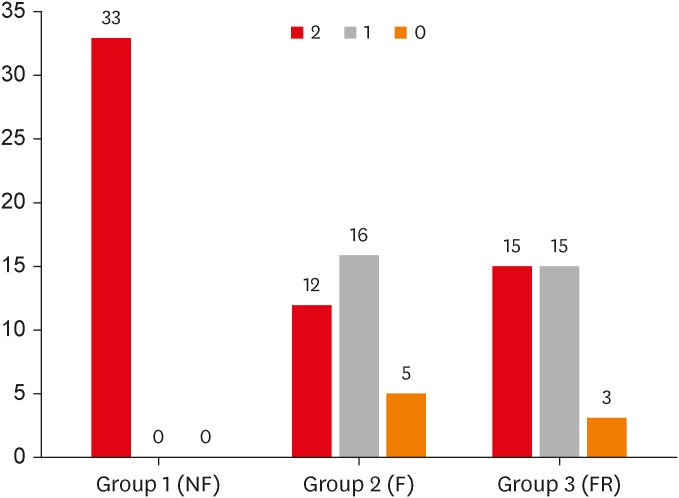
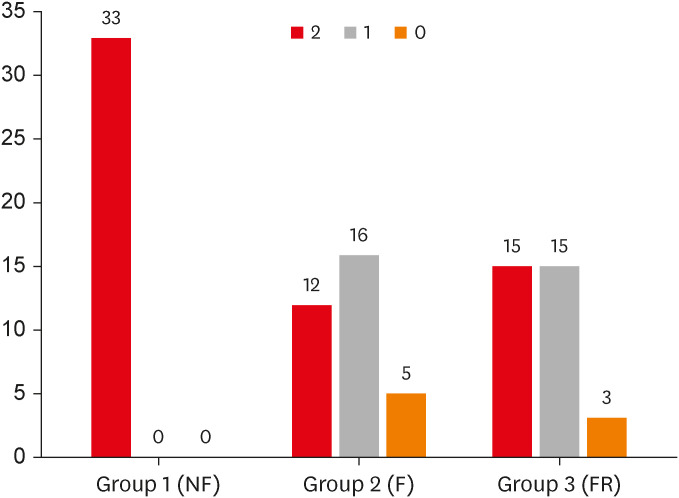
Figure 2. The frequencies of PSL core 2,1,0 were 12/33, 16/33, and 5/33 in Group 2, 15/33, 15/33, and 3/33 in Group 3, respectively, and in Group 1, they were all PSL 2.
Table 1 Pulse sound level score in steps 1 and 2
|
Steps |
No. |
Average |
Median |
|
Step 1 |
|
|
|
|
|
Group 1 |
33 |
2 (0.00) |
2 |
|
|
2 |
33 |
1.21 (0.70) |
1 |
|
|
3 |
33 |
1.36 (0.65) |
1 |
|
Step 2 |
|
|
|
|
NF |
|
|
|
|
|
Group 4 |
18 |
1.94 (0.24) |
2 |
|
|
5 |
18 |
1.89 (0.32) |
2 |
|
|
6 |
18 |
1.89 (0.32) |
2 |
|
|
7 |
18 |
2.00 (0.00) |
2 |
|
|
8 |
18 |
2.00 (0.00) |
2 |
|
F |
|
|
|
|
|
Group 4 |
18 |
1.94 (0.24) |
2 |
|
|
5 |
18 |
1.17 (0.70) |
1 |
|
|
6 |
18 |
1.00 (0.77) |
1 |
|
|
7 |
18 |
0.56 (0.70) |
0 |
|
|
8 |
18 |
0.44 (0.62) |
0 |
Table 2 Results of post hoc tests in steps 1 and 2 when flaps were elevated
|
Steps |
Z |
p
|
|
Step 1 |
|
|
|
G2–G1 |
−4.245 |
0.000*
|
|
G3–G1 |
−4.001 |
0.000*
|
|
G3–G2 |
−2.236 |
0.063 |
|
Step 2 (Flap) |
|
|
|
G5–G4 |
−3.276 |
0.000*
|
|
G6–G4 |
−3.153 |
0.000*
|
|
G7–G4 |
−3.624 |
0.000*
|
|
G8–G4 |
−3.739 |
0.000*
|
|
G6–G5 |
−0.812 |
0.449 |
|
G7–G5 |
−2.495 |
0.016 |
|
G8–G5 |
−3.127 |
0.001*
|
|
G7–G6 |
−1.780 |
0.098 |
|
G8–G6 |
−1.996 |
0.051 |
|
G8–G7 |
−0.816 |
0.750 |
Figure 2
Effect of flap on pulse sound level.
The number above the bar graph indicates the frequency.
NF, no flap; F, flap; FR, flap repositioned.
Step 2. The mean, standard deviation, and median of the PSLs of the groups are summarized in
Table 1. Friedman’s test results showed no difference between the groups when the flap was not elevated (χ
2[
4] = 6.667;
p = 0.30). However, there was a significant difference between the groups when the flap was elevated (χ
2[
4] = 42.959;
p < 0.001).
Post-hoc analysis with Wilcoxon signed-rank tests was conducted with Bonferroni correction, resulting in a significance level set at
p < 0.005. PSL was Group 4> 5 ≥ 6, and 7 ≥ 8 (
Table 2). The distribution of scores in each group is shown in
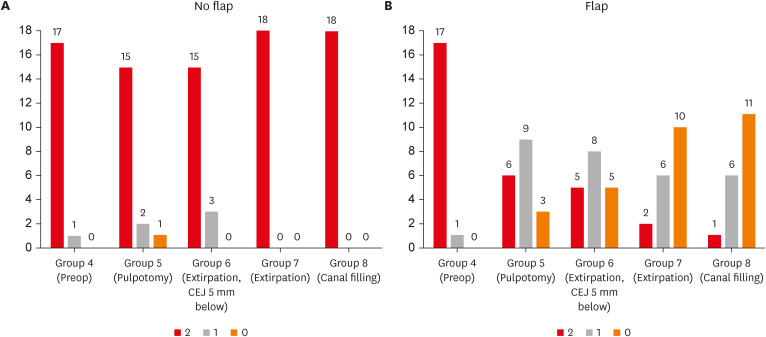
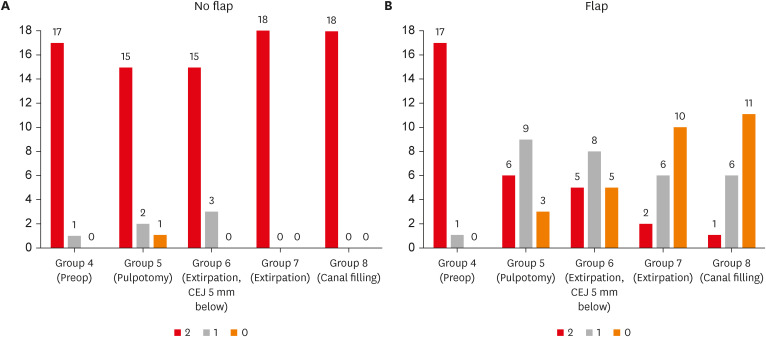
Figure 3. When flaps were not raised, the frequency of PSL score of 2, 1, 0 was 17/18, 1/18,0/18 in Group 4, 15/18, 2/18, 1/18 in Group 5, and 15/15, 3/18, and 0/18 in Group 6 respectively, and in groups 7 and 8, all 18 cases had a PSL score of 2. When flaps were elevated, the frequency of PSL scores of 2,1,0 was 17/18, 1/18, and 0/18 in Group 4, 6/18, 9/18, and 3/18 in Group 5, 5/15, 8/18, and 5/18 in Group 6, 2/18, 6/18, and 10/10 in Group 7, and 1/18, 6/18, and 11/18 in Group 8, respectively.
Figure 3 Effect of flap on pulse sound level in each endodontic procedure in the no flap groups (A) and flap groups (B). The number above the bar graph indicates the frequency.
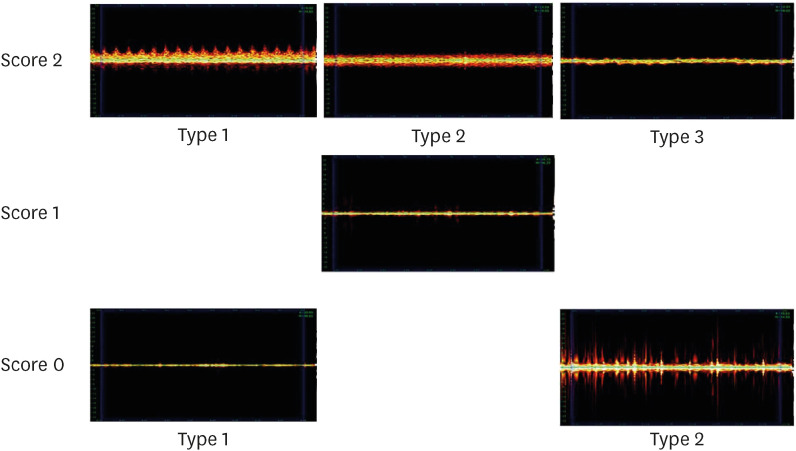
Representative PS for each PSL is shown in
Figure 4. In PSL 2, the following 3 types of PS 2 were observed.
Type 1: The difference between systole and diastole is clear; therefore, the peak is clearly visible.
Type 2: Systolic and diastolic differences were not observed; however, spray-like patterns were observed around them.
Type 3: No spray-like pattern was observed; however, a uniform peak was observed in the middle.
Figure 4 Representative pulse spectrum for each pulse sound level.
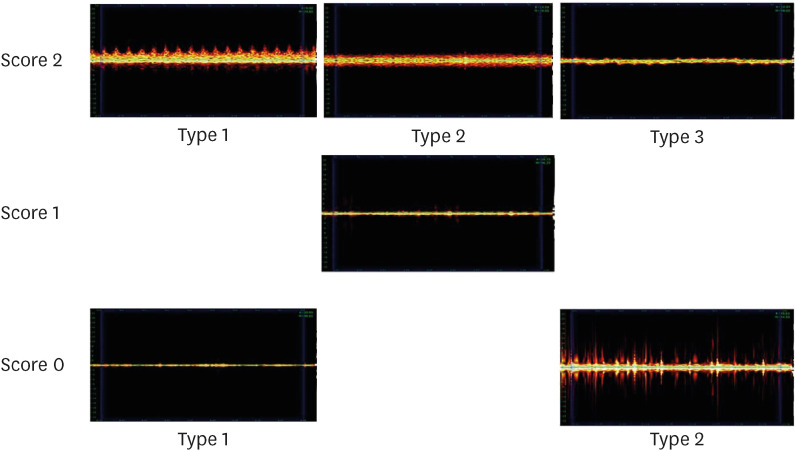
PS 1 for PSL 1 was observed to be low and uniform, with a few peaks and a slightly thick line.
In PS 0 for PSL 0, the following 2 types of PS could be observed.
Type 1: Spectrum is a narrow line, and peaks rarely appear.
Type 2: The spectrum appears broad; however, the interval between the peaks and the size of the peaks are not constant, and a significant amount of noise is observed.
No adverse health events occurred in the experimental dogs during the procedures.
DISCUSSION
In step 1, Group 1 showed a higher PSL than Group 2 (
Table 1). These findings suggest that the measurement of PBF in the UDF may be affected by the blood flow in the gingiva.
In step 1, the PSL of Group 3 was not different from that of Group 2 and was lower than that of Group 1 (
Table 2). The distribution of PSL also showed a similar pattern in Groups 2 and 3, which was different from that in Group 1 (
Figure 2). As for the blood flow supply to the attached gingiva adjacent to the tooth, there is a portion flowing into the attached gingiva from the unattached gingiva and a portion flowing into the gingiva from the alveolar bone through the periosteum. The reason for this result might be that the blood flow from the alveolar bone to the gingiva through the periosteum affects the PBF measurement of the UDF. If blood flow from the unattached gingiva to the attached gingiva had an effect, the PSL in Group 3 and its distribution would be the same as that in Group 1.
Since the tip of the UDF probe is directed toward the pulp when placed on the cervical part of the tooth, it would be difficult for gingival blood flow to directly affect PBF from a theoretical point of view. However, gingival blood flow may indirectly affect PBF because of the gel used in the present study. The gel helps transfer the ultrasonic energy from one material to another without loss and is essential when applying the UDF probe to the teeth. As part of the gel applied to the cervical region permeates into the gingival sulcus, ultrasonic energy from the UDF probe flows along the gel and detects blood flow through the periosteum and into the gingiva. It can be inferred that the false-positive responses reported by Ahn
et al. [
7] in previous studies were owing to this phenomenon.
In Group 2 of step 1, the PSL was 0 in 5/33 cases. Therefore, UDF exhibits approximately 85% sensitivity and approximately 15% false negatives in the measurement of PBF. In human teeth, the UDF probe is used for measurement by tilting it at an angle of approximately 60° to the long axis of the tooth. In humans, PBF measurement is relatively easy because the crown and root areas are parallel. In dogs, compared to humans, the root of the tooth is more curved; therefore, capturing at such an angle is challenging, and there is a high possibility that the measurement may be inaccurate [
23]. Additionally, the teeth of dogs are larger than those of humans, which was thought to be more advantageous for measuring PBF before initiating the study. However, the measurement was seemingly more time-consuming and challenging in dogs in this study. It can be speculated that the measurement may have been more difficult because the blood flow portion was relatively small compared with the size of the large tooth. However, further research is required. It is suspected that a false negative appeared because of several reasons.
In step 2, the PSL was found to be 2 or close to 2 in all groups measured without flap elevation, and there was no statistical difference between the groups. In contrast, there was a statistical difference between the PSLs of each group in the steps in which flaps were elevated and measured (
Tables 1 and
2). These results further support the results of step 1 that gingival blood flow significantly affects the measurement of PBF in the UDF. Additionally, it shows that the measurement result of PBF after flap elevation is much more objective than that without flap elevation.
In step 2, Group 5showed higher PSL than Group 8, and there was no statistical difference between Groups 5, 6, and 7 with flap elevation. Yoon
et al. [
24] reported that in a laboratory experiment using extracted teeth and simulated PBF, it was possible to measure PBF under the cementoenamel junction using UDF. In this study, the PSL in Groups 5 and 6 was approximately 1, and there was no statistical difference. To some extent, these results can be considered to agree with their study.
Although the PSL should theoretically be 0 in Groups 7 and 8 of step 2, the frequencies recorded as 2 and 1 were eight in Group 7 and seven in Group 8, that is, 44% and 39% of false positives appeared, respectively. Ahn
et al. [
7] reported in their clinical study that the tooth was necrotic, and false positives indicating a positive UDF measurement were found in 2/69 (3%) cases. However, in the present study, the false-positive percentage was significantly higher. In this study, all three measurements (Group 7, two times; Group 8, one time) indicated as PSL 2 were all PS 2 type 3. Therefore, further research is needed to determine whether teeth showing PS 2 type 2 are considered vital. The magnitude of the pulsuating sound may be more important than the magnitude of the simple sound. Additionally, many cases in Groups 7 and 8 indicated by PSL 1 showed a boundary between PS 1 and PS 0 type 1. This result is presumed to be because the dog’s teeth were curved, and the PSL was affected by blood flow around the root. Through additional research, a new method should be established to determine the vitality of teeth considering PSL and PS more specifically to improve results on the pulp vitality.
In this study, we found that isolation of the gingiva from the tooth is essential for correct measurement of PBF using UDF. For this, a custom-made splint, with only a part of the cervical tooth portion exposed, could be used, similar to the previous study. However, impression and splint-making processes are required, and a simpler and more effective method should be developed.
CONCLUSIONS
PBF is affected by gingival blood flow when measured with UDF. Thus, to correct the UDF measurement, the gingiva must be isolated from the tooth.
ACKNOWLEDGEMENTS
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (Ministry of Science and ICT; MSIT) (No. 2020R1H1A210120011).
-
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
-
Author Contributions:
Conceptualization: Park SH.
Data curation: Kim D.
Formal analysis: Kim D.
Funding acquisition: Park SH.
Investigation: Ko HS, Park SY, Ryu SY.
Methodology: Park SH, Kim D.
Project administration: Park SH.
Supervision: Park SH.
Validation: Kim D.
Visualization: Ko HS, Kim D.
Writing - original draft: Kim D.
Writing - review & editing: Park SH.
REFERENCES
- 1. Hock J, Nuki K, Schlenker R, Hawks A. Clearance rates of xenon-113 in non-inflamed and inflamed gingiva of dogs. Arch Oral Biol 1980;25:445-449.PubMed
- 2. Kim S, Schuessler G, Chien S. Measurement of blood flow in the dental pulp of dogs with the 133xenon washout method. Arch Oral Biol 1983;28:501-505.ArticlePubMed
- 3. Aukland K, Bower BF, Berliner RW. Measurement of local blood flow with hydrogen gas. Circ Res 1964;14:164-187.ArticlePubMed
- 4. Tönder KH, Aukland K. Blood flow in the dental pulp in dogs measured by local H2 gas desaturation technique. Arch Oral Biol 1975;20:73-79.ArticlePubMed
- 5. Emshoff R, Emshoff I, Moschen I, Strobl H. Laser Doppler flow measurements of pulpal blood flow and severity of dental injury. Int Endod J 2004;37:463-467.ArticlePubMed
- 6. Strobl H, Emshoff I, Bertram S, Emshoff R. Laser Doppler flow investigation of fractured permanent maxillary incisors. J Oral Rehabil 2004;31:23-28.ArticlePubMed
- 7. Ahn SY, Kim D, Park SH. Long-term prognosis of pulpal status of traumatized teeth exhibiting contradictory results between pulp sensibility test and ultrasound Doppler flowmetry: a retrospective study. J Endod 2018;44:395-404.ArticlePubMed
- 8. Roeykens HJ, Deschepper E, De Moor RJ. Laser Doppler flowmetry: reproducibility, reliability, and diurnal blood flow variations. Lasers Med Sci 2016;31:1083-1092.ArticlePubMedPDF
- 9. Roeykens HJ, De Coster P, Jacquet W, De Moor RJ. How standard deviation contributes to the validity of a LDF signal: a cohort study of 8 years of dental trauma. Lasers Med Sci 2019;34:1905-1916.ArticlePubMedPDF
- 10. Roeykens HJ, De Moor RJ. Diurnal variations and pulpal status: is there a need for FFT besides LDF? Lasers Med Sci 2018;33:1891-1900.ArticlePubMedPDF
- 11. Radhakrishnan S, Munshi AK, Hegde AM. Pulse oximetry: a diagnostic instrument in pulpal vitality testing. J Clin Pediatr Dent 2002;26:141-145.ArticlePubMedPDF
- 12. Schnettler JM, Wallace JA. Pulse oximetry as a diagnostic tool of pulpal vitality. J Endod 1991;17:488-490.ArticlePubMed
- 13. Nissan R, Trope M, Zhang CD, Chance B. Dual wavelength spectrophotometry as a diagnostic test of the pulp chamber contents. Oral Surg Oral Med Oral Pathol 1992;74:508-514.ArticlePubMed
- 14. Cotti E, Campisi G, Ambu R, Dettori C. Ultrasound real-time imaging in the differential diagnosis of periapical lesions. Int Endod J 2003;36:556-563.ArticlePubMedPDF
- 15. Rajendran N, Sundaresan B. Efficacy of ultrasound and color power Doppler as a monitoring tool in the healing of endodontic periapical lesions. J Endod 2007;33:181-186.ArticlePubMed
- 16. Yoon MJ, Kim E, Lee SJ, Bae YM, Kim S, Park SH. Pulpal blood flow measurement with ultrasound Doppler imaging. J Endod 2010;36:419-422.ArticlePubMed
- 17. Yoon MJ, Lee SJ, Kim E, Park SH. Doppler ultrasound to detect pulpal blood flow changes during local anaesthesia. Int Endod J 2012;45:83-87.ArticlePubMed
- 18. Cho YW, Park SH. Use of ultrasound Doppler to determine tooth vitality in a discolored tooth after traumatic injury: its prospects and limitations. Restor Dent Endod 2014;39:68-73.ArticlePubMedPMC
- 19. Cho YW, Park SH. Measurement of pulp blood flow rates in maxillary anterior teeth using ultrasound Doppler flowmetry. Int Endod J 2015;48:1175-1180.PubMed
- 20. Kim D, Park SH. Effects of age, sex, and blood pressure on the blood flow velocity in dental pulp measured by Doppler ultrasound technique. Microcirculation 2016;23:523-529.ArticlePubMed
- 21. Ahn SY, Kim D, Park SH. Efficacy of ultrasound Doppler flowmetry in assessing pulp vitality of traumatized teeth: a propensity score matching analysis. J Endod 2018;44:379-383.ArticlePubMed
- 22. Nagendrababu V, Kishen A, Murray PE, Nekoofar MH, de Figueiredo JA, Priya E, Jayaraman J, Pulikkotil SJ, Camilleri J, Silva RM, Dummer PM. PRIASE 2021 guidelines for reporting animal studies in endodontology: a consensus-based development. Int Endod J 2021;54:848-857.ArticlePubMedPDF
- 23. Rashed F. A comparative study of the dentition and temporomandibular joint anatomy and histology adult dogs. Biol Syst Open Access 2015;4:1000147.Article
- 24. Yoon MJ, Kim DH, Jung IY, Park SH. A laboratory study to detect simulated pulpal blood flow in extracted human teeth using ultrasound Doppler flowmetry. Int Endod J 2021;54:231-240.ArticlePubMedPDF
 , Hyoung-Seok Ko
, Hyoung-Seok Ko , Soo-Yeon Park
, Soo-Yeon Park , Seung-Yeon Ryu
, Seung-Yeon Ryu , Sung-ho Park
, Sung-ho Park





 KACD
KACD

 ePub Link
ePub Link Cite
Cite

